The regulatory variant rs17612742 confers the risk of large artery atherosclerotic stroke by increasing the expression of endothelin receptor type A
Di Luan,Xing-Lun Dang,Xi Chen,Zi-Xu Wang,Shi-Fei Ye,Yuan-Xiang Zhang,Li-Li Yuan,Kun Lian
1School of Medicine,Southeast University,Nanjing,Jiangsu 210009,China.2Key Laboratory of Animal Models and Human Disease Mechanisms of the Chinese Academy of Sciences&Yunnan Province,Kunming Institute of Zoology,Chinese Academy of Sciences,Kunming,Yunnan 650223,China.3Kunming College of Life Science,University of Chinese Academy of Sciences,Kunming,Yunnan 650204,China.4Department of Neurosurgery,The Second Affiliated Hospital of Kunming Medical University,Kunming,Yunnan 650101,China.5Department of geriatric psychiatry,Nanjing Brain Hospital Affiliated to Nanjing Medical University,Nanjing,Jiangsu 210024,China.6Department of Cerebrovascular Disease,Shanghai Fourth People’s Hospital affiliated to Tongji University School of Medicine, Shanghai 200000, China.7School of Pharmacy, Wannan Medical College, Wuhu, Anhui 241000, China.8Department of Neurology, First Affiliated Hospital of Wannan Medical College,Wuhu,Anhui 241000,China.9 Yunnan Clinical Research Center for Mental Disorders,the first Affiliated Hospital of Kunming Medical University,Kunming,Yunnan 650032,China.
Abstract Genome-wide association studies have found that rs17612742 increases the risk of large artery atherosclerotic stroke.The rs17612742 was located in the intron region of Endothelin receptor type A (NRA), which was cellular and gender-heterogeneous.Functional genomics studies of rs17612742 were carried out through some public databases, and it was found that rs17612742 was located in the chromatin open region and promoted the expression of NRA.Compared with the control group, EDNRA expression increased in middle cerebral artery occlusion exposed rodents.However, age heterogeneity of EDNRA expression under physiological and pathological conditions was not observed.In addition, we also discussed how to conduct further empirical studies to provide evidence that rs17612742 confers the risk of large artery atherosclerotic stroke by increasing the expression of NRA.
Keywords:large artery atherosclerotic stroke; rs17612742; EDNRA
Introduction
A stroke consists of 70% ischemic stroke and 30% intracerebral hemorrhage.Large artery atherosclerotic stroke accounts for 25% of ischemic stroke [1–3].Based on large-scale collaborative genome-wide association studies (GWAS), the heritability of LAS is about 40% [4, 5].Several GWAS studies have found that rs17612742 increases the risk of large artery atherosclerotic stroke [6–9].Specifically, to identify genetic factors contributing to stroke, the MEGASTROKE consortium collected data from 29 large-scale studies,including 67,162 patients who had experienced a stroke and 454,450 controls.Multiple stroke risk loci were identified using a genome-wide association meta-analysis, of which rs17612742 was found to be associated with an increased risk of large artery atherosclerotic stroke[6].Traylor et al.performed Bayesian multinomial regression analysis on 16,664 stroke cases and 32,792 controls and found that rs17612742 increases the risk of large artery atherosclerotic stroke and small vessel stroke while reducing the risk of intracerebral haemorrhage and cardioembolic stroke [7].In addition, Wang et al.found that rs17612742 was not associated with Alzheimer's disease[8].Moreover, Ishigaki et al.found that rs17612742 positively correlated with peripheral artery stenosis in the Japanese population[10].
The rs17612742 was located in the intron region ofEndothelin receptor type A(EDNRA)on chromosome 4q31.22(Figure 1 A).NRA is a receptor for endothelin-1, which is functional as vasoconstriction and involved in mandibulofacial dysostosis with alopecia [11, 12].In addition, EDNRA was involved in multiple signaling pathways,including calcium, cGMP-PKG, cAMP signaling pathway, and renin secretion [13].Endothelin-3/Ednra signaling pathway was implicated in axon growth and nervous system development [14].Edna-/-embryos showed craniofacial skeletal deformities and muscle defects[15–19].The craniofacial defect induced by Edna mutant can be rescued via Edna cDNA knock-in [20].
The function of EDNRA was cellular and gender-heterogeneous.Conditional knockout of vascular mural cells Edna in mice, but not in retinal neurons and macroglia,prevented endothelin-1-induced retinal ganglion cell death [21].Hypoxia increased EDNRA expression in male brain microvascular endothelial cells (BMVECs)but decreased in female BMVECs.NRA antagonists prevented endothelin-1-induced death of BMVECs in females but not males[22].Conditional knockout of vascular smooth muscle Edna in mice reduced blood pressure and vascular reactivity and affected vascular development [23].
Hypothesis/Theory
The allele frequencies of rs17612742
The 1000 Genomes Project Phase 3 data showed that the frequency of the C allele of rs17612742 ranged from 14% to 23% (Supplementary Figure 1).For the Chinese population, the C allele frequencies of rs17612742 in PGG.Han [24],China Metabolic Analytics Project[25],and WBBC[26]data were 24.10%,22.55%,and 22.53%,respectively.The analysis of east-west differentiation shows that low genetic differentiation of rs17612742 between Utah residents with Northern and Western European ancestry and Chinese Han populations (FST =0.012) (Figure 1 B).
Functional genomics annotation of rs17612742
Functional genomics annotation tools RegulomeDB v2.0.3[27],CADD v1.6[28],GWAVA[29],and INSIGHT[30]indicated that rs17612742 might be a regulatory variant (Figure 1 C).The DNA methylation quantitative trait loci and H3K9ac QTLs analysis showed that rs17612742 promotes the methylation of cg16264526(chr4:148401273, EDNRA) (Figure 1 D).

Figure 1 The rs17612742 was associated with an increased risk of large artery atherosclerotic stroke.A,The rs17612742 was located in the intron region of Endothelin receptor type A on chromosome 4q31.22; B, The analysis of east-west differentiation shows that low genetic differentiation of rs1950834 between CEU and Han populations; C, Functional genomics annotation tools indicated that rs17612742 might be a regulatory varian;D,The DNA methylation quantitative trait loci(mQTLs) and H3K9ac QTLs(haQTLs)analysis showed that rs17612742 promotes the methylation of cg16264526(chr4:148401273,EDNRA).mQTLs,methylation quantitative trait loci;haQTLs,H3K9ac QTLs;CEU,Utah residents with Northern and Western European ancestry, EDNRA, Endothelin receptor type A.
DNase-seq of neurogenic cell lines, in vitro differentiated neurons,primary brain, vascular endothelial cells, and brain tissue indicated that rs17612742 was located in the chromatin open region (Figure 2).H3K4me3, H3K27me3, H3K36me3, H3K9me3, H3K4me1, H3K9ac,and H3K27ac ChIP-seq of the fetal and adult brain suggested that the locus of rs17612742 was a regulatory region, and might promote the transcription ofEDNRA(Figure 3).
Chromatin interaction visualization analysis showed that rs17612742 physically interacted withEDNRA(including the promoter regions) in the human aorta and hippocampus (Supplementary Figure 2).

Figure 2 A, DNase-seq of neurogenic cell lines, in vitro differentiated neurons; B, primary brain and vascular endothelial cells;C, and brain tissue;D,indicated that rs17612742 was located in the chromatin open region.

Figure 3 H3K4me3, H3K27me3, H3K36me3, H3K9me3, H3K4me1, H3K9ac, and H3K27ac ChIP-seq of fetal(A) and adult brain (B)suggested that the locus of rs17612742 was a regulatory region.
The expression QTLs analysis of rs17612742
The rs17612742 has four nearby genes,EDNRA,protein arginine methyltransferase 9,transmembrane protein 184C, andRho GTPase activating protein 10.The eQTLs analysis of rs17612742 showed that it only affected the expression ofEDNRAbut not the other three genes.Compared with the T allele of rs17612742, the risk allele C of large artery atherosclerotic stroke promotedEDNRAexpression (Figure 4).
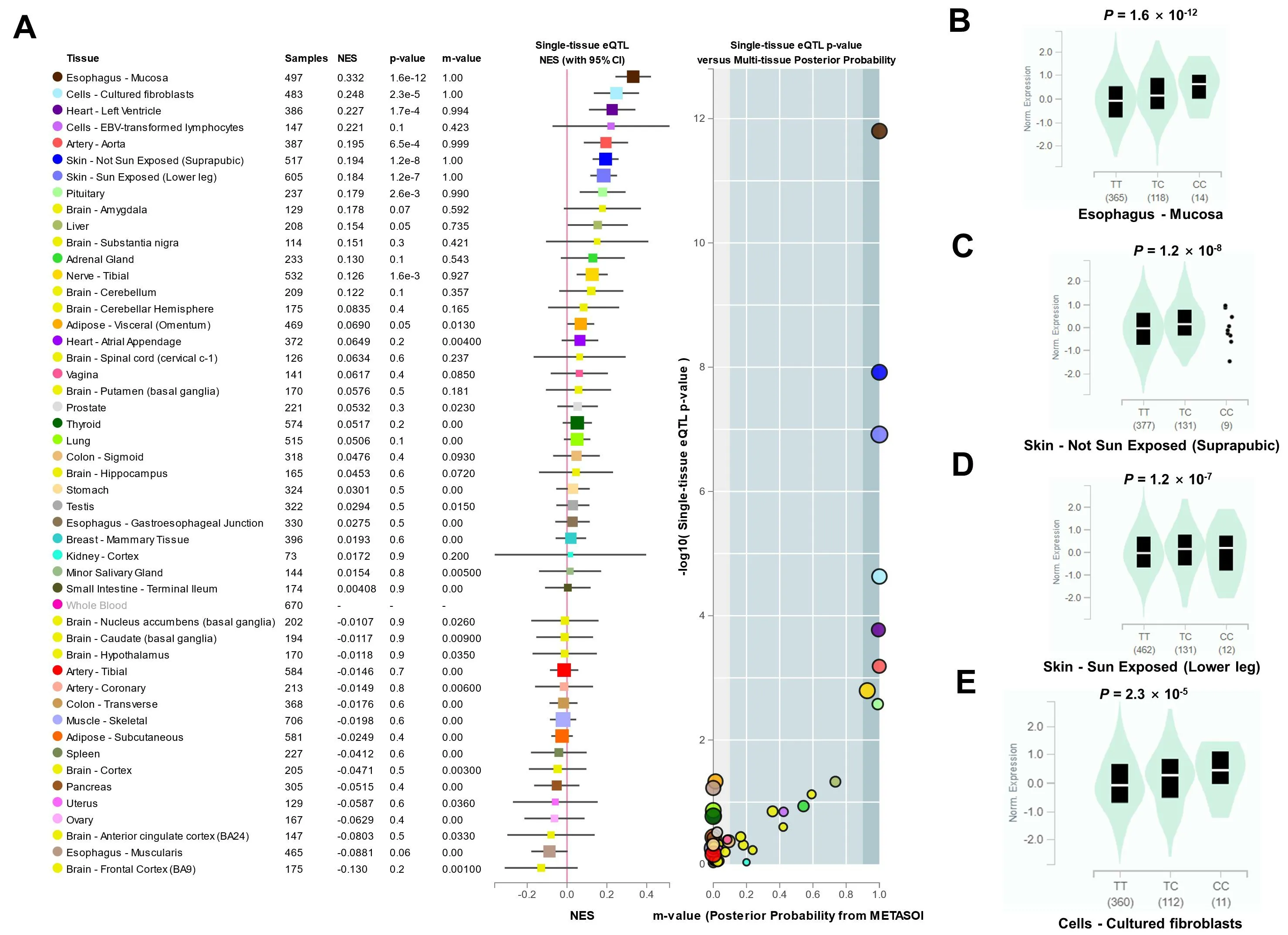
Figure 4 The eQTLs analysis of rs17612742 showed that it affected the expression of EDNRA.A, Multi-organization meta-analysis RE2 P value=1.41×10-28;B,The rs17612742 affected the expression of EDNRA in tissues esophagus-mucosa(B),skin-not sun exposed(suprapubic)(C),skin-sun exposed (lower leg) (D), and cells-cultured fibroblasts (E).
Discussion /Consequences of the hypothesis
GWAS found many disease-related potential risk variants without prior assumptions and bias.The main task in the post-GWAS era was to identify causal variations involved in the pathophysiological process of diseases from these associated variations discovered by GWAS [31–35].Most single nucleotide polymorphisms (SNPs) were located in non-coding regions, and the widespread linkage disequilibrium between SNPs increases the difficulty of finding causal variation.One of the potential solutions is to verify whether the discovery of GWAS is a causal variation by combining ENCODE [36,37], Roadmap [38], GTEx [39, 40], and other functional genomics-related databases with cell and animal experiments.
Whether rs17612742 was a regulatory variation needs further empirical research.For instance, use dual-luciferase reporter assays to assess whether rs17612742 had promoter or/and enhancer activity[41].Electrophoretic mobility shift assay was used to evaluate the binding ability of the C and T allele of rs17612742 to transcription factors [42–44].Further, whether knockdown transcription factors affect the expression ofNRA.Through gene editing knocked out the rs17612742 to detect whether the expression ofEDNRAwas affected[45–47].Due to the differences between rodent and human genomes,it was difficult to study the mechanism of rs17612742 participating in large artery atherosclerotic stroke pathophysiological processes in rodents.Induced pluripotent stem cells and iPSC-derived neural cell models of large artery atherosclerotic stroke patients with rs17612742 variant may be a potentially powerful research tool [48–50].In addition, neural rosettes [51], neurospheres [52], and cerebral organoids[53, 54] may also be powerful research tools.
Notably, the SNPs rs61202211 (R2 = 1.0), rs6841473 (R2 =0.961), rs10305839 (R2 = 0.932) and rs73855814 (R2 = 0.928)were in linkage disequilibrium with rs17612742 and affectedEDNRAexpression [55–58].Experiments were still needed to detect whether the SNPs mentioned above were causal variations of large artery atherosclerotic stroke.
Given that EDNRA was conserved in euteleostomi and the homology between humans and mice was 92.27%, the mouse middle cerebral artery occlusion (MCAO) model can be used to clarify the specific mechanism of EDNRA in the pathophysiological process of large artery atherosclerotic stroke (Figure 5) [59, 60].

Figure 5 EDNRA was conserved in euteleostomi (A), and the homology of human and mouse was 92.27% (B, dot matrix view of NP_001948.1 vs NP_034462.1; C, multiple alignment results-Alignments; D, multiple alignment results-Graphical overview).
To evaluate the physiological changes of Edna in large artery atherosclerotic stroke cases, the GEO datasets were searched with“MCAO” as the keyword [61, 62], and 28 rodent transcriptional data sets were included after reading the title, summary, and overall design.After the expression data of EDNRA in the MCAO group and control group were extracted, a meta-analysis was performed using Review Manager 5.4 [63, 64].The results showed that compared with the control group, the expression of Edna was increased in the MCAO group (Figure 6) (Supplementary Figure 3 A).The results of subgroup analysis showed that compared with the control group, the expression of Edna in the MCAO group brain tissue increased (Figure 6 B)(Supplementary Figure 3 B).At the same time, there was no statistical difference in the expression of EDNRA in blood and cell specimens(Figure 6 C) (Supplementary Figure 3 D).

Figure 6 Forest plots of comparison between MCAO group and control group in brain, blood, and cell specimens (A); brain specimens (B); blood specimens (C); cell specimens (D).
In addition,GSE166162[65]and GSE137482[66]were analyzed to assess the age heterogeneity of EDNRA under physiological and pathological conditions (Figure 7).Age heterogeneity of Edna expression was not found.However, due to the small sample size, the age heterogeneity of potential Edna expression may be masked, and more data were needed for future evaluation.

Figure 7 Age heterogeneity of Ednra expression was not found.A,GSE166162;B,GSE137482;the statistical method used was two independent sample T test.
NRA was a receptor for endothelin-1.Mediates its action by association with G proteins that activate a phosphatidylinositol-calcium second messenger system.The rank order of binding affinities for EDNRA was: endothelin-1 >endothelin-2 >> endothelin-3.Function prediction tools GeneMANIA and STRING v11.5 were used to predict the functional mechanism of EDNRA from gene and protein levels, respectively, as shown in Figure 8.
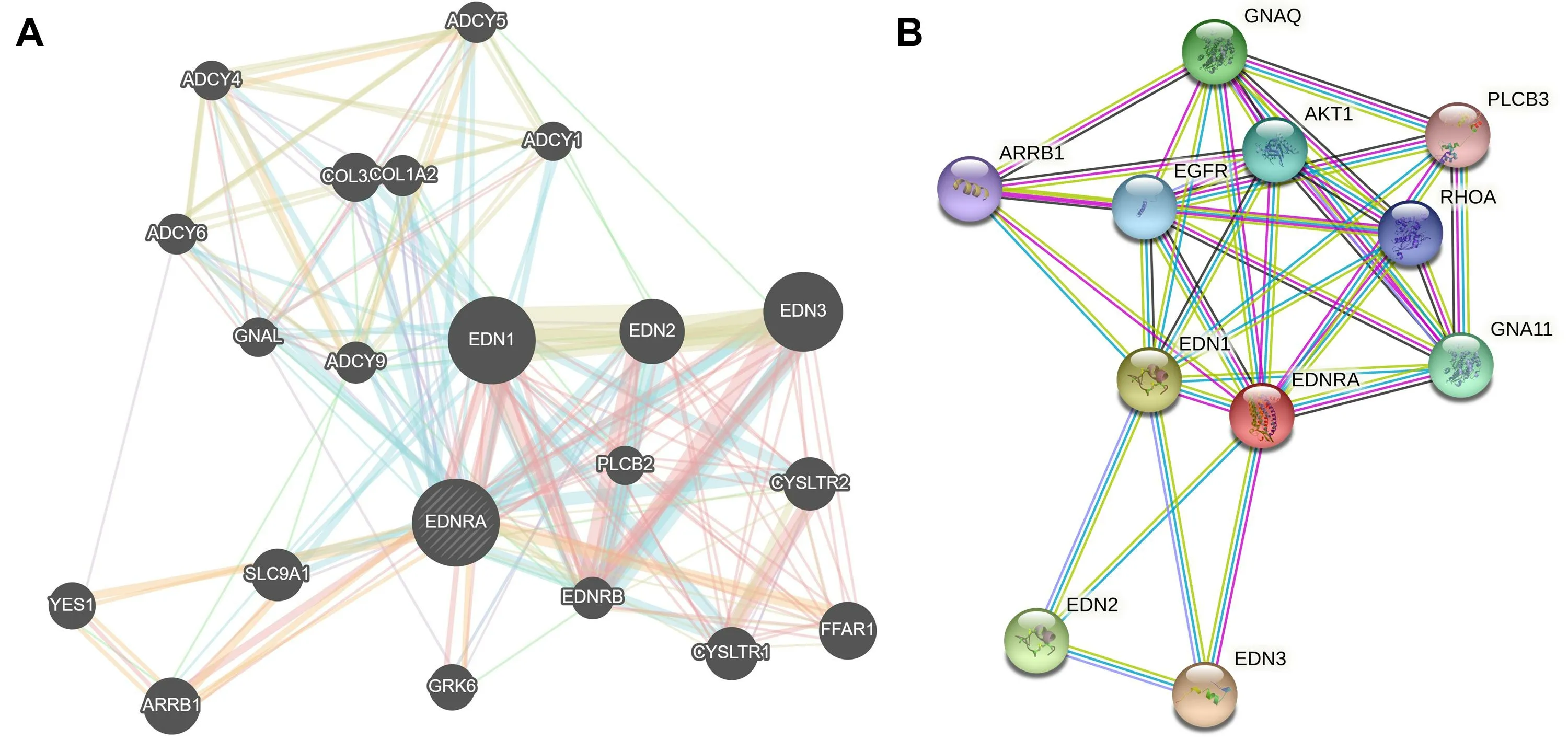
Figure 8 GeneMANIA (A) and STRING v11.5 (B)predicted the functional mechanism of EDNRA from gene and protein levels, respectively.
Fully elucidating the pathophysiological mechanism of rs17612742 regulating the risk of large artery atherosclerotic stroke by increasing the expression of NRA, as well as the age, tissue, and cell heterogeneity of EDNRA, may help to broaden the understanding of stroke, and rs17612742 and EDNRA may be potential targets for the treatment of stroke.
- Life Research的其它文章
- Exploring the practice of common oral diseases among the patients visiting in the selected dental college and hospital in Bangladesh
- Different levels of MUC5AC and MUC5B genes expression in severe allergic versus non-allergic asthma
- Research progress on iron homeostasis regulation and heart-related diseases
- The establishment of pancreatic islet isolation in India – an update on human pancreatic islet transplantation
- Physiological signal processing in heart rate variability measurement: A focus on spectral analysis
- Research status of persistent vegetative state in recent 20 years: A bibliometric analysis

