Bone marrow mesenchymal stem cells and exercise restore motor function following spinal cord injury by activating PI3K/AKT/mTOR pathway
Xin Sun , Li-Yi Huang , Hong-Xia Pan , Li-Juan Li , Lu Wang , Gai-Qin Pei , Yang Wang , Qing Zhang , Hong-Xin Cheng ,Cheng-Qi He , Quan Wei ,
Abstract Although many therapeutic interventions have shown promise in treating spinal cord injury, focusing on a single aspect of repair cannot achieve successful and functional regeneration in patients following spinal cord injury.In this study, we applied a combinatorial approach for treating spinal cord injury involving neuroprotection and rehabilitation, exploiting cell transplantation and functional sensorimotor training to promote nerve regeneration and functional recovery.Here, we used a mouse model of thoracic contusive spinal cord injury to investigate whether the combination of bone marrow mesenchymal stem cell transplantation and exercise training has a synergistic effect on functional restoration.Locomotor function was evaluated by the Basso Mouse Scale, horizontal ladder test, and footprint analysis.Magnetic resonance imaging, histological examination, transmission electron microscopy observation, immunofluorescence staining, and western blotting were performed 8 weeks after spinal cord injury to further explore the potential mechanism behind the synergistic repair effect.In vivo, the combination of bone marrow mesenchymal stem cell transplantation and exercise showed a better therapeutic effect on motor function than the single treatments.Further investigations revealed that the combination of bone marrow mesenchymal stem cell transplantation and exercise markedly reduced fibrotic scar tissue, protected neurons, and promoted axon and myelin protection.Additionally, the synergistic effects of bone marrow mesenchymal stem cell transplantation and exercise on spinal cord injury recovery occurred via the PI3K/AKT/mTOR pathway.In vitro, experimental evidence from the PC12 cell line and primary cortical neuron culture also demonstrated that blocking of the PI3K/AKT/mTOR pathway would aggravate neuronal damage.Thus, bone marrow mesenchymal stem cell transplantation combined with exercise training can effectively restore motor function after spinal cord injury by activating the PI3K/AKT/mTOR pathway.
Key Words: axon growth; bone marrow mesenchymal stem cell; exercise training; mTOR; neuroprotection; neurotrophin; remyelination; scar formation; spinal cord injury; synaptic plasticity
Introduction
Spinal cord injury (SCI) is a serious and complex disease that typically leads to lifelong disability and enormous healthcare burdens (Karsy and Hawryluk,2019).The pathophysiology of spinal cord injury can be divided into primary and secondary injuries (Fischer et al., 2020).The primary injury is the initial trauma to neurons, glial cells, and their surrounding vasculature caused by initial mechanical forces such as compression, shearing, and stretching (Ahuja et al., 2017b).After the primary injury event, a cascade of secondary events,including apoptosis of neurons, glial scarring, demyelination, excitotoxicity,and the release of proinflammatory factors, can further exacerbate neurological dysfunction and expand the zone of tissue injury (Ahuja et al.,2017a)
To date, many neuroregenerative strategies have been investigated as potential therapies for SCI.Previous studies demonstrated that the transplantation of stem cells has considerable potential for promoting tissue repair and functional improvement following SCI (Chiba et al., 2009;Ding et al., 2014; Lin et al., 2018; Li et al., 2021).In addition, bone marrow mesenchymal stem cells (BMMSC) have attracted attention because they are easy to harvest and preserve, have low immunogenicity, and reduce the risk of developing tumors (Stewart and Stewart, 2011).Additionally, BMMSC can migrate toward the injury site, exhibiting the property of “homing” (Kim et al., 2021; Ma et al., 2022).The most common mechanisms of cell therapy include neuroprotection, immunomodulation, neuronal relay formation,axonal regeneration, and remyelination (Assunção Silva et al., 2022; Fan et al.,2022).Although BMMSC show therapeutic promise according to preclinical findings, clinical trials still fail to demonstrate functional recovery and neural circuit restoration (Cofano et al., 2019).A randomized study tested the clinical efficacy of autologous BMMSC transplantation in subacute SCI patients, and the results showed that BMMSC transplantation into the injury site of SCI patients can be performed safely, but effects on motor function have not yet been observed (Karamouzian et al., 2012).Many other clinical trials have also failed to report satisfactory clinical improvements (Syková et al., 2006;Geffner et al., 2008; Mendonça et al., 2014).Stem cell grafts alone showed weak efficacy in functional recovery in human SCI.One possible explanation for this is that the transplanted cells could not form a sufficient number of functional connections and failed to sustain stable neuronal relays with the host circuitry for a long time (Hutson and Di Giovanni, 2019).Rehabilitationtraining seems to be an effective approach for locomotor function recovery after SCI, which may be related to the plasticity of spinal neurons below the level of injury, corticospinal tract growth, and enhancement of neurotrophic factor production (Foret et al., 2010; Flynn et al., 2013; Houle and Côté, 2013;Morawietz and Moffat, 2013; Wang et al., 2015; Hinahon et al., 2017; Yang et al., 2020).Studies in clinical trials have also shown that functional recovery after exercise is related to the degree of activation of the motor cortex (Jo and Perez, 2020; Bilchak et al., 2021; Chang et al., 2021).Permanent loss of function after SCI is caused by interruption of the ascending sensation and descending input of the spinal cord as well as a failure of axon regeneration and neural circuit reconstruction (Orr and Gensel, 2018).A large body of evidence has shown that the formation and remodeling of functional neural circuits depend on strengthening neuroplasticity (Torres-Espín et al., 2018;Yang et al., 2020).Combined treatment of mesenchymal stem cells and exercise presented better functional and morphological outcomes than treatment with mesenchymal stem cells or exercise alone after SCI (Massoto et al., 2020).However, the mechanisms underlying the efficiency of combined treatment with BMMSC and exercise in SCI remain unclear.
Mammalian target of rapamycin (mTOR), a serine/threonine protein kinase,can stimulate ribosomal protein translation (Song et al., 2012).Recent research has suggested that axonal growth in the injured CNS is mediated through the phosphatidylinositol 3-kinase (PI3K)/AKT/mTOR signaling pathway(Ding et al., 2021; Fan et al., 2022b).This pathway is suppressed in the injured CNS, which may limit the protein synthesis necessary for axon regeneration(Yin et al., 2018).PTEN is considered to be an essential factor that can negatively regulate the PI3K/AKT/mTOR pathway, and genetic deletion of this molecule has been shown to increase the intrinsic growth capacity of neurons(Danilov and Steward, 2015).
The purpose of this study was to determine whether the combination of BMMSC transplantation together with exercise training can improve the therapeutic efficacy of each treatment alone in mice after thoracic contusive SCI, and to investigate the potential mechanisms of combinatorial therapies in SCI repair.
Methods
BMMSC isolation and identification
Eight healthy wild-type C57BL/6 mice (female, 4 weeks, 14–16 g) were purchased from Chengdu Dossy Experimental Animals Co., Ltd.(Chengdu,Sichuan Province, China) (Yang et al., 2015; Nakamura et al., 2020).The mice were housed under specific pathogen-free (SPF) conditions in individually ventilated cages at a temperature of 22–24°C with a 12-hour light/dark cycle.C57BL/6 mice were anesthetized with 1% pentobarbital sodium (40 mg/kg i.p., Nembutal; Beijing Chemical Co., Beijing, China) and were then sacrificed by cervical dislocation.The femur and tibia from both sides were obtained with sterile forceps and surgical scissors, as previously described(Cunha et al., 2013).After the ends of the long bones were cut away, the bone marrow cavity was rinsed with minimal essential medium (alpha-MEM;Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (Gibco) and 1%penicillin-streptomycin solution (Invitrogen, Grand Island, NY, USA) using a 1-mL syringe.The obtained cells were inoculated into tissue culture flasks and cultured in a humidified incubator (Thermo Fisher Scientific, Waltham,MA, USA) at 37°C with 5% CO.Nonadherent cells were removed by changing the medium and the remaining adherent cells were passaged at 70–80%confluence.BMMSC of passages 3–5 were used in this study.Flow cytometry analysis (LSRFortessa; BD Biosciences, Franklin Lakes, NJ, USA) was used for BMMSC identification.Passage 3 cells were stained with the following fluorescence-conjugated antibodies: PE-CD29 (Cat# 562801), FITC-CD34 (Cat#553733), APC-CD44 (Cat# 559250), and PerCP/CY5.5-CD45 (Cat# 550994; all from BD Biosciences).
Culture of PC12 cells and primary cortical neurons
PC12 (Cat# CRL-1721, RRID: CVCL_0481), a rat pheochromocytoma cell line, was purchased from Shanghai Institutes for Biological Science, Chinese Academy of Sciences (Greene and Tischler, 1976).PC12 cells were cultured in RPMI-1640 medium (Gibco) containing 15% horse serum and 5% fetal bovine serum (FBS).To obtain differentiated PC12 cells, the cells were treated for 3 days with 50 ng/mL nerve growth factor (NGF; MilliporeSigma, Burlington,MA, USA) dissolved in RPMI-1640 medium containing 10% FBS.
Primary cortical neurons were obtained from the cerebral cortex of postnatal day 0 SD rats (Chengdu Dossy Experimental Animals Co., Ltd.).The rats were anesthetized with 0.8% pentobarbital sodium (40 mg/kg, i.p., Nembutal;Beijing Chemical Co.) and then sacrificed by cervical dislocation.The cortex was cut into small pieces (approximately 1 mm), digested with papain(Worthington, Lakewood, NJ, USA) and DNase I (MilliporeSigma) at 37°C for 30 minutes, and the digestion was then terminated with 10% FBS.After centrifugation, the pellets were resuspended in high-glucose Dulbecco’s modified Eagle’s medium (DMEM; Gibco) supplemented with 10% FBS (Gibco).Cortical neurons were plated in six-well plates coated with poly(lysine) and laminin (BD Labware, Bedford, MA, USA) at a density of 2.0 × 10cells/mL.After 4 hours, the culture medium was replaced with neurobasal medium containing 0.25% L-glutamine (Invitrogen), 1% penicillin/streptomycin solution (Invitrogen), and 2% B27 (Invitrogen).Half of the culture medium was changed every 3 days.
Drug treatment in PC12 cells and primary cortical neurons
To further investigate whether the benefits of combinatorial therapies in SCI repair occurred through the PI3K/AKT/mTOR pathway, we used the PC-12 cell line and primary cortical neurons, both treated with hydrogen peroxide (HO, 100 μmol, for 24 hours) to mimic neuronal injury.The PC-12 cell line and primary cortical neurons were cultured in six-well plates at a density of 3 × 10cells per well in the RPMI-1640 medium and cortical culture medium, respectively, for 12 hours.Then, the RPMI-1640 medium or cortical culture medium was replaced with corresponding medium containing HO(MilliporeSigma) for 24 hours.In this part of the study, the cells were randomly divided into a control group (with no HOtreatment), HOgroup(model), and PI3K inhibitor group (treated with HOand the PI3K inhibitor LY294002 [10 μmol; Calbiochem, San Diego, CA, USA]).
Animals and SCI model
Seventy-five healthy wild-type female (Funk et al., 2016; Plemel et al.,2017) C57BL/6 mice (8 weeks, 18–20 g) were purchased from Chengdu Dossy Experimental Animals Co., Ltd.The mice were housed under specific pathogen-free (SPF) conditions in individually ventilated cages at a temperature of 22–24°C with a 12-hour light/dark cycle.The animal study was approved by the Laboratory Animal Ethics Committee of the West China Hospital of Sichuan University (approval No.20211198A; approval date: June 22, 2021) and all animal procedures were conducted in accordance with Chinese national guidelines for the care and use of laboratory animals.
The mice were first anesthetized via the inhalation of 3% isoflurane (RWD,Shenzhen, China) for induction, and then anesthesia was maintained under 1.5% isoflurane during all surgical procedures.After laminectomy at T10,the mice were subjected to contusive SCI at the same level using an Infinite Horizon impactor (Precision Systems & Instrumentation, Fairfax Station, VA,USA).Contusion force was 70 kDyn (1 dyn = 10N) with tissue displacement to a depth of 500–600 μm and a dwell time of 0 seconds.After suturing the surgical incisions, penicillin (50,000 U/kg per day) was administered intramuscularly for 3 days and manual bladder expression was performed twice a day until spontaneous voiding returned.The sham group (n
= 15)underwent only laminectomy and the spinal cord remained intact.After surgery, 60 surviving SCI mice were randomly assigned to the injury, TMT(treadmill training), BMMSC, and combination therapy (BMMSC + TMT)groups (n
= 15 in each group) using a random number table method.A summary of the study design is shown in Figure 1.
Figure 1|Flowchart of the experiment.
BMMSC transplantation
Immediately after injury, 5 μL of phosphate-buffered saline (PBS; Thermo Fisher Scientific) or BMMSC (1 × 10cells) was injected directly into the contusion epicenter at a depth of 1 mm using a Hamilton syringe positioned with a stereotaxic instrument (RWD Life Science, Shenzhen, Guangdong,China) and driven by a syringe pump (KDS LEGATO 130, KD Scientific,Holliston, MA, USA) at a flow rate of 1 μL/min.
Treadmill training
Three days of exercise was implemented in all groups prior to SCI injury for acclimation.Treadmill training began 3 days after SCI.The mice in the TMT and BMMSC + TMT groups were allowed to run on the treadmill apparatus(ZH-PT; Anhui Zhenghua Biological Instrument Equipment Co., Ltd., China) for 8 weeks (20 minutes daily, 6 days weekly).In terms of the training speed, it was started at 4 m/min and gradually increased according to the tolerance of the mice (maximum speed of 9 m/min).
Behavioral evaluation
Locomotor recovery was evaluated using the Basso Mouse Scale (BMS),horizontal ladder test, and footprint analysis (Ma et al., 2001; Basso et al.,2006; Cummings et al., 2007).The BMS was assessed by two observers whowere blinded to the experimental treatments before injury, 1 day after the injury, and then weekly after the injury in an open field.The BMS was mainly used to analyze the particular behavioral components of locomotion, such as trunk stability, plantar stepping, coordination, and paw position.
The horizontal ladder test requires adequate sensorimotor function to contact and feel the ladder rungs when stepping, making it more sensitive to locomotor deficits (Soblosky et al., 2001).The ladder consisted of 30 metal rungs (2 mm in diameter) spaced regularly (1.3 cm apart) measuring 30 cm in height.Mice were exposed to the horizontal ladder at least once prior to surgery for acclimation to the testing procedure.The walking ability of each mouse across the 30 metal rungs was recorded by video during each testing period.The hindlimb error rate (wrong steps/total steps) was evaluated weekly by a blinded investigator.A correct step corresponded to plantar or toe placements on rungs without slipping or dragging.The mean hindlimb error rates were calculated by dividing the number of error steps by the total number of steps.
For the footprint analysis, mouse forepaws and hindpaws were painted with red and blue dyes, respectively.Mice were then allowed to walk in a straight path lined with white paper.
Magnetic resonance imaging
T2-weighted sagittal magnetic resonance images were acquired using a 7T small-animal MR system, Biospec USR70/30 (Bruker Corporation, Karlsruhe,Germany).During magnetic resonance imaging (MRI), the mice were anesthetized by inhalation of 3% isoflurane and maintained under anesthesia by inhalation of 1.5% isoflurane through a face mask.The parameters were as follows: repetition time (TR) = 3000 ms, echo time (TE) = 27 ms, field of view =40 × 30 mm, number of averages = 2, matrix size = 256 × 256, slice number =10, slice thickness = 0.8 mm, and slice gap = 0 mm.Six animals were observed for each group.The lesion area was measured manually using ImageJ (version 1.6; NIH, Bethesda, MD, USA; Schneider et al., 2012) in each slice, and lesion volumes were calculated by summing lesion areas in each slice multiplied by the thickness of the slice.
Tissue preparation
Eight weeks after the intervention, the animals were deeply anesthetized with isoflurane and transcardially perfused with saline followed by 4%paraformaldehyde (MilliporeSigma) in 0.1 M phosphate buffer (pH 7.2)or 2.5% glutaraldehyde in 0.1 M PBS.For histological examination and immunofluorescence, spinal cords (1 cm long for longitudinal sections and 0.5 cm long for transverse sections) centered at the injury epicenter were dissected and fixed in 4% neutral buffered paraformaldehyde for 24 hours,embedded in paraffin, and cut into 2.5-μm-thick slices using a microtome(Leica, Wetzlar, Germany).For western blot analysis, spinal cord tissue at the injury epicenter was flash frozen in liquid nitrogen.
Histological examination
Longitudinal sections of the injured spinal cord were stained with Masson’s trichrome (Solarbio, Beijing, China) to assess scar formation and with hematoxylin and eosin (H&E; Richard-Allan Scientific, Kalamazoo, MI, USA)for morphological detection (n
= 3 per group).Transverse sections were stained with Nissl (cresyl violet, Solarbio) to count neurons after injury (n
=3 per group).The sections were routinely deparaffinized, rehydrated, and washed.Then, Masson’s trichrome, H&E, and Nissl staining was performed in accordance with the manufacturer’s instructions.Finally, the sections were dehydrated with anhydrous ethanol (pure grade; Beijing Chemical Factory)two times for 5 seconds, penetrated with xylene (pure grade; Beijing Chemical Factory) two times for 5 minutes, and sealed with neutral gum.Images were acquired using Ni-E (Nikon, Tokyo, Japan).The collagenous area fraction was measured as the ratio of stained collagen area to total area.The number of Nissl bodies was calculated using Nikon NIS Analysis software 4.54.Transmission electron microscopy
Spinal cord tissue samples were collected and transverse sections of the spinal cord were fixed in 2.5% glutaraldehyde (Solarbio) in 0.1 M PBS at 4 °C for 24 hours.Then, samples were postfixed in 1% osmium tetroxide (Solarbio)in PBS, dehydrated in a graded series of alcohol, and embedded in epoxy resin(Epon 812; Serva, Wetzlar, Germany).Ultrathin sections of 50 nm (EM KMR2 ultramicrotome; Leica) were stained with uranyl acetate and lead citrate(MilliporeSigma) and finally examined with a JEM-1400 transmission electron microscope (JEOL, Tokyo, Japan).The G-ratio (the ratio of axon diameter to nerve fiber diameter) was calculated to analyze the myelin sheath thickness.
Western blot analysis
Western blotting was used to determine the expression of proteins related to axon and myelin integrity, synaptic plasticity, and the production of neurotrophic factors, as well as the PI3K/AKT/mTOR pathway.The animals were sacrificed 8 weeks after injury and spinal cord tissue at the injury epicenter was harvested for western blot analysis from four mice per group.Homogenates from spinal cord tissue were lysed in radioimmunoprecipitation assay buffer (Beyotime Biotechnology, Shanghai, China) containing protease and phosphatase inhibitor cocktails at 4°C.Protein concentrations were determined using a bicinchoninic acid (BCA) kit (Beyotime).After normalization, samples were subjected to 10% SDS-PAGE and then transferred to a PVDF membrane (Millipore, Bedford, MA, USA).These membranes were blocked with 5% nonfat dry milk (Yili Milk Company, Inner Mongolia,China) for 1 hour at room temperature and incubated with a specific primary antibody at 4°C overnight.After washing with Tris-buffered saline plus Tween(TBST), the membranes were incubated with anti-rabbit immunoglobulin G(IgG) horseradish-peroxidase-conjugated secondary antibodies for 1 hour.Immunoreactive bands were visualized using an enhanced chemiluminescent detection system (Bio-Rad, Hercules, CA, USA).The immunoreactive bands were quantified by densitometric analysis using ImageJ software.Relative protein expression levels were normalized to β-actin.The antibodies used in this study are summarized in Additional Table 1.
Immunofluorescence
Immunofluorescence was used to detect the markers of axon regeneration,myelin integrity, synaptic function, and neurotrophic factor secretion.Spinal cord tissue was prepared as described previously and sectioned in the longitudinal or transverse plane at a thickness of 2.5 μm using a Microtome(Leica).Sections were deparaffinized and rehydrated with xylene for 2× 10 minutes, 100% ethanol for 2× 5 minutes, and 96% ethanol, 80% ethanol, 70%ethanol, and HO for 5 minutes each.Antigen retrieval was performed with citrate buffer (pH 6.2; Beyotime Institute of Biotechnology, Shanghai, China)and the sections were blocked with 10% goat serum (Solarbio).Sections were then incubated at 4°C overnight with the following primary antibodies: glial fibrillary acidic protein (GFAP), myelin basic protein (MBP), neuronal nuclei(NeuN), neurofilament-200 (NF200), postsynaptic density protein 95 (PSD95),synaptophysin (SYN), and microtubule-associated protein 2 (MAP2) antibodies(all from Proteintech, Wuhan, Hubei Province, China) and NGF antibody(Abcam, Cambridge, MA, USA).After washing, sections were stained for 1 hour at 37°C with the following fluorescent secondary antibodies: fluorescein isothiocyanate (FITC)-conjugated and tetramethyl rhodamine isothiocyanate(TRITC)-conjugated secondary antibody (Proteintech).Nuclei were visualized by staining with DAPI.Images were acquired using an N-STORM & A1 (Nikon)microscope.The intensity was analyzed using Nikon NIS Analysis software 4.54.The antibodies used in this study are summarized in Additional Table 1.
Statistical analysis
No statistical methods were used to predetermine sample sizes; however, our sample sizes are similar to those reported in previous publications (Chen et al., 2019; Domínguez-Iturza et al., 2019; To et al., 2019).Data are expressed as the mean ± SEM.Statistical analysis was performed using GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, USA, www.graphpad.com).BMS scores and hindlimb error rates were analyzed using two-way analysis of variance followed by Tukey’spost hoc
test for multiple comparisons.All other experimental results were analyzed by one-way analysis of variance followed by Tukey’spost hoc
multiple comparison test.AP
-value < 0.05 was considered significant.Results
Identification of BMMSC and primary cortical neurons
After three passages, the cell surface markers of BMMSC were analyzed by flow cytometry.The results showed positive expression (> 99%) of CD29 and CD44 and negative expression (< 1%) of CD34 and CD45, which indicated the high purity of the extracted BMMSC (Additional Figure 1A).The purity of primary cortical neuron cultures was demonstrated by immunofluorescence staining of MAP2, a marker of mature neurons.The primary cultures of cortical neurons exhibited typical neuronal morphology with a dense network of neurites that could be visualized by MAP2-positive axons and dendrites and NeuN-positive somata (Additional Figure 1B).
Combination therapy enhances locomotor function after SCI
BMS score assessment and horizontal ladder and footprint analyses were performed to evaluate the functional recovery of mice subjected to the different treatments.On the first day after injury, BMS assessment showed that the hindlimbs of all mice were completely paralyzed (BMS score = 0);then, the mice showed different extents of recovery (Figure 2A).Starting at 3 weeks after injury, the BMS scores in the treatment groups (TMT:P
< 0.001,BMMSC:P
< 0.001, BMMSC + TMT:P
< 0.001) were significantly higher than that in the injury group.These differences remained statistically significant up to 8 weeks after SCI.The BMMSC + TMT group obtained higher scores than the BMMSC group at 6 weeks after SCI, which persisted until the end of the experiment (P
< 0.05).In addition, the BMS score in the BMMSC + TMT group significantly increased from the 7th week after injury (P
< 0.05) to the 8th week (P
< 0.05) compared with that in the TMT group.The footprint test intuitively reflected differences in the tracks of hindlimbs among the different groups.Mice in the injury group showed obvious dragging of hindlimbs compared with the clear footprints of mice in the sham group, whereas mice in the BMMSC+TMT group displayed a fairly consistent hindlimb track with a few stumbles at 8 weeks after SCI (Figure 2B).Animals in the TMT or BMMSC group partially recovered coordination of the fore- and hindlimbs, but still showed some dragging.We also measured the differences in motor function using a horizontal ladder test (Figure 2C).Over the 8-week testing period,all mice subjected to SCI showed a progressive decrease in the error rates(Figure 2D).The mean error rate was significantly lower in the BMMSC + TMT group than in the injury group at 4 weeks (P
< 0.05), which persisted until 8 weeks after SCI (P
< 0.01).However, no difference was found between the TMT or BMMSC alone group and the injury group at all time points examined.Taken together, these results show that the combined intervention can better promote recovery of locomotor function than individual therapy after SCI.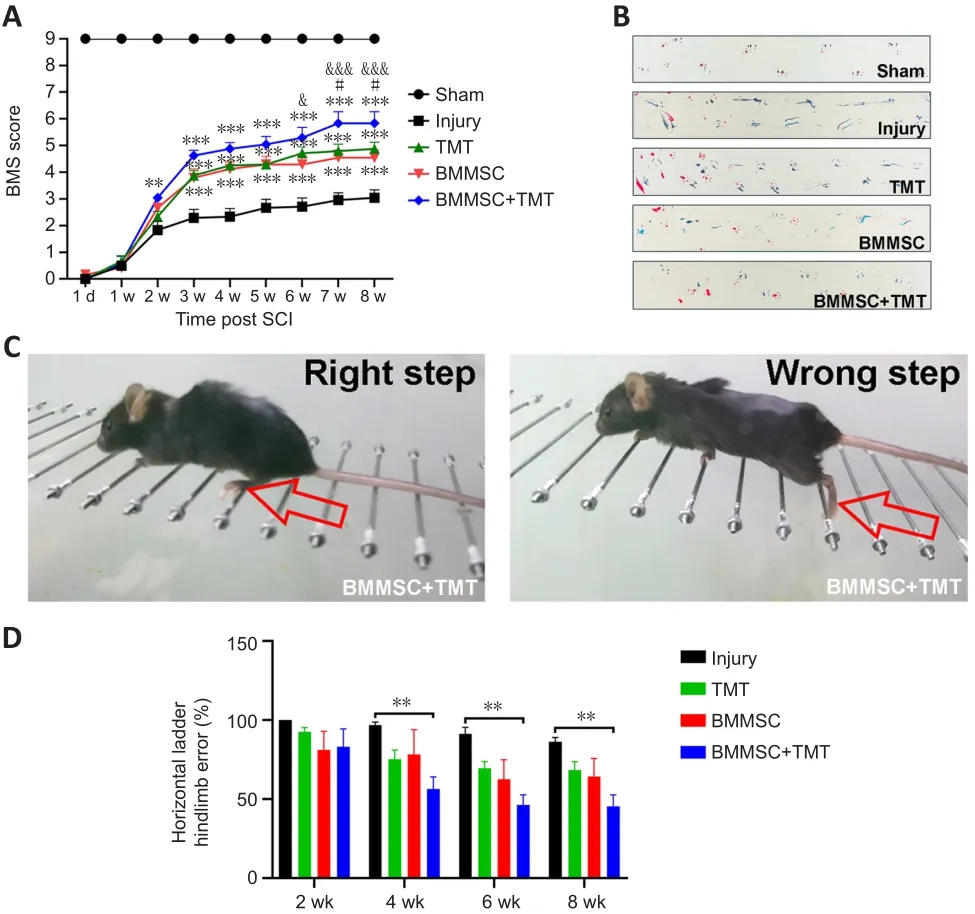
Figure 2| Combining BMMSC and TMT improves motor function in mice after SCI.
Combination therapy promotes histological repair of the damaged spinal cord and reduces fibrotic scar formation
We investigated the effect of the combined therapy on the structural repair of injured spinal cord tissue.The lesion volume of the injured spinal cord was measured at 8 weeks after injury using T2-weighted MRI (Figure 3A).Compared with that in the injury group, a significant reduction in lesion volume was found in mice that received treatment (TMT:P
< 0.01,BMMSC:P
< 0.001, BMMSC + TMT:P
< 0.001; Figure 3C).Nevertheless, the differences between the combined treatment and TMT or BMMSC alone were not statistically significant.The histological changes in morphology in the injured spinal cord at 8 weeks after injury were evaluated using H&E staining (Figure 3B).Severe tissue damage and infiltration of a large number of inflammatory cells were observed in the injury group.After TMT and/or BMMSC treatments, the integrity of the spinal cord was well preserved and the injured tissue showed only mild infiltration of inflammatory cells.Masson’s trichrome staining was also used to estimate scar formation at the site of injury (Figure 3B).TMT or BMMSC alone did not significantly affect the fibrotic area, whereas a significantly lower average percentage of fibrosis was observed in the BMMSC + TMT group than in the injury group (P
< 0.001;Figure 3D).These results suggest that TMT or BMMSC alone can promote the preservation of spinal cord tissue, but no significant synergism between them was noted.Furthermore, only the combination of BMMSC and TMT could result in a significant reduction in fibrotic scar formation.Combination therapy protects neurons and enhances the production of neurotrophic factors following SCI
To determine the neuroprotective effect of the intervention on SCI mice, we examined neuronal density in the anterior horn of the injured spinal cord at 8 weeks after SCI by double immunofluorescent staining for GFAP (an astrocyte marker) and NeuN (a neuronal marker) (Figure 4A).SCI induced a significant decrease in NeuN-positive neurons in the anterior horn of the injured spinal cord.The immunofluorescence results showed that the relative density of NeuN in the BMMSC + TMT group significantly increased compared with those in the Injury (P
< 0.001), TMT alone (P
< 0.05), and BMMSC alone groups (P
<0.05; Figure 4C).However, TMT or BMMSC alone did not significantly affect neuronal viability.We also investigated whether the combination treatment resulted in the enhanced production of NGF, which is a crucial neurotrophin for neuronal growth and survival (Figure 4B).TMT or BMMSC alone did not provide any obvious beneficial effects on NGF production.Only when the two strategies were combined was the NGF level at the injured epicenter region significantly increased compared with that in the injury group (P
<0.001, Figure 4D).For Nissl staining, as shown in Figure 4E, a large number of normal neurons in the sham group displayed an integrative and granularlike morphology, while in the injury group, their numbers were significantly decreased, and most of them exhibited shrunken cell bodies and Nissl granule dissolution.Quantitative comparison of Nissl bodies showed that only the BMMSC + TMT group exhibited a significant increase in the number of surviving neurons compared with the injury group (P
< 0.05; Figure 4G).Additionally, western blot analysis of the NeuN and NGF proteins further confirmed the above results (Figure 4F, H, and I).We also detected the protein levels of brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF), but the differences among all groups were not statistically significant (Additional Figure 2).These observations indicated that the combination therapy could exert a protective effect on neurons and promote the repair of damaged tissue after SCI.
Figure 3|Combining BMMSC and TMT promotes histological repair of the damaged spinal cord in mice after SCI.
Combination therapy enhances axonal and myelin protection in injured spinal cord
The fragmentation of axons at the lesion center of the spinal cord was evaluated by double immunofluorescent staining for GFAP and NF200 (an axonal marker) (Figure 5A and B).GFAP-positive astrocytes accumulated along the lesion border (Figure 5A).Notably, more NF200-positive, injured axons crossed the lesion border in the BMMSC + TMT group than in the TMT(P
< 0.05) or BMMSC alone group (P
< 0.05) and the injury group (P
< 0.05;Figure 5E).In addition, the expression of NF200 in the TMT or BMMSC group was significantly increased compared with that in the injury group (P
< 0.05).Double immunofluorescent staining for GFAP and MBP (a myelin marker)was performed to determine the effect of the intervention on remyelination(Figure 5C).We also detected the microstructure of myelin using transmission electron microscopy (Figure 5D).The immunofluorescence results suggested that TMT or BMMSC alone increased the expression of MBP (P
< 0.05), but the combination of the two strategies did not result in superior improvement compared with TMT or BMMSC alone (Figure 5F).Furthermore, transmission electron microscopy analysis revealed that only the BMMSC + TMT group had significantly increased myelin thickness, as quantified by the G-ratio,compared with the injury group (P
< 0.001; Figure 5G).Moreover, western blot results further confirmed the immunofluorescence findings of NF200 and MBP (Figure 5H–J).Overall, these findings indicate that the combination therapy can alleviate axon and myelin destruction in the injured site compared with TMT or BMMSC alone.Combination therapy improves synaptic function after SCI
Immunofluorescence staining of PSD (a presynaptic marker protein) and SYN(a postsynaptic marker protein) in the injured spinal cord was performed to evaluate synaptic function (Figure 6A and B).The expression levels of PSD and SYN at the injured epicenter region were substantially increased in theBMMSC + TMT group compared with those in the injury group (PSD:P
< 0.001,SYN:P
< 0.01).TMT or BMMSC alone tended to increase the expression levels of PSD and SYN, but no significant difference was observed between the TMT or BMMSC alone group and the injury group (Figure 6C and D).Western blot analysis for PSD and SYN (Figure 6E–G) also confirmed the above results, indicating that TMT and BMMSC acted synergistically to upregulate the expression of synaptic protein markers better than the individual interventions.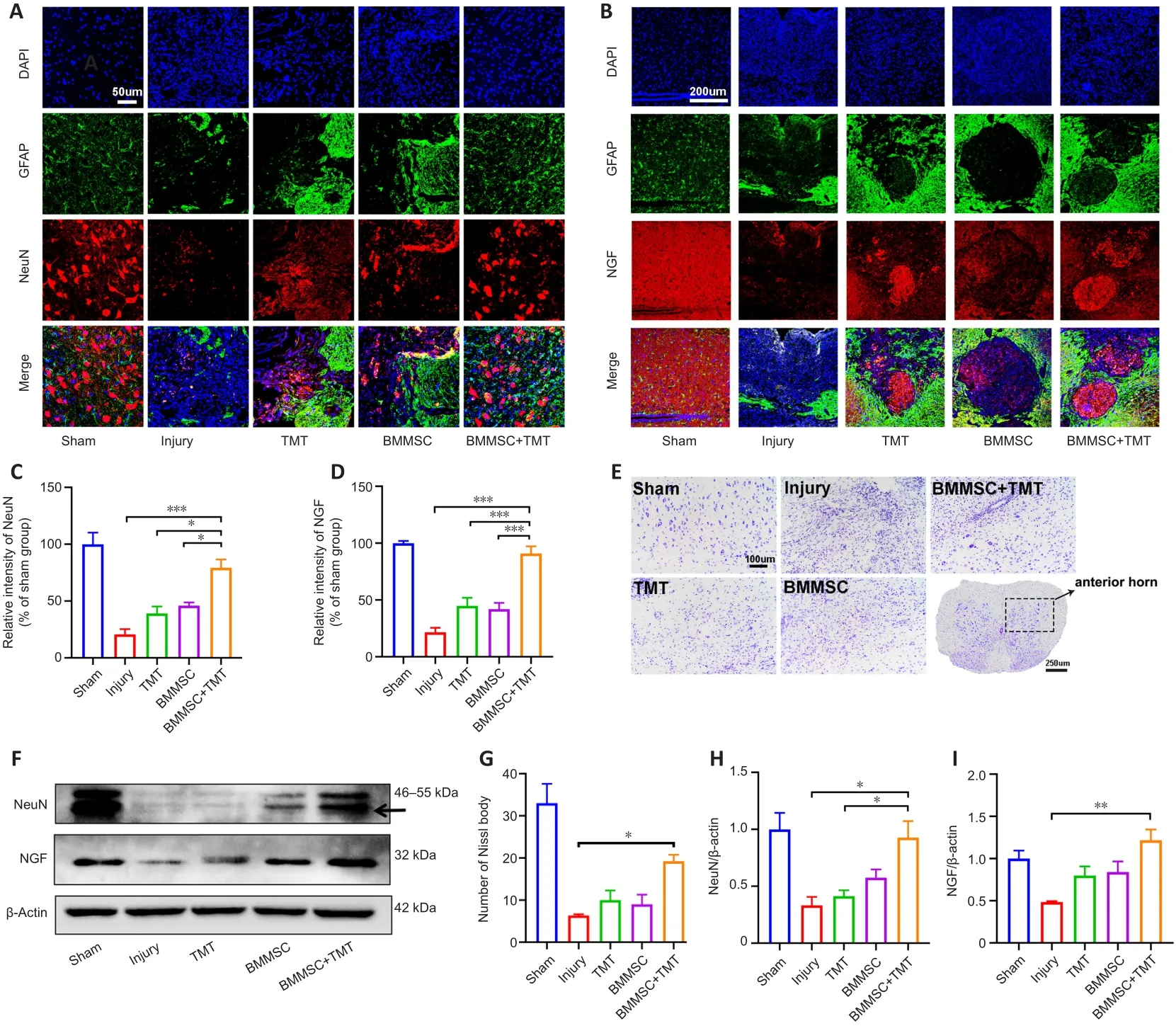
Figure 4|Combining BMMSC and TMT provides neuronal protection and enhances the expression of neurotrophic factors in mice after SCI.
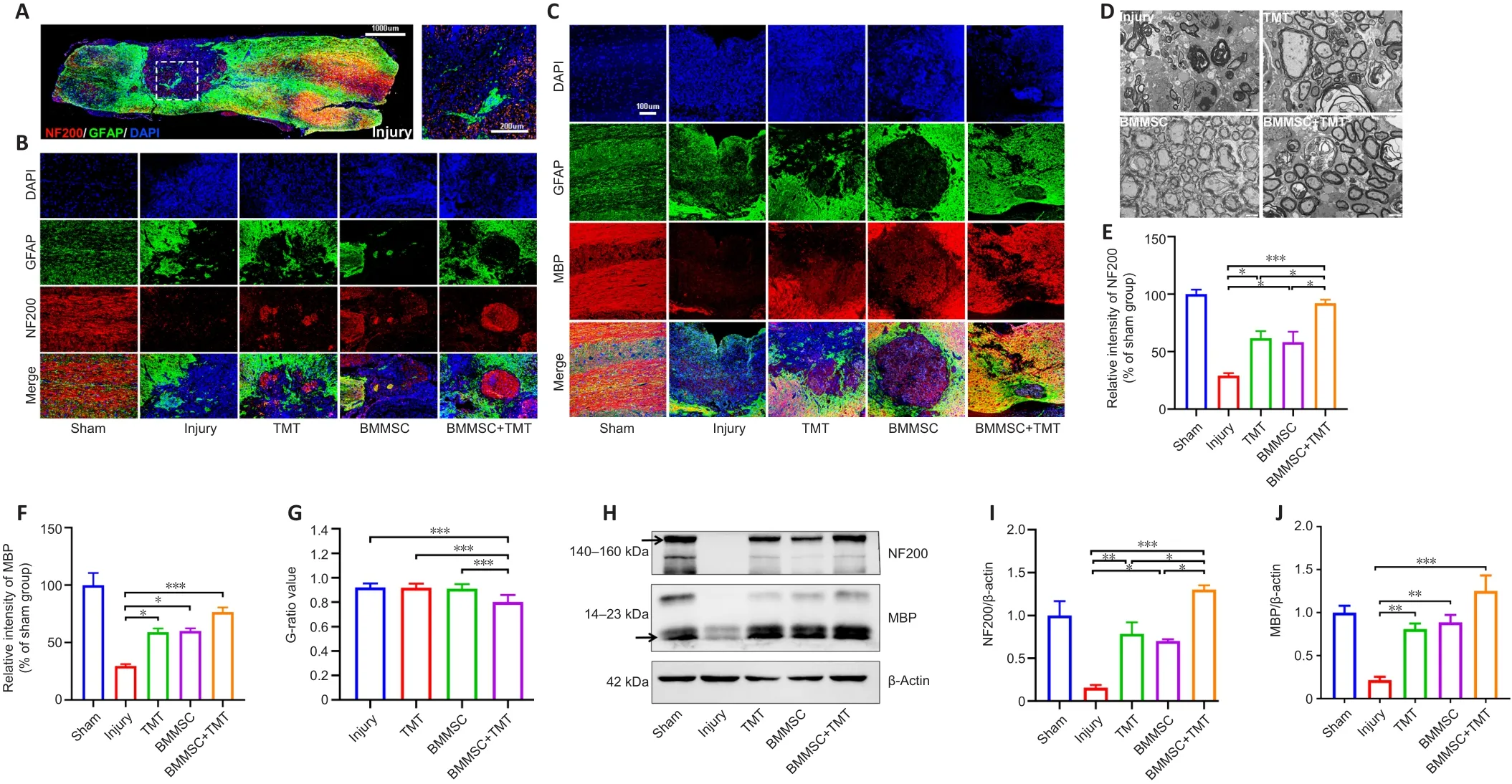
Figure 5 | Combining BMMSC and TMT promotes axonal and myelin protection in the injured spinal cord in mice after SCI.
BMMSC transplantation combined with TMT synergistically augments SCI recovery through activation of the PI3K/AKT/mTOR pathway
Studies have confirmed that the PI3K/AKT/mTOR signaling pathway is involved in the growth of central nervous system (CNS) axons during development(Berry et al., 2016; Li et al., 2020a).During CNS maturity, phosphatase and tensin homolog on chromosome 10 (PTEN) suppresses the activity of this signaling pathway, accounting for the failure of axon regeneration in the injured spinal cord (Berry et al., 2016).To further determine whether the combinatorial therapies promote SCI recovery by targeted activation of the PI3K/AKT/mTOR pathway, we performed western blot analysis to determine the protein levels of the key molecules in this pathway (Figure 7A).The results indicate that only the combination therapy significantly enhanced the expression of phospho- (p-)PI3K, p-AKT, and p-mTOR compared with the levels in the injury group (p-PI3K:P
< 0.05, p-AKT:P
< 0.05, p-mTOR:P
< 0.001;Figure 7B–D).Silencing of PTEN, a negative regulator of mTOR, was shown to promote axon growth in animal models after SCI.In our study, although the expression level of PTEN in the BMMSC + TMT group tended to slightly decrease compared with that in the injury group, the differences were not statistically significant.The above data revealed that BMMSC implantation combined with TMT promoted axonal regeneration and neuroplasticity via the PI3K/AKT/mTOR signaling pathway.Inhibition of the PI3K/AKT/mTOR pathway exacerbated neuronal injuryin vitro
To further investigate whether the synergistic effects of the combination therapy in SCI repair occurred through enhancing the activity of the PI3K/AKT/mTOR pathway, we analyzed whether blocking this pathwayin vitro
may influence neuronal injury.We used PC12 cells and primary cortical neurons stimulated by HOas a neuronal oxidative stress modelin vitro
.HOdecreased the phosphorylation of PI3K and AKT in the PC12 cell line compared with the levels in the control group (PI3K:P
< 0.001, AKT:P
< 0.01; Figure 8A–C).After pre-treatment with PI3K inhibitor (LY294002), the phosphorylation of PI3K and AKT was significantly inhibited compared with that in the HOgroup(PI3K:P
< 0.05; AKT:P
< 0.01).As shown in Figure 8D–G, the western blot analysis showed that NF200, NeuN, and BDNF protein expression in the PC12 cell line was significantly decreased in the HOgroup compared with that in the control group (NF200:P
< 0.01, NeuN:P
< 0.001, BDNF:P
< 0.01).Notably,blockage of the PI3K pathway by LY294002 treatment led to a significant decrease in the expression of NF200, NeuN, and BDNF compared with that in the HOgroup (NF200:P
< 0.05; NeuN:P
< 0.01, BDNF:P
< 0.01).Again,similar results were obtained in primary cortical neurons (Figure 9).Taken together, these results suggest that the PI3K/AKT/mTOR pathway is involved in neuronal injury.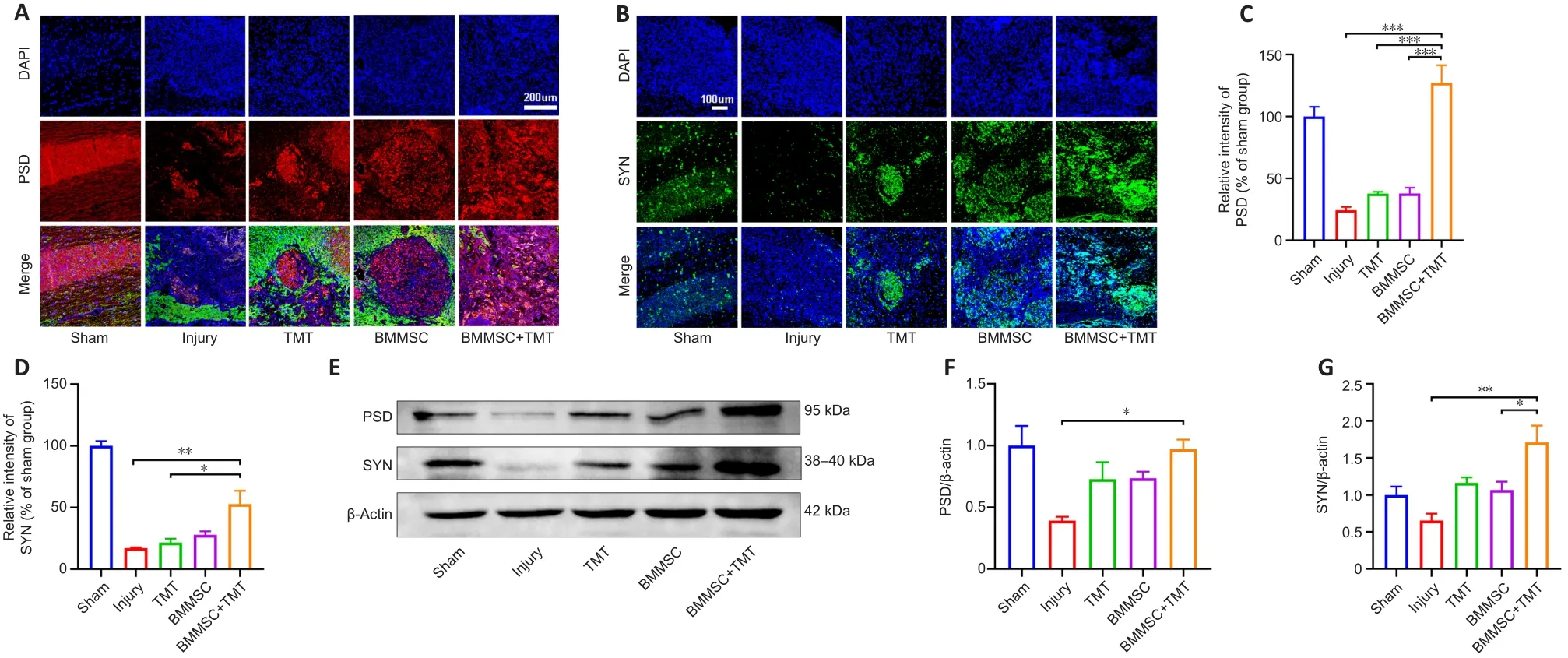
Figure 6 | Combining BMMSC and TMT promotes synaptic function in mice after SCI.

Figure 7|Combining BMMSC and TMT upregulates the PI3K/Akt/mTOR signaling pathway in the injured spinal cord in mice.

Figure 8|Blockage of the PI3K/AKT/mTOR pathway exacerbates neuronal injury in PC12 cells (western blot assay).

Figure 9|Blockage of the PI3K/AKT/mTOR pathway exacerbates neuronal injury in primary cortical neurons (western blot assay).
Discussion
The findings of the present study indicate that BMMSC transplantation combined with exercise training synergistically improved motor function in paralyzed hindlimbs and established favorable conditions for functional restoration after SCI in terms the following aspects: promoting axon and myelin protection, enhancing synaptic function and neurotrophin secretion,suppressing scar formation, and protecting neurons.We also found that the neuroprotective effects of BMMSC transplantation combined with TMT on SCI recovery occurred through activation of the PI3K/AKT/mTOR pathway.
Although many therapeutic interventions have shown promise in treating SCI, focusing on a single aspect of repair cannot achieve successful and functional regeneration in patients following SCI.Therefore, a combination of various interventions addressing the multiple aspects of SCI pathology is likely required.In this study, we opted for a combinatorial approach of neuroprotection and rehabilitation, capitalizing on cell transplantation and functional sensorimotor training to promote nerve regeneration and functional recovery.Treatment targets for SCI that can improve functional recovery include reduction of secondary damage, replacement of lost cells,removal of inhibitory molecules, axon regeneration through targeting neuronal mechanisms, resupply of trophic support, remyelination of demyelinated axons, and rehabilitation for circuit remodeling (Führmann et al., 2017; O’Shea et al., 2017; Griffin and Bradke, 2020).Thus, in the present study, multiple integrated lines of evidence derivedin vivo
were used to assess SCI recovery,including locomotor performance, histopathological lesions, scar formation,axon growth, synapse remodeling, and myelin regeneration.Anin vitro
model of HO-induced neurotoxicity in PC12 cells and primary cortical neurons was used to further confirm the involvement of the key signaling pathway.Our results indicated that the combined treatment with BMMSC and TMT achieved a better therapeutic effect on functional recovery than in the other groups.The enhancement of motor functional recovery by the combination therapy can potentially be explained as follows.The combination of BMMSC and TMT markedly reduced fibrotic scar tissue, protected neurons, promoted remyelination and axonal regeneration, and increased synapse formation, all to a larger extent than either TMT or BMMSC alone.More strikingly, although the BMS score of each single therapy was significantly higher than that of the injury group at the end of the trial, the mean error rates of hindlimbs did not differ significantly between TMT or BMMSC alone and the injury group.A possible explanation for this is that stepping across the rungs requires precise foot placement and grasping, which may present a challenge to mice with poor locomotor performance (Emerick and Kartje, 2004; Metz and Whishaw,2009).Additionally, we noted that both single therapy and combined therapy greatly reduced the tissue damage, as assessed by MRI and H&E staining,and the combined therapy did not obviously enhance these independent effects of each single therapy.Thus, physical exercise or cell transplantation alone can be reasonably considered to also promote tissue preservation.To our knowledge, a study by Massoto et al.(2020) investigated the efficacy of BMMSC transplantation combined with physical exercise after mouse SCI.However, that study only determined the morphological and functional improvements and did not explore the potential mechanisms by which combinatorial therapies act in SCI repair.Interestingly, our study found that the pro-regenerative effect of the combined treatment was achieved via activation of the PI3K/AKT/mTOR pathway.
Stem cell transplantation can promote SCI repair and functional improvement by these cells differentiating into neurons or glial cells to replace damaged cells and secreting a variety of neurotrophic factors to protect the injured tissue and enhance axon regeneration (Vismara et al., 2017; Veneruso et al., 2019).BMMSC are generally accepted to have the advantages of high biosafety, wide-ranging biological effects, and low immunogenicity (Shao et al., 2019).In this study, we also observed that BMMSC can protect axons and myelin against damage, which is similar to findings in previous studies(Assinck et al., 2017; Veneruso et al., 2019).Scar formation is a pivotal determinant of axonal regeneration after SCI (Gaudet and Popovich, 2014).Reactive astrogliosis is typically associated with the formation of compact scar borders around the inflammatory core (Li et al., 2020b).Moreover,border-forming astrocytes increase the deposition of chondroitin sulfate proteoglycans, the major matrix components of glial scars, which may act as a physical and chemical barrier to axon outgrowth (Canning et al., 1996;Schachtrup et al., 2010; Hara et al., 2017; Tran et al., 2018).Our findings in the present study show that only the BMMSC + TMT treatment led to a substantial decrease in scar formation, which may make a significant contribution to axon regeneration in the injured spinal cord.Indeed, we found that the combination of BMMSC and TMT led to the highest expression of neurofilaments in the injured spinal cord.
SCI leads to the disruption of neural connectivity, resulting in severe permanent neurological disability.Restoration of function relies on promoting the formation of new connections and circuits (Onifer et al., 2011; Tran et al., 2018).The remodeling of functional neural circuits in the spinal cord and brain may need to be driven by rehabilitation (Loy and Bareyre, 2019).Combined treatments targeting the promotion of neuronal plasticity seem to be an effective approach.Recent reports on the role of cell transplantation with exercise training are limited to those on several preclinical studies.A study in rats reported no evidence of functional recovery after bone marrow stromal cell transplantation or physical exercise alone, or after both treatments (Yoshihara et al., 2006).In a subsequent chronic SCI mousestudy, neural stem cell transplantation combined with TMT treatment was shown to significantly enhance functional recovery and facilitate neuronal differentiation of transplanted cells compared with either treatment alone(Tashiro et al., 2016).Another recent study reported the functional and morphological benefits of a combinatorial approach with BMMSC and early TMT treatment in a compression SCI mouse model (Massoto et al., 2020).Therefore, further investigations are still needed to explore the detailed mechanisms of the combined treatment.
An intriguing finding in our study is that the BMMSC + TMT mouse model group exhibited remarkable upregulation of the PI3K/Akt/mTOR pathway.Evidence from the PC12 cell line and rat primary cortical neuron cultures also demonstrated that the PI3K/Akt/mTOR pathway is involved in neuronal injury.PI3Ks are a class of lipid kinases that convert phosphatidylinositol(4,5)-bisphosphate into phosphatidylinositol (3,4,5)-trisphosphate and then activate AKT and mTOR, ultimately mediating neuroprotection and axogenic protein synthesis (Liu and Xu, 2012).After SCI, the upregulation of PTEN can restrict the binding of AKTs to membranes by dephosphorylating phosphatidylinositol (3,4,5)-trisphosphate to phosphatidylinositol(4,5)-bisphosphate, leading to inactivation of the PI3K/AKT/mTOR pathway(Berry et al., 2016).In our study, we also found that the expression of PTEN was upregulated in spinal cord tissues derived from mice subjected to SCI.We hypothesized that our interventions would reduce the expression of PTEN and then promote activation of the PI3K/AKT/mTOR pathway.The results indicated that, in the combination group, the expression of PTEN tended to be downregulated, but the differences were not significant.One speculation is that the combinatorial approach of BMMSC transplantation and exercise training may not target PTEN to regulate the PI3K/AKT/mTOR signaling pathway.Notably, neurotrophic factors are crucial for supporting the viability of neurons and the growth of axons during mammalian CNS development (Castilla-Cortázar et al., 2019).Furthermore, the binding of neurotrophic factors to tyrosine kinase receptors (Trk) triggers their dimerization and the autophosphorylation of tyrosine residues within the intracellular kinase domain, which can activate the PI3K/AKT/mTOR intracellular signaling pathway (Berry et al., 2016).As expected, the NGF level at the lesion center was significantly increased in the BMMSC + TMT group.Based on these findings, we speculated that BMMSC combined with TMT exert a neuroprotective effect by elevating NGF levels and then activating PI3K/AKT/mTOR signaling.Additionally, BDNF and VEGF levels were not obviously altered by either single therapy or combination therapy in this study.However, previous studies demonstrated that both BMMSC and TMT have the potential to increase BDNF levels in injured spinal cord (Tashiro et al., 2016; Massoto et al., 2020).This discrepancy might be due to differenttime points being examined.mTOR and its downstream signaling pathways are also involved in the regulation of synaptic plasticity (Hou and Klann, 2004;Jaworski and Sheng, 2006; Hoeffer and Klann, 2010).Numerous studies have shown that several forms of protein synthesis-dependent synaptic plasticity require the activity of ERK, PI3K, and mTOR (Tang et al., 2002; Liu et al., 2018).It was reported that the PI3K/Akt/mTOR pathway can regulate translation initiation through downstream targets such as ribosomal protein S6 kinase(S6K) and eukaryotic initiation factor 4E (eIF4E)-binding protein (4E-BP) (Jain et al., 2021; Maracci et al., 2022; Wright and Lannigan, 2022).In this report,we have shown that the combined treatment can dramatically increase PSD and SYN expression.However, precise delineation of the signaling cascades occurring during synaptic plasticity is important and remains to be addressed in future work.Further studies will also be required to identify the complex interactions between exercise training and cell transplantation and determine the best temporal window when this combinatorial treatment can be applied.Collectively, our findings demonstrated that BMMSC transplantation combined with exercise training synergistically promotes hindlimb motor functional recovery in mice with incomplete spinal cord contusive injury and exerts its neuroprotective roles after SCI via the PI3K/AKT/mTOR signaling pathway.
Author contributions:
XS and QW conceived the idea and designed the research studies.XS and LYH conducted the experiments.HXP, LJL, LW, and GQP participated in SCI model preparation and data analysis.YW, QZ, and HXC acquired and analyzed the data.XS drafted the initial manuscript.QW and CQH reviewed and revised the manuscript.All authors approved the final version of manuscript.
Conflicts of interest:
All authors declare no competing interests.
Author statement:
This paper has been posted as a preprint on Research Square with doi: https://doi.org/10.21203/rs.3.rs-880133/v1 which is available from: https://www.researchsquare.com/article/rs-880133/v1.
Availability of data and materials:
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:
Seth Herr, Purdue University, USA.
Additional files:
Open peer review report 1.
Primary and secondary antibodies used in this study.
Identification and characterization of BMMSCs and primary cortical neurons.
Representative western blot images and quantification of PTEN, BDNF, and VEGF in the injured spinal cord in mice at 8 weeks after SCI.
- 中国神经再生研究(英文版)的其它文章
- Patient-specific monocyte-derived microglia as a screening tool for neurodegenerative diseases
- Molecular hallmarks of long non-coding RNAs in aging and its significant effect on aging-associated diseases
- Inflammation in diabetic retinopathy: possible roles in pathogenesis and potential implications for therapy
- Targeting the nitric oxide/cGMP signaling pathway to treat chronic pain
- Neurosteroids as stress modulators and neurotherapeutics: lessons from the retina
- Myelinosome organelles in pathological retinas:ubiquitous presence and dual role in ocular proteostasis maintenance

