Neurosteroids as stress modulators and neurotherapeutics: lessons from the retina
Yukitoshi Izumi , Makoto Ishikawa , Toru Nakazawa , Hiroshi Kunikata Kota Sato , Douglas F.Covey ,Charles F.Zorumski
Abstract Neurosteroids are rapidly emerging as important new therapies in neuropsychiatry, with one such agent, brexanolone, already approved for treatment of postpartum depression, and others on the horizon.These steroids have unique properties, including neuroprotective effects that could benefit a wide range of brain illnesses including depression, anxiety, epilepsy, and neurodegeneration.Over the past 25 years, our group has developed ex vivo rodent models to examine factors contributing to several forms of neurodegeneration in the retina.In the course of this work, we have developed a model of acute closed angle glaucoma that involves incubation of ex vivo retinas under hyperbaric conditions and results in neuronal and axonal changes that mimic glaucoma.We have used this model to determine neuroprotective mechanisms that could have therapeutic implications.In particular,we have focused on the role of both endogenous and exogenous neurosteroids in modulating the effects of acute high pressure.Endogenous allopregnanolone, a major stress-activated neurosteroid in the brain and retina, helps to prevent severe pressure-induced retinal excitotoxicity but is unable to protect against degenerative changes in ganglion cells and their axons under hyperbaric conditions.However, exogenous allopregnanolone, at a pharmacological concentration, completely preserves retinal structure and does so by combined effects on gamma-aminobutyric acid type A receptors and stimulation of the cellular process of macroautophagy.Surprisingly, the enantiomer of allopregnanolone, which is inactive at gamma-aminobutyric acid type A receptors, is equally retinoprotective and acts primarily via autophagy.Both enantiomers are also equally effective in preserving retinal structure and function in an in vivo glaucoma model.These studies in the retina have important implications for the ongoing development of allopregnanolone and other neurosteroids as therapeutics for neuropsychiatric illnesses.
Key Words: allopregnanolone; autophagy; enantiomers; excitotoxicity; gamma-aminobutyric acid type A receptors; glaucoma; optic nerve; oxysterols
Introduction
Neurosteroids are important modulators of neuronal stress and use a variety of mechanisms to help neurons establish homeostasis following insults.Neurosteroids are synthesized endogenously in the brain from cholesterol or sterol precursors, and are a subset of a broader class of steroids that modulate brain function, referred to as neuroactive steroids (NAS) (Paul and Purdy,1992).Increasing evidence indicates the importance of NAS as therapeutics for neuropsychiatric illnesses (Zorumski et al., 2013), and has led to FDA approval of brexanolone, for treatment of postpartum depression (Meltzer-Brody and Kanes; 2020).Brexanolone is a formulation of the neurosteroid,allopregnanolone (AlloP), in cyclodextrin (Captisol) for intravenous infusion.Another NAS, zuranolone, is in late-stage human trials for postpartum depression and major depression (Gunduz-Bruce et al., 2019; Deligiannidis et al, 2021).The approval of brexanolone has prompted increasing interest in understanding mechanisms contributing to the action of AlloP and related NAS.For over 25 years, our group has examined models of neuronal injury and stress in retinal preparations and these studies provide new insights into the actions of AlloP as both an endogenous stress modulator and as a pharmacological therapeutic.In this review, we describe our studies in the retina, with emphasis on glaucoma models, and ways in which this work may inform use of NAS in neuropsychiatry.
Search Strategy and Selection Criteria
This review focuses upon work done in our laboratories over an extended period that includes the development of anex vivo
retinal preparation for studying various forms of retinal degeneration including extension to a model of glaucoma.To put this work in context of the field, our review of the literature used PubMed as the search engine with search terms that included retina, glaucoma, neurosteroids, and excitotoxicity, focusing on work pertinent to the experiments described.Relevance of the Retina and Glaucoma to Neuropsychiatry
Multiple neuropsychiatric illnesses involve changes in axons and white matter in the brain.These include neurodegenerative illnesses such as Alzheimer’s and Huntington’s diseases, but also primary psychiatric illnesses including schizophrenia, major depression, and autism, among others (Fields, 2008;Alnaes et al., 2018).Axonal changes contribute to altered connectivity within and across brain regions underlying mental functions of cognition,emotion, and motivation, and can be observed early in the course of illness,particularly in disorders with developmental origins.Given that glaucoma involves damage to retinal ganglion cells (RGCs) and their axons that form the optic nerve, glaucoma could provide insights into understanding axonal dysfunction and its treatment.Glaucoma is the second leading cause of blindness worldwide and the number one cause of blindness among African Americans.Glaucoma is also associated with more than two-fold increase in major depression (Yoshikawa et al., 2019).
A second reason for interest in the retina involves increasing evidence that RGCs mediate effects of light on mood and behavior, including risk of depression.It is now clear that there are multiple subtypes of RGCs, including RGCs that express melanopsin and have intrinsic photosensitivity (ip) akin to photoreceptors (Tran et al., 2019).Different subtypes of ipRGCs connect to different subcortical regions and mediate unique behavioral effects (Fernandez et al., 2018; Do, 2019).One subclass connects to the suprachiasmatic nucleus and secondarily to the hippocampus, mediating effects of light on learning and memory (Fernandez et al., 2018; Huang et al., 2021).Asecond subtype connects to the perihabenular region and secondarily to the dorsomedial striatum and nucleus accumbens to mediate effects of light on mood (Fernandez et al., 2018; Huang et al., 2019).Other studies indicate that ipRGCs drive a light-activated pathway to ventral lateral geniculate and the intergeniculate leaflet that results in inhibition of lateral habenula and mediates antidepressant effects of light, a finding of importance for seasonal mood disorders and disorders of circadian rhythms (Huang et al., 2019).
An Ex Vivo Retinal Preparation and Glaucoma Model
Work in the retina has had a major impact on understanding mechanisms of neurodegeneration, particularly glutamate-mediated excitotoxicity.Some of the earliest work on glutamate toxicity came from observations in the retina (Lucas and Newhouse, 1957) and was later extended to the brain by John Olney (Olney, 1969).In the 1980s, Olney and colleagues used anex vivo
embryonic chick retinal preparation to define mechanisms underlying glutamate-induced excitotoxicity (Olney et al., 1986, 1990; Romano et al.,1998).Because of limitations of the chick model including challenges in doing studies across a range of ages, Yukitoshi Izumi developed anex vivo
model for these studies using rat retinas (Izumi et al., 1995a).This model proved useful for histological studies across a range of developmental ages (Izumi et al., 1995b) including work on the effects of excitotoxic conditions on Muller glial cells (Izumi et al., 2003) and the role of glutamate transporters (Izumi et al.2002).Other studies examined the mechanisms of various forms of retinal phototoxicity (Tokuda et al., 2007, 2009).In the early 2000s, Makoto Ishikawa and colleagues adapted theex vivo
rat preparation to study the effects of elevated pressure as a model of acute closed angle glaucoma.The initial model incubated retinas under conditions designed to mimic acute increases in hydrostatic pressure.For these studies,retinal eye cups from 30-day-old rats were incubated at the bottom of cylinders filled to varying degrees with oxygenated artificial cerebral spinal fluid (ACSF) to mimic normal pressure (10 mmHg), moderately elevated pressure (35 mmHg) and high pressure (75 mmHg) (Ishikawa 2010; Figure 1).Retinas incubated under normobaric conditions exhibited intact retinal histology with no evidence of damage.However, retinas incubated for 24 hours at 75 mmHg exhibited damage to RGCs (shrinkage) and swollen axons of these neurons without significant injury to other retinal layers (Ishikawa et al.,2010, 2011).Damage to the optic nerve head was also observed.Importantly,damage caused by high pressure differed distinctly from excitotoxic damage produced by glutamate or AMPA under normobaric conditions (Ishikawa et al.,2010), and was histologically distinct from the effects of simulated ischemia(oxygen-glucose deprivation) (Izumi et al., 2003; Figure 2).Nonetheless,damage to RGCs and axonal swelling resulting from hyperbaric incubation was blocked completely by a combination of glutamate receptor antagonists acting at NMDA and non-NMDA ionotropic receptors (Ishikawa et al., 2010).The initial glaucoma model described above was an open hydrostatic system in which pressure on the retinal eyecups was controlled by the height of fluid in the incubation cylinders.This model had several limitations and was eventually replaced by a closed incubation system in which volume in the chamber was kept constant and pressure was regulated by influx and efflux of gas (95% oxygen-5% carbon dioxide) via separate valves, using a manometer to monitor intra-chamber pressure (Ishikawa et al., 2016; Figure 1).Retinas were incubated in standard volumes of ACSF for 24 hours and showed similar changes in RGCs and axons in response to hyperbaric pressure without damage to other retinal layers.
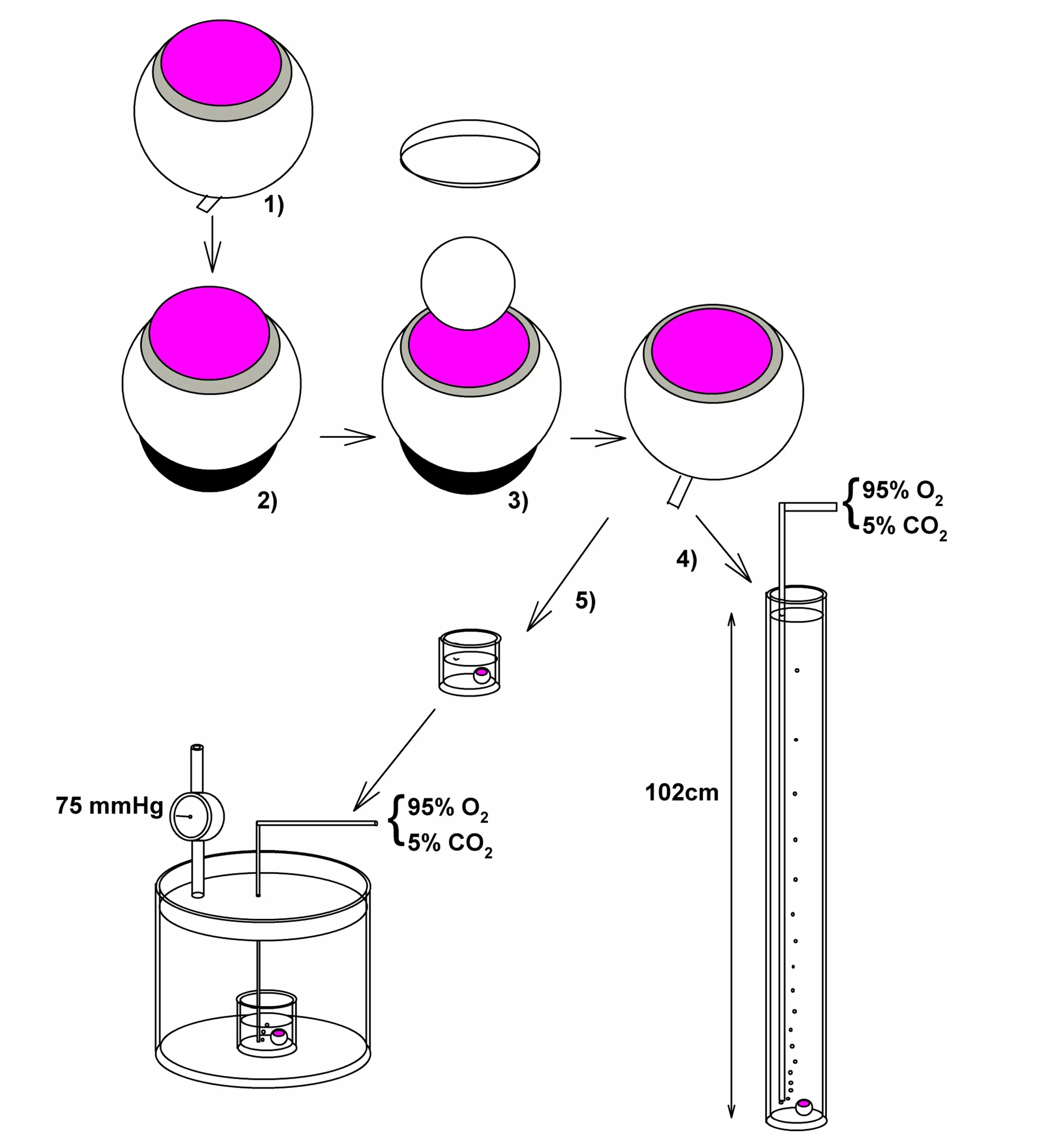
Figure 1|The diagram depicts methods for ex vivo rat retinal studies including the methods used to mimic glaucoma.
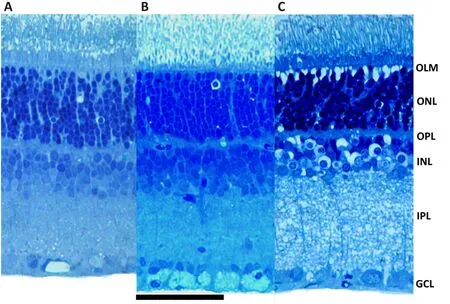
Figure 2|Retinal histology in the ex vivo model.
Neurosteroids and Effects of High Pressure in the Retina
Why neurosteroids?
Neurosteroids, including AlloP, are synthesized locally from cholesterol in excitatory neurons and glia cells in response to a variety of stressors.In the hippocampus, these stressors include metabolic insults (altered energy metabolism) and toxins (ethanol and acetaldehyde) along with the stress hormone, corticosterone (Zorumski and Izumi, 2012; Zorumski et al., 2014).Importantly these stressors cause acute accumulation of glutamate and tonic activation of NMDA receptors (Tokuda et al., 2011).Even brief exposures to these stressors (5–30 minutes) cause untimely activation of NMDA receptors and, as a result, impair induction of long-term potentiation (LTP)in the CA1 hippocampal region through an NMDA receptor-mediated form of metaplasticity (Zorumski and Izumi, 2012).These changes occur in the absence of histological damage or changes in baseline synaptic transmission,but involve upregulation of the synthesis of 5-alpha reduced neurosteroids,particularly AlloP.These observations, coupled with the role of cellular stress (high pressure) and glutamate in the glaucoma model, led us to consider whether AlloP and other sterols might be important modulators of stress on retinal structure and function, serving perhaps as homeostatic neuroprotective mechanisms.Furthermore, the retina is neurosteroidogenic and RGCs express key enzymes in neurosteroid synthesis including CYP11A1,a mitochondrial cholesterol side chain cleavage enzyme that generates pregnenolone as the first step in neurosteroid synthesis (Guarneri et al., 1994,1998, 2003; Cascio et al., 2015).RGCs express other steroidogenic proteins including the 18 kDa translocator protein, TSPO, as well as 5-alpha reductase(5AR), a key synthetic enzyme (Ishikawa et al., 2016).
Neurosteroids as neuroprotectants
Because of the therapeutic potential of AlloP and other NAS, we initially examined whether exogenous AlloP alters injury to RGCs and axons in the nerve fiber layer (NFL).Co-incubation of retinas for 24 hours with high pressure and varying concentrations of AlloP resulted in concentrationdependent retinal protection.At 10 nM AlloP, there was no clear change in pressure-induced damage; some neuroprotection was observed at 100 nM, with complete retinal preservation at 1 μM (Ishikawa et al., 2014), a concentration above physiological levels.
In related studies, we examined the role of endogenous AlloP in modulating effects of high pressure.Using liquid chromatography and tandem mass spectrometry to measure AlloP, we found very low levels of the steroid under basal conditions when retinas were incubated for 24 hours at 10 mm Hg.At 35 mm Hg, only marginal increases of AlloP were observed, while incubation at 75 mm Hg resulted in 60–70-fold increases in AlloP compared to normobaric conditions (Ishikawa et al., 2014).These increases in AlloP at high pressure were partially inhibited (approximately 50% reduction) by finasteride, a 5AR antagonist that acts at a subset of 5ARs.More complete inhibition was observed with dutasteride, a broader spectrum 5AR antagonist(Pinacho-Garcia et al., 2020), and with the competitive NMDA receptor antagonist, 2-amino-5-phosphonovalerate (Ishikawa et al., 2014).Using an antibody against 5-alpha-reduced steroids that primarily recognizes AlloP(Tokuda et al., 2010, 2011), we also observed immunohistochemical evidence of increases in neurosteroids in RGCs; these changes were dampened by the antagonists described above (Ishikawa et al., 2014).
Changes in neurosteroid levels led us to consider whether endogenous 5-alpha-reduced steroids play a protective role in the retina under stress.When retinas were incubated with 5AR antagonists under normobaric conditions, no significant histological changes were observed.However, 5AR antagonists led to marked damage in multiple retinal layers during exposure to high pressure.These latter changes included RGC and axonal damage as well as excitotoxic changes in other retinal layers.Excitotoxic changes included marked swelling of neurons resulting in “bulls-eye” appearances of cells in the inner nuclear layer (INL) and “Swiss-cheese” (edematous) changes in the inner plexiform layer.This severe excitotoxicity was blocked by the NMDA receptor antagonist, 2-amino-5-phosphonovalerate, and by exogenous AlloP at 1 μM (Ishikawa et al., 2014).
We also found that high pressure increases expression of genes and proteins thought to be involved in neurosteroidogenesis, including TSPO and 5AR.Increases in 5AR, mostly type II, a form of the enzyme inhibited by dutasteride and finasteride (Pinacho-Garcia et al., 2020), were observed in RGCs and INL (Ishikawa et al., 2016), consistent with what we observed with changes in AlloP levels described above.TSPO has a complex relationship to steroidogenesis.While it is clearly a protein of importance for mitochondrial function, its role in neurosteroid synthesis is less certain based on studies in TSPO knockout mice (Selvaraj et al., 2015), although other studies suggest some role in neurosteroidogenesis (Tokuda et al., 2010; Ishikawa et al., 2016;Mages et al., 2019).
How is AlloP neuroprotective? Roles of gamma-aminobutyric acid-A receptors and autophagy
AlloP is a complex neuromodulator produced on-demand under stressful conditions in the brain and nervous system.It has several known mechanisms that could contribute to neuroprotection.Most prominently, AlloP is a potent and effective positive allosteric modulator (PAM) of gamma-aminobutyric acid(GABA)-A receptors, the receptors that mediate the majority of fast inhibitory transmission (Zorumski et al., 2019).Potentiation of GABA-A receptors occurs at mid-nanomolar concentrations; at higher nanomolar to micromolar concentrations, AlloP can directly open GABA-A channels in the absence of GABA (called direct channel gating).GABA-A receptors are heteropentamers with 19 subunits identified (Belelli and Lambert, 2005).Different subtypes of GABA-A receptors are expressed at synapses where they mediate phasic forms of inhibition.Other GABA-A receptors are expressed at extrasynaptic sites where they sense ambient levels of GABA and mediate tonic inhibition(Belelli et al., 2009).Importantly, AlloP and related NAS modulate both phasic and tonic inhibition, giving them the unique ability to regulate the balance of excitation and inhibition throughout the brain.Thus, modulation of GABA transmission is a major mechanism likely contributing to the effects of AlloP.Beyond GABA, AlloP also has effects on other ion channels and intracellular processes.These include inhibition of low voltage-activated (T-type) calcium channels that mediate burst firing in certain neurons and help to regulate oscillatory firing and pain (Pathirathna et al., 2005).AlloP is a highly lipophilic steroid and accesses intracellular compartments where it modulates inflammation (Balan et al., 2019, 2021), mitochondrial function, and energy utilization (Grimm et al., 2014).
We examined the role of GABA-A receptors in the effects of AlloP in our glaucoma model using picrotoxin, a non-competitive GABA-A receptor antagonist.The protective effects of exogenous AlloP were completely blocked by co-administration of picrotoxin, indicating a prominent role for GABA-A receptors in neuroprotection.In addition to overcoming protection of RGCs and axons in the NFL, picrotoxin promoted more severe damage in the retina including excitotoxic changes in the INL and inner plexiform layer(Ishikawa et al., 2014).
Because neuroprotective effects of AlloP in other studies involve mechanisms in addition to GABA-A receptor modulation (Langmade et al., 2006), we alsoexamined whether alternative mechanisms contribute to effects in the retina focusing on the possible role of macroautophagy (autophagy).Autophagy is a mechanism by which cells recycle debris to help control stress responses,preserve energy and promote survival, although, when unchecked, excessive autophagy can also lead to cell death (Kroemer et al., 2010; Zhou et al., 2019).Under hyperbaric conditions, AlloP increased levels of the lipidated form of microtubule-associated protein 1 light chain 3 beta (LC3B-II) and decreased levels of sequestosome 1 (p62), consistent with enhancement of autophagy.At the ultrastructural level, AlloP increased the number of autophagosomes and degenerative autophagic vacuoles in the NFL.Inhibitors of autophagy,including bafilomycin A1 and SAR405, overcame the protective effects of AlloP and induced severe neurodegeneration.Autophagy activators, rapamycin and Torin 2, produced partial protection against high pressure, consistent with a protective role of autophagy in our model (Ishikawa et al., 2021).
Taken together, these results indicate that effects on GABA-A receptors and activation of autophagy contribute to retinal protection by AlloP in theex vivo
glaucoma model (Figure 3).Whether other actions of AlloP contribute to retinal preservation remains uncertain, but are areas for further investigation.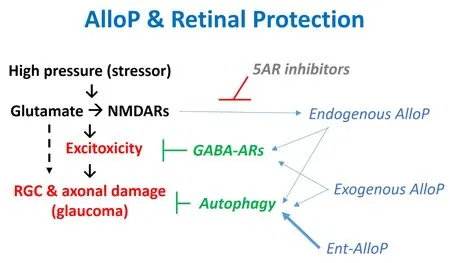
Figure 3 | AlloP and effects of high pressure on the retina.
The enantiomer of AlloP is also neuroprotective in the retina
In an effort to determine the importance of GABA-A receptor PAM activity in neuroprotection by AlloP, we used its unnatural enantiomer (ent-AlloP).Earlier work demonstrated that effects of AlloP on GABA-A receptors are highly enantioselective with the enantiomers differing by at least an order of magnitude in potency (Wittmer et al., 1996; Covey et al., 2001).However, enantiomers accumulate identically in cell membranes and intracellular compartments (Chisari et al., 2009, 2010; Jiang et al., 2016).To our surprise, but consistent with a prior study in a model of developmental neurodegeneration (Langmade et al., 2006), we found that ent-AlloP was at least as effective as AlloP in protecting the retina from damage produced by high pressure at an equimolar concentration (1 μM).Furthermore, and consistent with its lack of effects on GABA-A receptors, retinal protection by ent-AlloP was not altered by picrotoxin, unlike natural AlloP (Ishikawa et al.,2022).However, ent-AlloP increased the number of autophagosomes and degenerative autophagic vacuoles in the NFL, and enhanced protein levels of LC3B-II while decreasing levels of p62, indicating that ent-AlloP, like AlloP,promotes autophagy.The autophagy inhibitor, 3-methyladenine, blocked neuroprotection by ent-AlloP, but did not alter protection by AlloP.This latter result is intriguing and suggests that it is possible to dissociate the role of GABA-A receptors (or other mechanisms) from autophagy in the effects of natural AlloP.Furthermore, these results indicate that ent-AlloP promotes autophagy as a major mechanism of retinal protection, but these effects are independent of GABA-A receptors in contrast to AlloP (Figure 3).In direct comparisons using biochemical markers of autophagy, ent-AlloP appears to be more effective than AlloP in activating autophagy at a concentration of each that is fully neuroprotective (Ishikawa et al., 2022).We note that there is presently controversy concerning the role of autophagy in glaucoma and other neurodegenerative illnesses (Spalding et al., 2005; Kroemer et al.,2010), but there is considerable interest in whether agents targeting this mechanism can be developed for use in neuropsychiatric illnesses (Hui et al.,2021).Additionally, the targets and mechanisms by which NAS stimulates autophagy remain undefined, and it is presently unclear whether autophagy is a primary action or the result of another modulated cellular process.
AlloP enantiomers are protective in an in vivo glaucoma model
To determine whether effects observed in theex vivo
glaucoma model translate to animals, we used a rat model of increased intraocular pressure(IOP) produced by intracameral injection of polystyrene microbeads.In this model, IOP increases from about 10 mmHg to 30 mmHg over 3 days after bead injection and IOP remains elevated at this level for the remainder of the three-week experiment (Ishikawa, 2021, 2022).In control rats, elevated IOP results in damage to RGCs and axons in the NFL by 21 days after injection.Animals also exhibit impaired visual responses as measured by the retinal scotopic threshold response.Effects of AlloP and ent-AlloP were examined using a single injection of the steroids on day 7 after bead injection, at a time when elevated IOP was fully established.The steroids were solubilized in β-cyclodextrin and administered as a 1 μL injection into the vitreous using a 0.05% (w/v) saline solution.Neither steroid altered the increase in IOP caused by microbead injections.Nonetheless, both enantiomers produced strong neuroprotection based on histology and measures of apoptosis of RGCs, and both promoted autophagy based on measures outlined in theex vivo
studies above.Similarly, both steroids preserved the retinal scotopic threshold response, indicating preserved visual function as well as histology (Ishikawa et al., 2021, 2022).Lessons Learned from the Retina
Studies in the retina raise several important considerations for therapeutic use of neurosteroids in neuropsychiatric illnesses.Below we outline several key points that we take from the studies described.We provide additional commentary for certain points that may be relevant to considering how neurosteroids are used clinically going forward.
1.Rodent retinal models are useful for understanding mechanisms contributing to neurodegeneration and neuroprotection.In particular, theex vivo
system provides a useful tool for detailed mechanistic studies.2.Neurosteroids, particularly AlloP, are important endogenous modulators that help to preserve neuronal structure and function under stressful conditions.This is likely true throughout the brain as it is in the retina.
3.Extracellular glutamate accumulation and tonic activation of NMDA receptors are key drivers of the effects of stress on synthesis of neurosteroids.This has been observed in both retina and hippocampus.
4.Removal of acute protection by endogenous 5-alpha reduced steroids renders neurons highly vulnerable to excitotoxic neurodegeneration involving multiple retinal layers.However, there are limits on neuroprotection provided by endogenous AlloP, as evidenced by glaucomatous changes even in the presence of increases in endogenous neurosteroid.
5.Pharmacological effects of neurosteroids are required for full neuroprotection, even in the presence of elevated endogenous steroid levels.These pharmacological effects may require concentrations above those that are considered physiological as evidenced by full protection with 1 μM but not 100 nM exogenous AlloP.Furthermore, a single injection of neurosteroid at a pharmacological dose can have lasting neuroprotective effectsin vivo
even when the primary neuronal stressor (high pressure) persists.We note that current use of brexanolone (AlloP) for postpartum depression involves a 60-hour infusion in which the dose is escalated to a high level observed in pregnancy (approximately 100 nM) and maintained at that level for 28 hours before tapering off by the end of infusion (Meltzer-Brody and Kanes, 2020).Another NAS, zuranolone, is administered orally as a single, 30–50 mg daily dose (Gunduz-Bruce et al., 2019; Deligiannidis et al., 2021).Levels achieved by these steroids and duration of exposure at high levels in key brain regions may be important in determining clinical efficacy, and high doses may be required for maximal results, based on studies in the retina.It is also intriguing that even transient and perhaps intermittent exposures to GABAergic NAS can have persisting effects both in the glaucoma model and in humans with major depressive syndromes.These observations make it important to understand the longer-term effects of NAS following transient exposures; studies in the retina can help this effort based on results to date.6.The enantiomer of AlloP is an intriguing pharmacological tool, providing unexpected neuroprotection via the cellular mechanism of autophagy.Neuroprotection by ent-AlloP does not involve effects on GABA-A receptors and may thus obviate certain side effects of AlloP including sedation and loss of consciousness, perhaps allowing administration of higher doses.Metabolism of ent-AlloP and other enantiomeric steroids will likely differ from AlloP and possibly contribute to longer-lived effects.Side effects of enantiomeric neurosteroids are presently unknown.A diagram of the roles of endogenous and exogenous AlloP in the studies described is shown in Figure 3.We also note that in ourin vivo
glaucoma model (Ishikawa et al., 2021;2022) and in a model of developmental neurodegeneration (Langmade et al., 2006), both AlloP enantiomers provide neuroprotection following a single administration that persists for weeks in rodents, while having no effect on the causative mechanisms that underlie neuropathology.Future Directions in Neurosteroid Pharmacology
Neuroprotective effects of both endogenous and exogenous AlloP in the retina are unequivocal.Future studies are required to define the full mechanisms by which neurosteroids produce their protective effects including the role of GABA-A receptors and alternative targets.It is presently unclear how AlloP and its enantiomer stimulate autophagy, but it is clear that GABA-A receptors are not the entire story, particularly for ent-AlloP.Based on work with AlloP analogues that are photoaffinity labels, both endogenous and exogenously applied NAS interact with several known intracellular targets.These steroids interact strongly with Golgi and this interaction, as well as intracellular and membranous accumulation, is not enantioselective(Jiang et al., 2016).Specific intracellular targets identified to date include the microtubule protein, β-tubulin, at Cys-354 (Chen et al., 2012), and the mitochondrial proteins voltage-dependent anion channel-1 and -2 (VDAC) at Glu-73 of VDAC-1 along with four additional sites (Darbandi-Tonkabon et al.,2003, 2004; Budelier et al., 2017; Cheng et al., 2019).The site on β-tubulin is shared by colchicine, and the VDAC-1 sites are shared with cholesterol(Budelier et al., 2019).These are potentially important intracellular targets that could play a role in neuroprotection, but their roles in effects of NAS are still undefined.Earlier studies indicated no role for VDACs in GABA-A receptor effects of NAS or anesthesia (Darbandi-Tonkabon et al., 2004) and NAS do not alter gating of VDAC-1 (Cheng et al., 2019).Ongoing work with photoaffinity labels will no doubt identify other targets.
Going forward, it will be important to define other mechanisms that contribute to homeostatic and protective effects of NAS.In particular,neuroinflammation is a mechanism contributing to the pathogenesis of neuropsychiatric illnesses including retinal disorders.Increasing evidence indicates that NAS, and AlloP in particular, have anti-inflammatory effects that may be important for therapeutic effects (Balan et al., 2019, 2021).Other potential contributors could include effects on cellular stress responses including the folded protein response and integrated stress response.Autophagy interacts with integrated stress response, making this an intriguing potential target (Kroemer et al., 2010).It will also be important to define more clearly the role of GABA-A receptor modulation in the effects of AlloP and whether the enantiomers have additive or synergistic effects when applied in combination.It is also unknown at present whether mechanisms other than GABA-A receptors contribute to psychotropic actions.
This review has focused almost exclusively on AlloP and its enantiomer.While AlloP is a major cholesterol-derived neuromodulator, a host of other steroids are produced in the retina and brain (Guarneri et al., 2003;Belelli and Lambert, 2005).Some of these agents have unique properties including effects on other ion channels, receptors, and intracellular targets.Additionally, the class of side chain oxidized derivatives of cholesterol(oxysterols) are also neuromodulators with 24S-hydroxycholesterol (24SHC) being the major metabolite of cholesterol in the brain (Sun et al., 2016).24S-HC has multiple effects including potent and effective PAM activity at NMDA receptors (Paul et al., 2013).This oxysterol has complex effects on neurons including bidirectional effects on neurotoxicity.In some studies,24S-HC fosters neurodegeneration, as would be expected of an NMDAR PAM, while in other studies this oxysterol is neuroprotective (Sun et al.,2016).In theex vivo
glaucoma model, we found 24S-HC is neuroprotective by mechanisms that are not yet defined.Akin to AlloP, synthesis of 24S-HC via CYP46A1 appears to be stress-activated in response to reactive oxygen species (Sodero et al., 2011) and plays a role in regulating autophagy (Nobrega et al., 2019).An analogue of 24S-HC, SAGE-718, is currently in clinical trials as a cognitive enhancer in Huntington’s, Parkinson’s and Alzheimer’s diseases.Another oxysterol, 25-hydroxycholesterol is also intriguing as a modulator of neuroinflammation, and is synthesized in the brain primarily in microglia(Wong et al., 2020; Izumi et al., 2021); 25-hydroxycholesterol also has weak partial agonist PAM effects on NMDA receptors and can serve as a functional inhibitor of 24S-HC (Linsenbardt et al., 2014).In summary, studies in the retina clarify some of the protective mechanisms of AlloP and offer potential to identify other important effects going forward.It also appears that we are presently only scratching the surface of cholesterol-derived modulators and their potential roles as therapeutics in neuropsychiatry, including how these modulators interact to promote neuronal homeostasis.
Acknowledgments:
The authors thank Ann Benz and Kazuko Izumi from the Department of Psychiatry, Washington University School of Medicine, St.Louis, MO, USA for technical assistance, and members of the Taylor Family Institute, St.Louis, MO, USA for helpful comments and advice.
Author contributions:
YI, MI and CFZ conceived, designed and wrote the manuscript.All authors edited and revised the paper and approved the final version of the manuscript.
Conflicts of interest:
CFZ serves on the Scientific Advisory Board of Sage Therapeutics.DFC was a co-founder of Sage Therapeutics.DFC and CFZ have equity in Sage Therapeutics.Sage Therapeutics did not fund this research.Other authors have no conflicts to declare.
Availability of data and materials:
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:
Yasushi Kitaoka, St.Marianna University School of Medicine, Japan.
Additional file:
Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- Patient-specific monocyte-derived microglia as a screening tool for neurodegenerative diseases
- Molecular hallmarks of long non-coding RNAs in aging and its significant effect on aging-associated diseases
- Inflammation in diabetic retinopathy: possible roles in pathogenesis and potential implications for therapy
- Targeting the nitric oxide/cGMP signaling pathway to treat chronic pain
- Myelinosome organelles in pathological retinas:ubiquitous presence and dual role in ocular proteostasis maintenance
- Anti-IgLON5 disease: a novel topic beyond neuroimmunology

