Double-target neural circuit-magnetic stimulation improves motor function in spinal cord injury by attenuating astrocyte activation
Dan Zhao , Ye Zhang , Ya Zheng Xu-Tong Li, Cheng-Cheng Sun Qi Yang Qing Xie , Dong-Sheng Xu
Abstract Multi-target neural circuit-magnetic stimulation has been clinically shown to improve rehabilitation of lower limb motor function after spinal cord injury.However, the precise underlying mechanism remains unclear.In this study, we performed double-target neural circuit-magnetic stimulation on the left motor cortex and bilateral L5 nerve root for 3 successive weeks in a rat model of incomplete spinal cord injury caused by compression at T10.Results showed that in the injured spinal cord, the expression of the astrocyte marker glial fibrillary acidic protein and inflammatory factors interleukin 1β, interleukin-6, and tumor necrosis factor-α had decreased, whereas that of neuronal survival marker microtubule-associated protein 2 and synaptic plasticity markers postsynaptic densification protein 95 and synaptophysin protein had increased.Additionally, neural signaling of the descending corticospinal tract was markedly improved and rat locomotor function recovered significantly.These findings suggest that double-target neural circuit-magnetic stimulation improves rat motor function by attenuating astrocyte activation, thus providing a theoretical basis for application of double-target neural circuit-magnetic stimulation in the clinical treatment of spinal cord injury.
Key Words: astrocyte; inflammatory response; microtubule-associated protein 2; motor function; motor-evoked potential; neural circuit-magnetic stimulation;neural repair; neuromodulation technique; spinal cord injury; synaptic plasticity
Introduction
Spinal cord injury (SCI) is a leading cause of serious motor, sensory, and autonomic dysfunctions (Cao et al., 2022; Estrada et al., 2022).Treating it and promoting SCI rehabilitation remain a worldwide challenge.The pathophysiological process of SCI is complex and associated with different types of cellular and molecular responses (Anwar et al., 2016; Alizadeh et al., 2019), including edema, local ischemia, inflammation, ion imbalance,demyelination, and glial scarring.However, it is clear that astrocytes play a significant role in regulating the spinal cord microenvironment after SCI (Fan et al., 2018; Gaudet and Fonken, 2018; Okada et al., 2018).After SCI, naive astrocytes are rapidly activated into reactive astrocytes and undergo changes in protein expression, such as increased levels of glial fibrillary acidic protein(GFAP), nestin, and vimentin, while showing cell hypertrophy and proliferation and increased cytokine secretion (Zamanian et al., 2012; Sun et al., 2021).Reactive astrocytes are both beneficial and harmful (Karimi-Abdolrezaee and Billakanti, 2012; Lukovic et al., 2015).Briefly, reactive astrocytes can prevent lesion spread and drive the expression of trophic factors, thus alleviating secondary injuries (Faulkner et al., 2004).However, as SCI progresses,excessive numbers of reactive astrocytes can form a substantial glial scar and release harmful levels of inflammatory factors like tumor necrosis factor-alpha(TNF-α), interleukin-1 beta (IL-1β) and interleukin-6 (IL-6), as well as inhibitory molecules like chondroitin sulfate proteoglycans.Gradually, the tangled glial scar and the inflammatory responses it causes act as physical and chemical barriers to neural repair (Yiu and He, 2006).Therefore, modulating reactive astrogliosis can help optimize the microenvironment around the injury for neuroprotection and repair and improve the rehabilitation of motor function.
Transcranial magnetic stimulation (TMS) is being used in the prognostic assessment and treatment of SCI (Awad et al., 2015).The principle of TMS is to use a rapidly changing magnetic field to induce electrical currents and control the action potentials in neurons, thereby leading to different regulatory effects (Hallett, 2007).Strong evidence suggests that TMS has major therapeutic potential for motor dysfunction, neuropathic pain,spasticity, and bladder dysfunction in incomplete SCI (Korzhova et al., 2018;Krishnan et al., 2019; Nardone et al., 2019; Sun et al., 2019).Furthermore,previous studies have reported that TMS can decrease the expression of GFAP in the injured spinal cord to improve SCI.Yang et al.(2018) suggested that 20 Hz repetitive TMS can inhibit astrocyte activation to relieve neuropathic pain in SCI rats.Moreover, GFAP expression was down-regulated by about 30%when SCI rats were treated with repetitive TMS at 25 Hz for 8 weeks (Kim et al., 2013).However, clinical and basic research have shown that a single transcranial cortical target is not sufficient for neural circuit reconstruction(Zheng et al., 2020).Consequently, based on TMS and the underlying neural circuits, our team developed the neural circuit-magnetic stimulation (NCMS) technique for treating incomplete SCI.The specific protocol consists of stimulating the cerebral motor cortex to activate the descending corticospinal tract and synchronously or non-synchronously stimulating the downstream nerve root to generate sensory signals.Stimulation of the cerebral cortex effectively activates the descending corticospinal tract, whereas stimulation of the downstream nerve roots activates sensory pathways from the bottom up, which may further activate specific cortical regions or corticospinal tracts.This maximizes activation of the sensory-motor loop and promotes the reconstruction of motor-related functions.This protocol has been shown to have a positive effect on patient behavior (Mao et al., 2022).
However, we must understand how NC-MS treatment achieves these results.We hypothesized that one of the mechanisms through which NC-MS promotes functional recovery, neural survival, and neural regeneration was to attenuate the activation of astrocytes.Consequently, the current study applied NC-MS to rats with incomplete SCI and investigated the resulting motor function, electrophysiology, astrocyte activation, inflammation, neural survival and repair, and synaptic plasticity.
Methods
Animals
Considering sex and the effects of hormone changes, we selected male adult rats for the study.We used 36 adult male Sprague-Dawley rats (specialpathogen-free level, 10 weeks old, weighing 220–250 g), purchased from Shanghai Jiesijie Experimental Animal Co., Ltd., China (No.SCXK (Hu) 2018-0004).All animals were housed two per cage in a 12-hour light/dark cycle under standard conditions (25°C with 45–65% humidity), with ad libitum water and food for 1 week while they adapted to the new environment.After that, rats were split randomly into three groups, each with 12 animals: sham operation + sham stimulation (Sham + SS), SCI + sham stimulation (SCI + SS),and SCI + NC-MS (SCI + NC-MS) treatment groups.The study was approved by the Animal Ethics Committee of Tongji Hospital in Shanghai, China (approval No.2019-DW-(036)) on August 29, 2019.All surgeries and experimental protocols were conducted according to the Care and Use of Laboratory Animals (National Institutes of Health Publications No.8023, revised 2011).
SCI model
SCI model rats were created using the modified clip-compression method according to Rivlin and Tator (Rivlin and Tator, 1978).After habituation, all rats underwent preoperative fasting and water deprivation.Then, they were anesthetized intraperitoneally with 1% pentobarbital sodium (0.4 mL/100 g,Pharmaceutical Group Chemical Reagent Co., Ltd., Shanghai, China), and the skin and surrounding tissues were incised and carefully separated to expose the T9–T11 vertebrae.The spinal cord was exposed at T10 after bilateral laminectomy.Then, an aneurysm clip (50 g, Fine Science Tools, Heidelberg,Germany) was used to span the exposed spinal cord and compress it vertically for 20 seconds (Figure 1A and B).Sudden and violent convulsions of the hind limb and tail swing were considered signs of successful SCI modeling.The muscle and skin were aseptically sutured after the incision was washed with normal saline.Finally, the rats were injected with 2 mL of normal saline and placed on a heating pad until fully awake.Rats in the sham group underwent the same surgery except for the spinal cord compression.
Post-surgery care entailed providing sufficient clean and dry padding, as well as adequate food and water.All rats received an intraperitoneal injection of penicillin (200,000 IU/d, Zhongnuo Pharmaceutical Co., Ltd., Binzhou, China)daily for 1 week to prevent infection and an abdominal massage to help bladder urination at 8 a.m.and 8 p.m.until recovery of autonomous urination function.
NC-MS treatment
Motor thresholds were measured by stimulating the left motor cortex (M1).The resting motor threshold was defined as the lowest stimulation intensity that led to a motor response in the right gastrocnemius more than 50% of the time (≥ 5 of the 10 stimulations) (Luo et al., 2017).From day 3 after SCI surgery, rats in the SCI + NC-MS group received NC-MS treatment using a magnetic stimulator (Mag TD, Yiruide Co., Wuhan, China) with a circular coil with a 64-mm diameter and a 75-mm 8-shaped coil.NC-MS treatment had two stimulation targets (Figure 1A and C).The first was the left motor cortex (M1, 0.5 mm lateral to the vertex on the biauricular line), which was stimulated using the 8-shaped coil at 40% maximum intensity (80% of resting motor threshold; 6 Telsa peak output; 25 Hz; 5000 pulses per day; five times per week) 15 times in total.The second target was the bilateral L5 nerve root,which was simultaneously stimulated with the circular coil at 40% maximum intensity (25 Hz, 15 trains).Animals in the sham stimulation groups also underwent the same protocol, but the coils were placed perpendicularly 2 cm above the target regions.During the treatment, the rat was placed on a homemade fixator (China Patent No.202010093329.2) in a quiet environment.
Motor function assessment
Changes in motor function were assessed using the Basso-Beattie-Bresnahan(BBB) scale and the inclined-plane test by two independent experimenters who were blind to the experimental groups.Assessments were performed 1day before surgery and 1, 3, 7, 14, and 21 days after surgery (Figure 1A).For each assessment, two rats in each group were placed on a 2-m diameter open field and allowed to move freely for 5 minutes.The experimenters observed the hind limb joint movements, coordination, stability, and fine function and then assigned an assessment score ranging from 0 to 21 (Basso et al., 1995).Next, the rats were placed on a rubber-covered plane (China Patent No.ZL 202020173423.4).The plane angle was gradually increased in 5° steps until the rats could no longer stay still for 5 seconds.The tests were performed three times and the average last angle was recorded (Duan et al., 2018).Higher scores and greater angles indicate better motor function recovery.
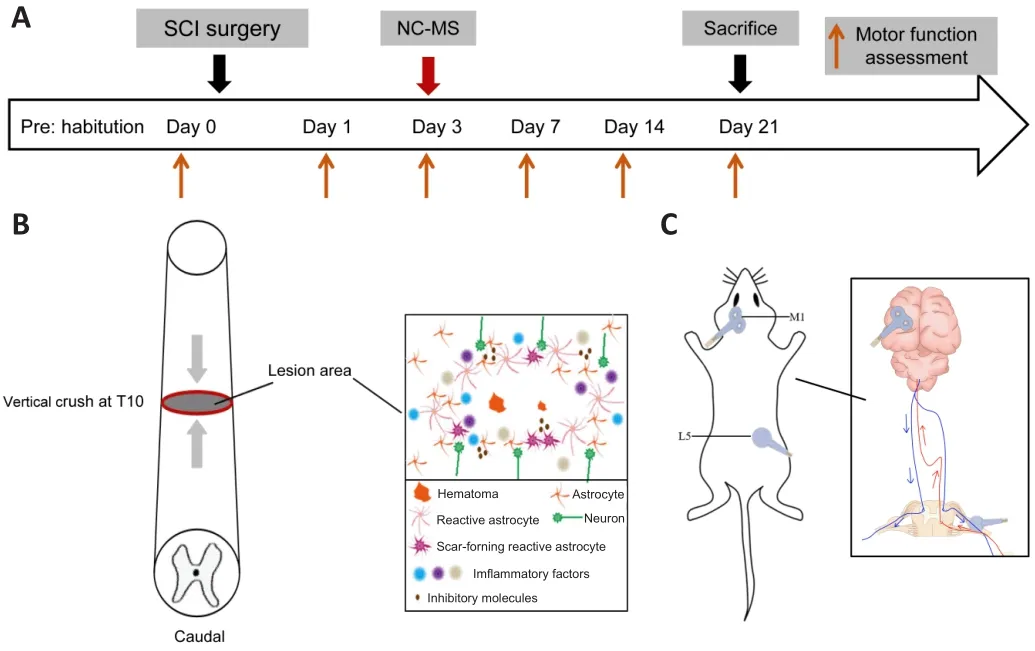
Figure 1 | Experimental timeline.
Western blot assay
GFAP is an astrocyte marker, and microtubule-associated protein 2 (MAP2),postsynaptic densification protein 95 (PSD95), and synapsin are neural survival and axon regeneration markers.Changes in the expression levels of these proteins in the injured spinal cord tissue were measured by western blot assay.On day 21 after SCI surgery, rats (n
= 5/group) were euthanized via full anesthesia with 2% pentobarbital sodium.The injured spinal cord(1 cm in length) was harvested quickly and put on ice.Proteins in the supernatant were collected after treatment with lysis buffer; then the protein concentrations were measured using BCA Protein Assay (Beyotime, Shanghai,China).Western blot assay was conducted based on a previous protocol(Zong et al., 2020).Protein samples (35 μg/well) were loaded on 6% and 10%sodium dodecyl sulfate-polyacrylamide electrophoresis gels (Beyotime) and then wetly transferred onto a 0.45 μm polyvinylidene fluoride membrane(Millipore, Billerica, MA, USA).After blocking with 5% skim milk, membranes were incubated with primary antibodies including MAP2 (mouse, 1:1000,Abcam, Cambridge, UK, Cat# ab11267, RRID: AB_297885), PSD95 (mouse,1:500, Abcam, Cat # ab2723, RRID: AB_303248), synapsin (rabbit, 1:1000,CST, Danvers, MA, USA, Cat# 2312, RRID: AB_2200102), GFAP (rabbit, 1:5000,Abcam, Cat# ab7260, RRID: AB_305808), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; rabbit, 1:1000, Yishan Biotechnology Co., Ltd.,Shanghai, China, Cat# ES_Pab001H) overnight at 4°C on a shaker.Then,they were washed three times with Tris-buffered saline with 0.1% Tween-20 and incubated with anti-rabbit (goat, 1:1000, Beyotime, Cat# A0208, RRID:AB_2892644) or anti-mouse secondary antibody (goat, 1:1000, Beyotime,Cat# A0216, RRID: AB_2860575).Finally, protein blots were captured using DRAFT-FluorChem Q (Alpha Innotech Corporation, San Leandro, CA, USA), and protein expression levels were analyzed using ImageJ software 1.6.0 (National Institutes of Health, Bethesda, MD, USA) (Schneider et al., 2012), with GAPDH as a reference control.Immunofluorescence
GFAP and MAP2 in the injured spinal cord tissue were detected using immunofluorescence.On day 21 after SCI surgery, rats (n
= 4/group) were anesthetized with 1% pentobarbital sodium (0.4 mL/100 g) intraperitoneally and then perfused intracardially with 0.9% normal saline followed by 4%precooled paraformaldehyde.Injured spinal cords (1 cm in length) were removed and fixed in the 4% paraformaldehyde overnight at 4°C and then continuously dehydrated in a 10%, 20%, and 30% sucrose gradient.Next,tissues were embedded with the optimal cutting temperature compound and cut into continuous 8-μm-thick longitudinal sections.Immunofluorescence staining was performed as previously described (Okuda et al., 2017).After blocking in Tris-buffered saline with 0.1% Tween-20 with 10% goat serum(Solarbio, Beijing, China) for 1 hour at 37°C, sections were incubated overnight at 4°C with the following antibodies: MAP2 (mouse, 1:1000, Abcam,Cat# ab11267, RRID: AB_297885) and GFAP (rabbit, 1:500, Proteintech Group,Chicago, IL, USA, Cat# 16825-1-AP, RRID: AB_2109646).Then sections were washed with phosphate-buffered saline three times and incubated at 25°C for 1 hour with anti-rabbit (goat, 1:1000, Jackson ImmunoResearch, Amish,PA, USA, Cat# 111-165-144, RRID: AB_2338006) or anti-mouse IgG H&L(Alexa Fluor®488, goat, 1:1000, Jackson ImmunoResearch, Cat# 115-545-003,RRID: AB_2338840).Subsequently, 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride (1:1000, Invitrogen, Carlsbad, CA, USA) was used to stain the cell nuclei.Finally, all the sections were imaged with an immunofluorescence microscope (Nikon, Tokyo, Japan).Enzyme-linked immunosorbent assay
Rats (n
= 3/group) were euthanized on day 21 after SCI surgery and the injured spinal cord (1 cm in length) was dissected quickly to reflect the inflammation level in the spinal cord.Enzyme-linked immunosorbent assay kits (Westang Bio-Tech, Shanghai, China) were used to measure IL-1β, IL-6 and TNF-α expression levels as previously described (Wang et al., 2019).Optical density values were measured at 450 nm using a DENLEY DRAGON Wellscan MK 3 (Thermo Fisher Scientific, Waltham, MA, USA).Finally, IL-1β, IL-6 and TNF-α concentrations were calculated based on optical density values and standard curves.Evaluation of motor-evoked potential
The motor-evoked potential (MEP) was evaluated on day 21 after surgery following the motor function assessments.Based on previous research(Oudega et al., 2019), rats (n
= 7/group) were first anesthetized with 5%chloral hydrate (0.5 mL/100 g).The stimulating electrode (a pair of needle electrodes) was inserted into the left motor cortex (M1), the recording electrode was placed in the gastrocnemius of the right hind limb, the reference electrode was placed on the distal Achilles tendon, and the ground electrode was placed between the stimulating and recording electrodes.The latency of MEPs was recorded after stimulation with a single square wave(stimulation intensity 25–35 mA) by an electrodiagnostic machine (5 ms/D,Keypoint, Skovlunde, Denmark).Each measurement was repeated no less than three times to ensure test stability.Statistical analysis
The sample sizes were based on previous experience (Xu et al., 2017) and evaluations were blind to grouping.Data analysis was performed using SPSS 20.0 software (IBM Corporation, Armonk, NY, USA) and GraphPad Prism software (version 8.0.1; GraphPad Software Inc., San Diego CA, USA, www.graphpad.com).Values are reported as mean ± standard deviation (SD).We used a two-way repeated-measures analysis of variance (ANOVA) followed by the Sidak multiple comparisons test to compare motor function among the three groups.A one-way ANOVA followed by Tukey’spost hoc
test was used to analyze the results of the western blot, the enzyme-linked immunosorbent assay, and the MEP latencies.Differences were considered significant atP
< 0.05.Results
NC-MS treatment improves motor function in rat models of incomplete SCI
To assess the effect of NC-MS treatment on motor function in rats after incomplete SCI, we assessed motor function in all groups using the BBB scale and inclined plane test at different time points.Over time, the BBB scores in all three groups increased, but as expected, scores for the SCI + NC-MS and SCI + SS groups were significantly lower than those for the Sham + SS group(P
< 0.001; Figure 2A).Among the two SCI groups, scores for the SCI + NC-MS group were significantly higher than those for the SCI + SS group (P
< 0.001;Figure 2A).The inclined-plane angles followed a similar trend, as illustrated in Figure 2B.Angles for the SCI + SS group were consistently lower than those for the SCI + NC-MS group from day 7 to day 21 (P
< 0.001; Figure 2B).These results indicate that NC-MS treatment improved the motor function in rat models of incomplete SCI.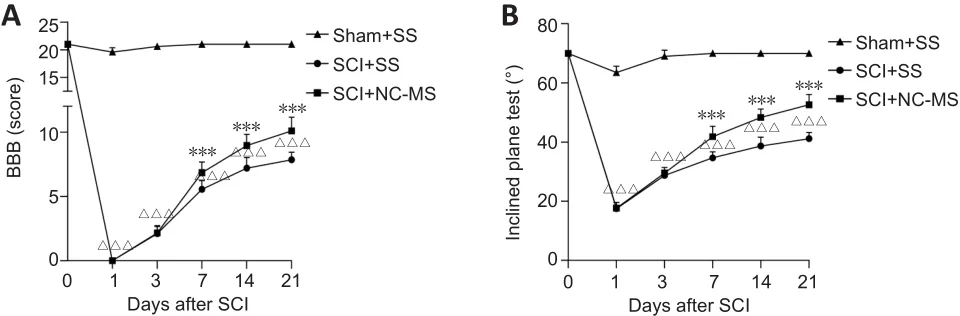
Figure 2 | NC-MS treatment improves motor function in rat models of incomplete SCI.
NC-MS treatment attenuates astrocyte activation in rat models of incomplete SCI
To assess the effect of NC-MS treatment on astrocyte activation in incomplete SCI, we performed western blot assays and immunofluorescence staining in three groups on day 21 after SCI to determine the degree to which the astrocyte-specific marker GFAP was expressed.Western blot results showed that GFAP expression was significantly higher in the SCI groups than in the Sham + SS group (P
< 0.01; Figure 3A and B).Among the two SCI groups, GFAP expression was significantly lower in the SCI + NC-MS treatment group than in the SCI + SS group (P
< 0.05).Immunofluorescence staining yielded similar results (Figure 3C).On day 21 after SCI, we observed high GFAP expression around the injured area as well as activated astrocytes that were swollen and hypertrophic.The active astrocytes exhibited thickened and elongated branches that were tangled into the reticulate structure, forming a dense and irregular glial scar.However, compared with the SCI + SS group, NC-MS treatment resulted in less GFAP expression and a smaller degree of swelling and astrocyte activation.These results indicate that NC-MS treatment attenuated astrocyte activation and reduced glial scar formation.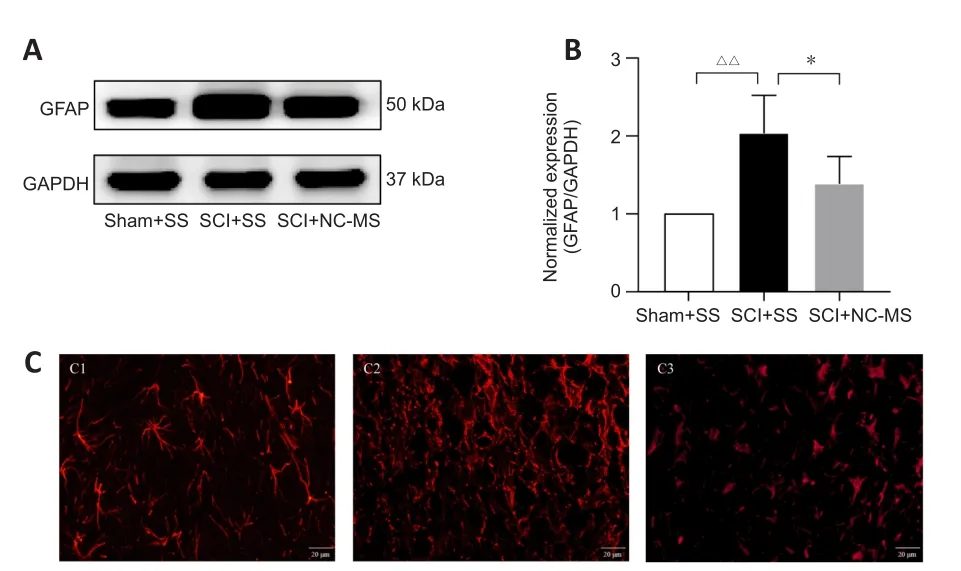
Figure 3 | NC-MS treatment attenuates the astrocyte activation in rat models of incomplete SCI.
NC-MS treatment reduces the inflammatory responses in rat models of incomplete SCI
Reactive astrocytes release a large number of inflammatory factors, which causes a cascade of inflammation, thus aggravating nerve damage (Liao et al.,2022).Because NC-MS treatment can attenuate astrocyte activation, we tested whether it could also reduce the inflammatory responses after SCI.Results of the enzyme-linked immunosorbent assays indicated that levels of IL-1β, IL-6, and TNF-α were significantly higher in injured spinal cord tissue on day 21 after SCI than in uninjured spinal cords (P
< 0.001).However, expression levels of these cytokines in damaged spinal cords were significantly less after NC-MS treatment than after sham stimulation (P
< 0.001; Figure 4).These data show that NC-MS treatment reduced the inflammatory responses after SCI.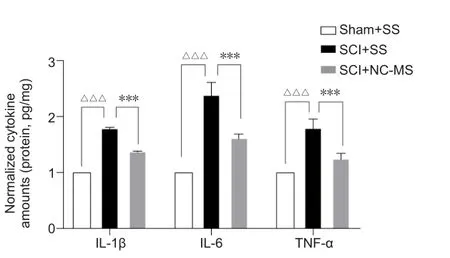
Figure 4|NC-MS treatment reduces the inflammatory responses in rat models of incomplete SCI.
NC-MS treatment enhances the neural survival, repair, and synaptic plasticity in rat models of incomplete SCI
TMS can promote neural repair and synaptic plasticity in many neurological disorders (Iglesias, 2020).Hence, we performed immunofluorescence staining and western blot assay to confirm whether NC-MS treatment can have the same effect after SCI.Immunofluorescence staining (Figure 5A) and western blot (Figure 5B and C) analysis showed that MAP2 expression was lower in injured spinal cord tissue after SCI.However, significantly higher MAP2 levels were observed in the SCI + NC-MS group than in the SCI + SS group (P
< 0.05;Figure 5C).Furthermore, we explored the influence of NC-MS on PSD95 and synapsin expression, both of which are related to synaptic plasticity.Western blot data suggested that NC-MS treatment leads to significantly more up-regulation of synaptic plasticity-related proteins than occurs without stimulation (P
< 0.05; Figure 5D and E).Thus, these findings confirmed that NC-MS treatment can enhance factors related to neural survival, repair, and synaptic plasticity in rat models of incomplete SCI.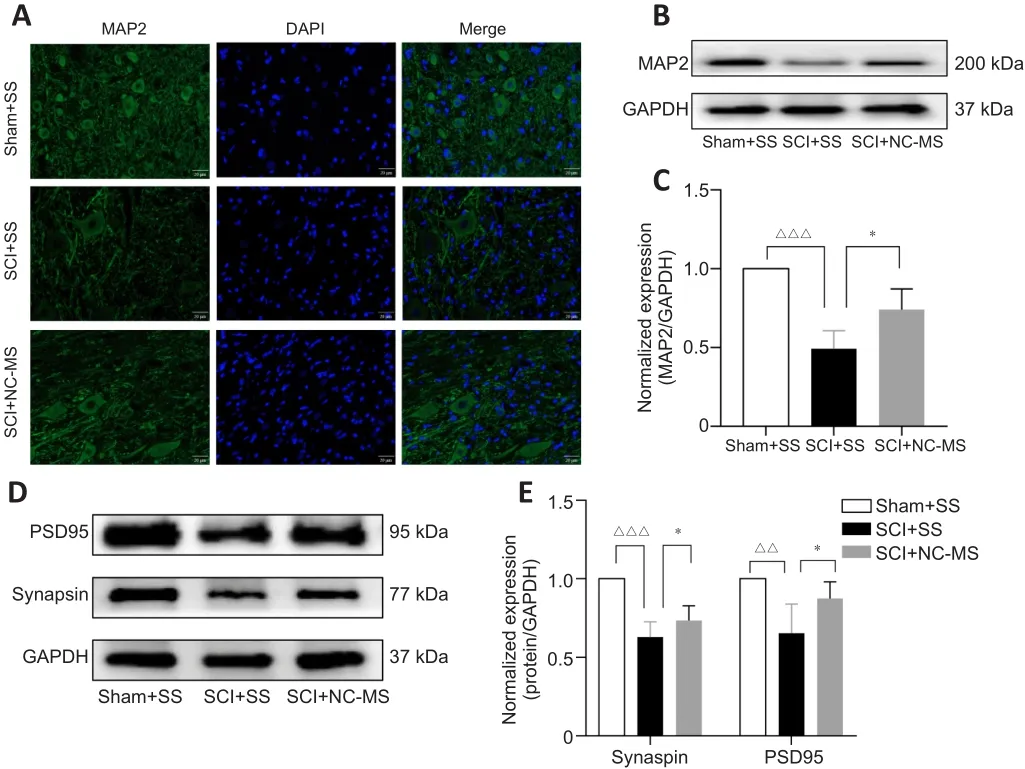
Figure 5|NC-MS treatment enhances the neural survival and repair, and synaptic plasticity in rat models of incomplete SCI.
NC-MS treatment promotes the recovery of the descending motor pathway
To evaluate the recovery of the descending motor pathway, MEPs were elicited in the gastrocnemius of the right hind limb on day 21 after SCI through stimulation of the contralateral motor cortex (Figure 6A).Examples of raw MEPs from the three groups are shown in Figure 6B.Compared with the Sham + SS group, MEP waveforms were unclear or even absent, and latencies were significantly longer in the SCI + SS group (P
< 0.001).However,the latencies for rats in the SCI + NC-MS group were significantly shorter than those in the SCI + SS group (P
< 0.001; Figure 6C).This suggests that NC-MS treatment can promote recovery of the descending motor pathway.
Figure 6|NC-MS treatment promotes the recovery of the descending motor pathway in rat models of incomplete SCI.
Discussion
In this study, we investigated the influence of double-target NC-MS treatment as an early intervention for rats with incomplete SCI.Our results revealed the following about the treatment in this rat model: (1) NC-MS treatment improved motor function and promoted recovery of the descending motor pathway; (2) NC-MS treatment attenuated astrocyte activation and inflammatory responses in the injured spinal cord tissue; and (3) NC-MS treatment enhanced factors related to neuronal survival, repair, and synaptic plasticity.Thus, we concluded that attenuating astrocyte activation is a mechanism of SCI recovery after NC-MS treatment.
Severe dysfunction is a lifelong sequela for patients with SCI.Therefore, motor function recovery is the primary metric used to assess the effectiveness of SCI treatments (Deng et al., 2020).Additionally, MEPs are considered as an important objective indicator of motor function (Brum et al., 2015).In our previous study, we found that magnetic stimulation of nerve roots can enhance the conduction within the sensory neuronal pathways and increase the excitability of the corticospinal tract (Zheng et al., 2022).In the current study, results from the BBB scale and inclined-plane test showed improved hind limb motor function when SCI was treated with NC-MS compared with when no treatment was given.Additionally, MEP latency in NC-MS-treated group was much shorter than in the untreated group.Therefore, NC-MS treatment can improve motor function and nerve conduction.
Astrocytes show a series of complex dynamic changes after SCI.Astrocytes have been shown to proliferate in the spared white matter from days 1 to 7 following SCI, with a peak on day 3, and after further proliferation,they transformed into dense glial scars (Alizadeh et al., 2019).The main impediments to the repair of neural circuits and motor function are large amounts of dense glial scars and the large amounts of their accompanying inflammatory factors and inhibitory molecules, which are released by the glial scar tissue.The result is a physical and chemical barrier (Yiu and He,2006).Therefore, targeting the regulation of early-stage astrocyte activation is a promising direction in SCI therapy.Previous studies have indicated that TMS is a good choice for decreasing the activation of astrocytes and improving SCI (Kim et al., 2013; Yang et al., 2018; Guo et al., 2020).Our team developed the double-target NC-MS treatment for incomplete SCI as a means to repair connections between the sensory cortex and the motor neurons by stimulating the cerebral cortex and the downstream nerve root synchronously or non-synchronously.In the present study, we found that NC-MS treatment on day 3 after SCI could attenuate astrocyte activation in the injured spinal cord tissue, as assessed through western blot assay and IF staining.Regrettably, the transformation and ratio change of the involved reactive astrocytic phenotypes (A1/A2) requires further study.In terms of the underlying mechanism, high-frequency TMS (25 Hz) has been shown to modify the voltage-dependent Cachannels in hippocampal CA1 pyramidal neurons in aged mice (Wang et al., 2015).Because Cheli et al.(2016) reported that inhibiting Cachannels can effectively prevent astrocyte activation,we hypothesize that the mechanism through which NC-MS regulates astrocytes might be through altering voltage-gated Cachannels in astrocyte membranes.This is our team’s current focus of research.
Inflammation is known to play a significant role in the development of SCI (Donnelly and Popovich, 2008).Astrocytes and microglia release proinflammatory factors such as IL-1β, IL-6, and TNF-α after SCI, which aggravates the secondary injury and further activates astrocytes and other glial cells,thus accelerating SCI development (Goldshmit et al., 2015).Therefore,repairing the microenvironment around the injury by regulating the glial cells is an important process for reconstructing neural circuits after SCI.We demonstrated that NC-MS treatment reduced inflammatory responses in the injured spinal cord tissue, thereby creating a microenvironment conducive to neural survival and repair.The levels of inflammatory factors including IL-1β, IL-6, and TNF-α were markedly down-regulated in the SCI + NC-MS group,consistent with the results of a previous study showing that TMS can reduce the level of TNF-α, IL-1β, and IL-6 in the hippocampus of depressed and anxious rats (Tian et al., 2020).Thus, we speculate that NC-MS might reduce inflammatory responses by attenuating astrocyte activation.
Furthermore, we observed the expression of MAP2 (a marker neuronal growth) and PSD95 and synapsin (markers of synaptic plasticity) in the injured spinal cord tissue.MAP2 is mainly distributed in the soma and dendrites of neurons (Yu et al., 2000) and plays an important role in their growth,differentiation, and plasticity (Johnson and Jope, 1992).MAP2 up-regulation implies more and healthier neurons, which is a key step in neural repair and neural circuit reconstruction.Similar to our previous research results (Gao et al., 2020), NC-MS treatment significantly increased the expression of PSD95 and synapsin, both of which are involved in synaptic plasticity (Duman,2002).Although neuronal and synaptic plasticity are considered the basis of magnetic stimulation efficacy (Ferreri and Rossini, 2013; Thomson et al.,2020), astrocytes are likely to be vital intermediaries (Cullen and Young,2016).Astrocytes might modulate glutamate release (Tani et al., 2014) and calcium levels (Paixão and Klein, 2010) via tripartite synapses (Perea et al.,2009) to build close contact with synapses.Hence, NC-MS treatment can promote neural survival and repair, as well as synaptic plasticity, by regulating astrocyte activation.However, additional studies such as biotin dextran-amine tract-tracing are needed to determine whether the neural circuits are actually reconstructed and the modes of neural repair.
Although the results showed that double-target NC-MS treatment has a positive therapeutic effect after SCI, our study has some limitations.First,although we demonstrated the effectiveness of double-target neural circuit magnetic stimulation for incomplete spinal cord injury, we did not compare it with single-target therapy.Whether double-target neural circuit magnetic stimulation is more effective than single-target therapy still needs to be further explored.In addition, indicators of neural regeneration and neural reconstruction were not studied in detail.It is not clear how NC-MS stimulation regulates astrocyte activation.Finally, although we chose the smallest coil, its size is still large for rats.Further studies should address these limitations.
In this study, we demonstrated that double-target NC-MS treatment can attenuate astrocyte activation to help optimize the injury microenvironment for neuronal survival and axon regeneration, thereby improving motor function.Therefore, NC-MS has the potential to be a treatment for motor function rehabilitation in incomplete SCI.
Acknowledgments:
We thank Wuhan Yiruide Co., China for providing access to advanced instruments needed for this study.
Author contributions:
Study design: DZ, DSX; experiment implementation: DZ,YZhang, YZheng, XTL; data analysis and supervision: DZ, CCS, QY; manuscript draft: DZ, YZhang; manuscript revision: DSX.All authors approved the final version of the manuscript.
Conflicts of interest:
All authors declare that have no competing interests.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Patient-specific monocyte-derived microglia as a screening tool for neurodegenerative diseases
- Molecular hallmarks of long non-coding RNAs in aging and its significant effect on aging-associated diseases
- Inflammation in diabetic retinopathy: possible roles in pathogenesis and potential implications for therapy
- Targeting the nitric oxide/cGMP signaling pathway to treat chronic pain
- Neurosteroids as stress modulators and neurotherapeutics: lessons from the retina
- Myelinosome organelles in pathological retinas:ubiquitous presence and dual role in ocular proteostasis maintenance