Contemporary techniques and outcomes of surgery for locally advanced renal cell carcinoma with focus on inferior vena cava thrombectomy:The value of a multidisciplinary team
Rirdo Cmpi *,Polo Brzghi ,Alessio Peorro ,Mri Lui Gllo ,Dmino Stri ,Alberto Mriotti ,Sverio Ginne ,Simone Agostini ,Vinenzo Li Mrzi ,Arngelo Sebstinelli ,Pietro Sptfor ,Muro Gi ,Grzino Vignolini ,Frneso Sess Polo Muiesn ,Sergio Serni ,b
a Unit of Urological Robotic Surgery and Renal Transplantation,University of Florence,Careggi Hospital,Florence,Italy
b Department of Experimental and Clinical Medicine,University of Florence,Florence,Italy
c Department of Radiology,Unit of Urogenital,Nephrological and Kidney Transplantation Imaging,Careggi Hospital,University of Florence,Florence,Italy
d Hepatobiliary Unit,Department of Clinical and Experimental Medicine,University of Florence,AOU Careggi,Florence,Italy
e Liver Unit,Queen Elizabeth Hospital,Birmingham,UK
KEYWORDS
Abstract Objective:To report the outcomes of surgery for a contemporary series of patients with locally advanced non-metastatic renal cell carcinoma(RCC)treated at a referral academic centre,focusing on technical nuances and on the value of a multidisciplinary team.
1.Introduction
Renal cell carcinoma(RCC)represents about 3%of all adult malignancies and 95% of diagnosed renal masses[1,2].Although the incidence is increasing during the last decades due to the common use of abdominal imaging,the mortality rate appears stable over time;and RCC represents one of the most lethal urological cancers[3].
According to European Association of Urology(EAU)and American Urology Association guidelines,surgery is still the gold standard treatment for non-metastatic RCC[4,5].
Despite an increasing interest in both neoadjuvant and adjuvant systemic therapies in recent years[6-8],for locally advanced non-metastatic RCC(stage cT3-T4 N0-1 M0),nephrectomy remains a key therapeutic step to control the disease,aiming to achieve cure,within a multidisciplinary approach.Nonetheless,surgical strategies in such clinical scenarios are often challenging and highly demanding,considering the heterogeneity of patient’s and tumour’s characteristics(ranging from RCC with clinical lymphadenopathies to cases with extensive inferior vena cava[IVC]thrombosis)[9].Furthermore,the available evidence on the outcomes of surgery for locally advanced RCC is sparse,leaving several issues such as the indications and templates of lymph node dissection,patient selection for minimally-invasive surgical approaches,and the optimal management of tumours involving adjacent organs still highly controversial,and being the object of debate within the urology community[10-12].
In this study,we sought to report the outcomes of surgery for a contemporary series of patients with locally advanced non-metastatic RCC treated at a referral academic centre,focusing on technical nuances and the value of a multidisciplinary team.
2.Patients and methods
2.1.Patients and dataset
After ethical committee approval(reference number 16120_oss,Careggi University Hospital),data from consecutive patients undergoing surgical treatment for nonmetastatic locally advanced renal masses suspected of RCC(cT3-T4 N0-1 M0)at our centre between January 2017 and December 2020 were prospectively collected.The chronic Kidney Disease Epidemiology Collaboration formula was used to calculate estimated glomerular filtration rate[13].Patient’s comorbidities were reported using the Charlson Comorbidity Index[14]and American Society of Anaesthesiologists Physical Status Classification[15].Intraoperative complications were reported according to the Intraoperative Adverse Incident Classification by the EAU ad hoc Complications Guidelines Panel[16],while postoperative surgical complications were classified according to both the modified Clavien-Dindo system[17]and the Charlson Comorbidity Index(using an online calculator:https://www.assessurgery.com/about_cci-calculator/)[18].Histopathological findings were reported including histological subtypes,pTNM stage[19],International Society of Urological Pathology grade[20],and the Leibovich prognostic scores for clear-cell RCC[21].
The follow-up schedule was based on the recommendations provided by the EAU guidelines[4].
2.2.Preoperative patient assessment
All patients underwent a contrast-enhanced computed tomography scan of the abdomen(or magnetic resonance imaging,if contraindicated)and chest for preoperativetumour staging.In selected cases,preoperative magnetic resonance imaging was required to better characterise the thrombus extension into the IVC and the potential infiltration of IVC walls(Fig.1).All imaging studies were reviewed by our dedicated team of experienced uro-radiologists.In case of RCC with evidence of IVC thrombosis extending above the diaphragm,a trans-esophageal echocardiography and a diagnostic coronarography were required to carefully assess the extension and characteristics of the thrombus as well as to exclude the presence of critical coronary artery stenosis(Fig.1).
Preoperative staging was defined according to the EAU guidelines[4],while tumour complexity was evaluated according to Preoperative Aspects and Dimensions Used for an Anatomical(PADUA)classification[22]and the Simplified PADUA REnal(SPARE)nephrometry system[23].Moreover,the level of IVC thrombosis was defined according to the Mayo staging system[10].In particular,level I was defined as a tumour thrombus<2 cm apart from the orifice of the renal vein,level II as a tumour thrombus extending to the IVC>2 cm above the renal vein but below the hepatic veins,level III as a tumour thrombus extending above the hepatic veins but below the diaphragm,and level IV as a tumour thrombus located above the diaphragm.
All clinical cases were discussed at our multidisciplinary tumour board after a comprehensive preoperative work-up including cardiac,renal,and respiratory assessments according to our institutional protocol[24].In addition,for selected elderly and/or frailer patients,a formal multidimensional geriatric evaluation was requested.In cases of locally-advanced RCCs with suspicion of adjacent organ infiltration(i.e.,liver,adrenal,spleen,etc.)and/or with level≥3 IVC thrombosis,consultation with general and/or vascular surgeons experienced in IVC surgery and liver transplant techniques was routinely pursued.
2.3.Decision-making regarding surgical approach and technique
Decision-making for patients with locally-advanced RCC was carried out taking into careful consideration several patientand tumour-related factors.In particular,for selected cT3a cases(i.e.,those with lower complexity tumours with suspicion of perirenal fat or sinus fat invasion),an open or minimally-invasive partial nephrectomy was performed following a standardised technique[25]in case of imperative indications and/or if deemed technically feasible according to surgeon’s preference and skills.Alternatively,an open or minimally-invasive radical nephrectomy was performed following established principles if partial nephrectomy was considered oncologically unsafe.
For≥cT3b cases(especially if IVC replacement was foreseen,or in cN1 cases whom a template-based lymph node dissection was planned),an open approach was routinely preferred thanks to the increased operative field and facilitation of the cooperation with other surgical teams(i.e.,vascular or general surgeons).In these scenarios,a multidisciplinary surgical team involving urologists as well as general vascular and cardiothoracic surgeons was always employed.
Before the surgery,the whole team discussed each case in a multidisciplinary meeting to plan all the steps of the intervention(including the need for ipsilateral adrenalectomy,template-based retroperitoneal lymph node dissection[26],and/or additional resections of adjacent organsaccording to EAU guidelines[4]).For thrombi invading the caval wall,IVC resection and reconstruction were performed using Gore-text prostheses(GORE-TEX® Vascular Grafts,W.L.Gore & Associates,Inc.,Flagstaff,AZ,USA).Preoperative renal artery embolisation was carefully considered according to the single patient-and tumourrelated characteristics,especially in cases where extensive lymphadenopathies made early ligation of the renal artery challenging.

Figure 1 Overview of preoperative imaging techniques to diagnose,characterise,and stage locally-advanced RCC.(A and B)Coronal and axial magnetic resonance images showing a case of RCC with level IV IVC thrombosis;(C)Axial contrast-enhanced computed tomography images showing a right-sided RCC with level II IVC thrombosis;(D and E)Selected snapshots from preoperative trans-oesophageal echocardiography showing a case of RCC with level III IVC thrombosis;(F)Coronal contrast-enhanced computed tomography images showing a right-sided RCC with level II IVC thrombosis.RCC,renal cell carcinoma;IVC,inferior vena cava.T,tumour.Arrow,level III IVC thrombosis showed during trans-oesophageal echocardiography.*Thrombus.
Surgery was carried out by two experienced urological surgeons(Serni S and Vignolini G)with the assistance of an experienced liver transplant surgeon(Muiesan P),if needed.
2.4.Technical nuances of radical nephrectomy and IVC thrombectomy
For patients with cT3b N0-1 RCC with Mayo level I-II IVC thrombosis,radical nephrectomy and IVC thrombectomy were performed following established surgical principles[9].
For patients with cT3b N0-1 RCC with Mayo level III-IV IVC thrombosis,specific technical nuances were employed by the surgical team to allow proper mobilisation of the infra-and retro-hepatic IVC as well as to manually reduce the extent of IVC thrombosis(i.e.,from level IVC to level III or even level II thrombosis),if possible,according to the characteristics of the thrombus.For such cases,extensive liver mobilisation(with ligation of the short hepatic veins and division of hepatic ligaments allowing full rotation of the liver toward the left,applying liver transplant mobilisation techniques[27])and the Pringle manoeuvre were often necessary(Figs.2-3).
An L-shaped modified Makuuchi incision(extending from the xiphoid process toward the umbilicus and extending laterally to the midaxillary line)was always performed to gain a wide access to the operative field.A Rochard selfretaining retractor(Condor® MedTec GmbH,Salzkotten,Germany)was placed,elevating the costal margins and splaying them laterally toward the axillae.After mobilisation of the tumour-bearing kidney,vessel loops were placed around the main renal vein and artery to provide mobilization of the vessels and their safe control in case of intraoperative adverse events.If possible,early ligation of the renal artery was routinely pursued aiming to reduce intraoperative bleeding.Arterial ligation results in decompression of collateral circulation and decreases blood loss.For venous management,full exposure and dissection of the IVC,contralateral renal vein,and part of the lumbar vein site at the location of the tumour thrombus is key.
If the IVC thrombosis was extended toward the suprahepatic veins or the right atrium,complete mobilisation of the liver was needed.To achieve this goal,accurate dissection and section of the ligamentum teres and the falciform ligament were performed.Then,the liver was gradually rolled to the left,as performed for liver transplantation[11,28,29],following the principles of the‘‘piggy-back’’technique[27].Small hepatic veins passing from the right and caudate lobe were ligated and carefully divided.In addition,the lesser omentum was opened,providing a better access to place a Rummel tourniquet(Integra®,Integra LifeScience Corporation,Washington,DC,USA)around the porta hepatis to perform a Pringle manoeuvre,if needed(Fig.2).In this fashion,the infrahepatic,intrahepatic,and suprahepatic portions of the IVC were completely exposed,and the liverwas dissected off the IVC until it lay in a“piggy-back”fashion,attached to the IVC only by the major hepatic veins.

Figure 2 Intraoperative snapshots showing the main steps of open surgery for locally advanced RCC.(A)Skin incision(modified Makuuchi incision)routinely performed at our institution for cases of RCC with suspected IVC thrombosis/infiltration,and/or suspected involvement of adjacent organs;(B)Overview of the operative field after placement of the Rochard retractor;(C)Intraoperative snapshot showing the final surgical result after radical nephrectomy with IVC thrombectomy and placement of a Gore-Tex prosthesis;(D)Exposure of a right-sided large RCC;(E)Exposure of the IVC and right renal loggia after retroperitoneal lymph node dissection;(F)Intraoperative snapshot showing the Pringle manoeuvre;(G)Intraoperative snapshot showing the operative field after left radical nephrectomy plus retroperitoneal lymph node dissection involving paraaortic and inter-aorto-caval templates.RCC,renal cell carcinoma;IVC,inferior vena cava.
For level III IVC thrombi(above the hepatic veins but below the diaphragm),we employed a milking technique aiming to reduce the thrombus below the major hepatic veins,and then applied a vascular clamp below these veins.This technique is often feasible,because ligation of the renal artery reduces the blood supply to the tumour thrombus.The technique serves a dual function:first,it allows the liver to drain into the IVC,avoiding hypotension from decreased venous return;second,by not clamping the major hepatic veins or porta hepatis,liver congestion and postoperative hepatic dysfunction are avoided[30].
In selected cases of floating level IV thrombi,thanks to the complete exposure of the IVC and the extensive liver mobilisation,the surgical team could even reduce the level of the thrombus by manually milking it toward more caudal portions of the IVC.The central tendon of the diaphragm was dissected until the supradiaphragmatic intrapericardial IVC was identified.The right atrium was then gently pulled beneath the diaphragm,and the Pringle maneuver performed to temporarily occlude the vascular inflow to the liver.After waiting the decompression of the liver,the vascular clamps were placed in the following order:the infrarenal vena cava and the contralateral renal vein were controlled,and then a Satinsky clamp(Surtex®,Surtex Instruments,New Malden,Surrey,England)was placed across the right atrium under echocardiography monitoring(for left-side tumours,while the right adrenal vein was also clamped).The IVC was incised from the diaphragm to the renal vein,and the tumour was removed(mobile tumour thrombus)or dissected sharply off the atrial wall(adherent tumour thrombus)and/or IVC.The three major hepatic veins could be directly visualised,their orifices inspected,and the tumour removed.Following removal of the tumour thrombus and closure of the upper cava,the vascular clamp was repositioned below the hepatic veins;the Pringle was released;and normal liver blood flow was reestablished.The remaining IVC below the hepatic veins was sutured closed[31,32].The diaphragm was closed with interrupted sutures,as previously described(Fig.3).
Employing such a liver-transplant technique may allow to safely avoid the need for sternotomy and extracorporeal circulation(ECC),also for level IV IVC thrombus RCC.Alternatively,for level IV IVC thrombus for which the above-described technique appears not feasible(based on preoperative imaging and/or intraoperative inspection),cardiothoracic surgeons are needed to perform a sternotomy with or without ECC,and cardiopulmonary bypass or deep hypothermic circulatory arrest can be required[33].
Lymph node dissection was performed mainly in case of pre-and/or intra-operative suspicion of lymph node metastases,according to surgeon’s preference.For right-sided tumours,the template included the hilar,para-caval(on the lateral side of the vena cava),and pre-caval(on the anterior side of the vena cava)lymph nodes,from the crus of the diaphragm to the aortic bifurcation.For left-sided tumours,the dissected anatomical templates were the renal hilar,pre-and/or para-aortic region(on the anterior and lateral side of the aorta)from the crus of the diaphragm to the aortic bifurcation[26](Fig.2).

Figure 3 Technical nuances of radical nephrectomy and IVC thrombectomy for locally-advanced RCC.(A)Exposure and complete mobilisation of the IVC and left or right renal veins;(B)Intraoperative snapshot showing the early ligation of the right renal artery in the inter-aorto-caval space;(C,D and E)Step-by-step overview of IVC thrombectomy for a RCC with level III thrombus;(C)Cavotomy with cold knife;(D)Caudal extension of the cavotomy to progressively remove the tumour thrombus;(E)Closure of the IVC with running Prolene sutures.RCC,renal cell carcinoma;IVC,inferior vena cava;LRV,left renal vein;RRV,right renal vein;RRA,right renal artery;T,tumour.Arrow,the incision direction on inferior cava vein(venotomy).Circle,the renal hilum.
In patients with suspected cT4 N0-1 tumours,surgical techniques were tailored to the specific tumour characteristics and could potentially involve liver segmentectomies(Fig.4)or resections of other adjacent organs,as appropriate.
3.Results
Overall,of 556 patients who underwent surgical procedures for suspected RCC at our institution during the study period,32(5.8%)patients harboured locally advanced RCC and were included in the analytic cohort.Of these,12(37.5%)tumours were staged as cT3a,8(25.0%)as cT3b,5(15.6%)as cT3c,and 7(21.9%)as cT4;6(18.8%)patients had preoperative evidence of lymph node involvement(cN1 status).All patients had solid renal masses with a median diameter of 8.0 cm(interquartile range[IQR]5.0-10.0 cm).The complexity of the tumour was evaluated using both the PADUA score(median 11[IQR 9-12])and SPARE score(median 7[IQR 4-8]),as shown in Table 1.Among enrolled patients,both the median Charlson Comorbidity Index and American Society of Anaesthesiologists score were 2(IQR 1-3 and 2-3,respectively).

Figure 4 Postoperative pictures showing a variety of specimens after radical nephrectomy for locally-advanced RCC.(A and B)RCC with level II IVC thrombosis;(C)pT4 RCC infiltrating the liver(in this case,en-bloc radical nephrectomy plus liver segmentectomy was needed);(D)RCC with level III IVC thrombosis(in this case,radical nephrectomy plus adrenalectomy was performed for suspected metastasis of the right adrenal at preoperative imaging;final stage pT3c pM1);(E and F)RCC with level II IVC thrombosis.RCC,renal cell carcinoma;IVC,inferior vena cava.
Nine patients(28.1%)underwent nephron-sparing surgery while 23(71.9%)received radical nephrectomy.A template-based lymphadenectomy was performed in 12(37.5%)cases,with evidence of disease in 3/12(25.0%)at definitive histopathological analysis(pN1 status).Surgical approach was open in 13(40.6%)patients and robotic in 19(59.4%),with a median operative time of 185 min(IQR 150-210 min),as reported in Table 2.Ipsilateral adrenalectomy was performed in 4(12.5%)cases,but all adrenal glands were negative for tumour involvement at histopathological analysis.
Four cases of RCC with level IV IVC thrombosis were successfully completed using liver transplant techniques without the need of ECC,as previously described.
Intraoperative complications were recorded in 3(9.4%)patients,all Grade 1 according to the IntraoperativeAdverse Incident Classification(Table 2).Regarding the postoperative course,the median overall length of hospitalisation was 4 days(IQR 4-6 days).The overall complication rate was 43.8%,with no major surgical complications(Clavien-Dindo>3)during the postoperative course;the overall median comprehensive complication index was 0.0(IQR 0.0-20.9).At histopathological analysis,2(6.3%)patients who underwent partial nephrectomy harboured a benign renal mass(oncocytoma),while the most common malignant histotype was clear cell RCC(62.5%),with a median Leibovich score of 6(IQR 5-7),as shown in Table 3.

Table 1 Preoperative baseline patients’and tumours’characteristics.

Table 2 Intraoperative outcomes in patients undergoing surgery for locally-advanced renal cell carcinoma in our series.
At a median follow-up of 24 months(IQR 18-37 months),eight(25.0%)patients experienced disease recurrence(six local recurrence;two distant metastases)while 2(6.2%)patients died of RCC(Table 4).
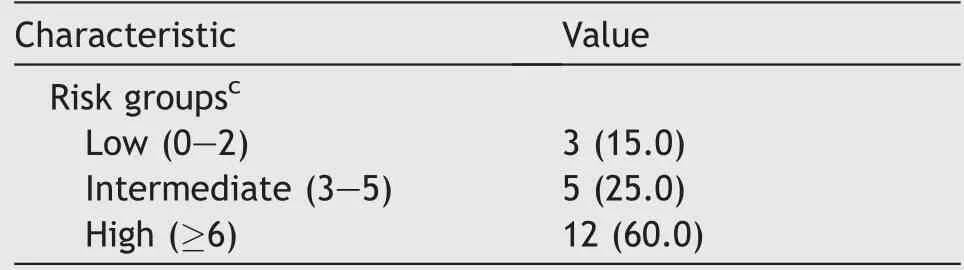
Table 3(continued)

Table 3 Postoperative outcomes and histopathologic results in patients undergoing surgery for locally-advanced RCC in our series.
4.Discussion
The optimal management of locally advanced nonmetastatic RCC remains challenging in clinical practice due to the relative rarity of the disease,the heterogeneity of clinical scenarios(Fig.4),and the demanding surgical procedures needed to achieve oncological efficacy.Moreover,although patients with locally advanced RCC are those
IQR,inter-quartile range;ICU,intensive care unit;POD,postoperative day;eGFR,estimated glomerular filtration rate;ISUP,International Society of Urologic Pathologists;CKD-EPI,Chronic Kidney Disease Epidemiology Collaboration;pM1 stage,pathological metastatic stage 1;pN,pathological lymph node stage;RCC,renal cell carcinoma.
aAccording to the Clavien-Dindo classification.
bWhere applicable.
cOne case-intraoperative finding of suspicious extraregional lymph nodes.
dAccording to Leibovich et al.[21].who may benefit the most from neoadjuvant or adjuvant systemic therapy(in light of an increased risk of recurrence and cancer-specific mortality)[7,8],how to integrate such therapies within multidisciplinary management strategies is still debated.As such,surgery remains the paradigm of care to achieve cure in this subset of patients[11,12].However,given the relative lack of evidence on standardised surgical techniques to manage advanced and complex RCC[12,34],intraoperative planning is often tailored according to patient-and tumour-related factors as well as surgeon preference.In this regard,our experience provides key insights to optimise the surgical strategy and technique to treat carefully selected patients with locally advanced nonmetastatic RCC.
The surgical approach should be personalised based on a careful preoperative evaluation of both patient and tumour factors.In particular,locally advanced tumours staged as cT3a N0 M0 are often suitable for minimally-invasive surgery,with even nephron-sparing approaches,if technically feasible[25].This patient cohort represented approximately one third of cases in our experience(Table 1).Nonetheless,the radiological definition of cT3a status(as compared to cT1-2)is still controversial and potentially highly variable across radiologists[35].

Table 4 Follow-up data in patients undergoing surgery for locally-advanced RCC in our series.
On the contrary,tumours which are staged as>cT3b and/or cN1 might pose several technical challenges for surgical teams and are currently being predominantly managed with an open approach[11,12].While during the last decade,a few studies have shown the safety and feasibility of robot-assisted laparoscopic radical nephrectomy and IVC thrombectomy,especially for level I-II IVC thrombosis[9,36,37];such techniques have been developed at selected high-volume institutions by highly experienced surgeons,limiting their reproducibility in broader healthcare contexts.
In our experience,all cases of cT3b/c-cT4 or cN1 tumours were managed with an open approach involving a multidisciplinary surgical team,as previously reported[11,12].An open approach allows indeed to achieve an optimal visualisation of the operative field,a safe and constant control of all portions of the IVC and the tumour thrombus,and promptly manage potential intraoperativeadverse events(such as massive bleeding and/or systemic tumour embolisation)that might be life-threatening in these clinical scenarios.An open approach allows the surgeon also to safely perform an extended,template-based lymphadenectomy,which might have a therapeutic benefit in selected patients[38](Fig.2).However,the role of lymphadenectomy for RCC is still controversial[39,40]given the lack of knowledge and relative unpredictability of lymphatic drainage[41,42],as well as lack of consensus on the optimal templates of dissection[26].
Lastly,as compared to minimally-invasive approaches,open surgery allows multiple surgeons to more easily collaborate during a case,such as liver transplant surgeons and urologists alternating for specific steps of the procedure(Fig.3).In this regard,the involvement of cardiothoracic surgeons in case of RCC with level IV IVC thrombosis,as well as of surgeons with prior experience in liver transplant techniques in case of RCC with level III IVC thrombosis is key to facilitate the most challenging surgical steps.Of note,liver transplant techniques might even besuccessfully employed to avoid the need for sternotomy and ECC in carefully selected patients,if technically feasible[30,31](Fig.3).
It is important to note that,as recently highlighted[36],surgery for locally advanced RCC should be carried out in high-volume centres offering a multidisciplinary preoperative,intraoperative,and postoperative care.Our experience confirms that provided careful patient selection,adequate surgeon experience,comprehensive diagnostic work-up(Fig.1),accurate anaesthesiologic assessment,and thorough postoperative monitoring,surgery for locally advanced RCC can achieve favourable perioperative,oncological,and functional outcomes(Tables 2-4).
Our experience should be interpreted in light of the study limitations.First,this is a retrospective analysis including a relatively small number of(carefully selected)patients with a relatively short follow-up;as such,larger series are needed to confirm our findings.Second,our findings might not be entirely generalisable to lower volume centres and/or in other healthcare contexts.Lastly,decision-making schemes regarding surgical approach(open vs.robotic),treatment type(partial vs.radical nephrectomy),and the need to involve additional surgical specialties were pursued on a case-by-case basis according to surgeon’s preference and skills.Acknowledged these limitations,our study provides additional evidence on the safety of surgery for selected patients with locally advanced RCC taking advantage of multidisciplinary teams.
Further research is needed to explore the impact of perioperative systemic therapy to improve oncologic outcomes in patients with advanced RCC and adverse pathologic features,to define the role of centralisation of care to optimise intra-and post-operative outcomes,and to clarify the best indications for multidisciplinary care and minimally-invasive surgery for RCC with IVC thrombosis.
5.Conclusion
Locally advanced RCC is a complex and heterogenous disease posing several challenges to surgical teams.In this study,we reported our contemporary techniques and outcomes of surgery for locally advanced RCC,focusing on the value of a multidisciplinary team.Our experience confirms that provided careful patient selection,surgery in experienced hands can achieve favourable perioperative,oncological,and functional outcomes.Larger studies are needed to confirm our findings and to standardise decision-making schemes and surgical strategies aiming to tailor the operative approach according to the single patient and tumour scenario in the context of multimodal treatment of RCC.
Author contributions
Study concept and design:Riccardo Campi,Alessio Pecoraro.
Data acquisition:Maria Lucia Gallo,Damiano Stracci,Alberto Mariotti.
Data analysis:Alessio Pecoraro,Riccardo Campi.
Drafting of manuscript:Paolo Barzaghi,Riccardo Campi.
Critical revision of the manuscript:Sergio Serni,Saverio Giancane,Simone Agostini,Vincenzo Li Marzi,Arcangelo Sebastianelli,Pietro Spatafora,Mauro Gacci,Graziano Vignolini,Francesco Sessa,Paolo Muiesan.
Conflicts of interest
The authors declare no conflict of interest.
 Asian Journal of Urology2022年3期
Asian Journal of Urology2022年3期
- Asian Journal of Urology的其它文章
- Burned-out testicular seminoma with retroperitoneal metastasis and contralateral sertoli cell-only syndrome
- Endoscopic management of adolescent closed Cowper’s gland syringocele with holmium:YAG laser
- Transcutaneous dorsal penile nerve stimulation for the treatment of premature ejaculation:A novel technique
- Bilateral calcified Macroplastique® after 12 years
- Culture-positive urinary tract infection following micturating cystourethrogram in children
- A phase II study of neoadjuvant chemotherapy followed by organ preservation in patients with muscle-invasive bladder cancer
