Three-dimensional imaging reconstruction of the kidney’s anatomy for a tailored minimally invasive partial nephrectomy:A pilot study
Dniele Amprore *,Angel Pecorro Feerico Pirmie ,Polo Verri ,Enrico Checcucci ,c,Sbrin De Cillis ,Alberto Pin ,Mrino Burgio ,Michele Di Dio ,Mtteo Mnfrei ,Cristin Fiori ,Frncesco Porpigli
a Department of Urology,San Luigi Gonzaga Hospital,University of Turin,Orbassano,Turin,Italy
b European Association of Urology(EAU)Young Academic Urologists(YAU)Renal Cancer Working Group,Arnhem,Netherlands
c European Association of Urology(EAU)Young Academic Urologists(YAU)Uro-technology and SoMe Working Group,Arnhem,Netherlands
d Division of Urology,Department of Surgery,SS Annunziata Hospital,Cosenza,Italy
KEYWORDS Three-dimensional;Kidney cancer;Renal cell carcinoma;Robotic;Laparoscopic;Nephron-sparing surgery;Complex renal mass
Abstract Objective:The aim of the study was to evaluate three-dimensional virtual models(3DVMs)usefulness in the intraoperative assistance of minimally-invasive partial nephrectomy in highly complex renal tumors.
1.Introduction
In the current urological scenario,the development of new technologies[1-3]allows to perform surgeries tailored on the features of each single case,leading urologists to enter the era of“precision surgery”[4,5].
However,in this context of technological advancements,even if imaging can be considered one of the most useful tools to improve the quality of our procedures,sometimes it may be insufficient to plan and manage intraoperatively each step of the surgery[6-8].
To obtain the most from radiological exams,in fact,bidimensional images(e.g.,computed tomography[CT]or magnetic resonance imaging)must be mentally transformed and adapted to the three-dimensional(3D)intraoperative environment[9-11].
The need to overcome this issue has led to the creation of 3D virtual models(3DVMs),with the aim to skip the“building in mind”process necessary to seize all the details of the human body[12,13].
As already demonstrated by several studies,thanks to 3DVMs,surgeon’s perception of the target anatomy has been improved,parallelly to his/her ability to navigate and orient in the surgical field[14-16].
The aim of the study was to evaluate their usefulness in the intraoperative assistance of minimally-invasive nephron sparing surgery in highly complex renal tumors.
2.Patients and methods
2.1.Study population
Within our institutional database we prospectively considered patients diagnosed with organ-confined unilateral complex renal masses(with Preoperative Aspects and Dimensions Used for an Anatomical[PADUA]classification score≥10),treated with minimally-invasive(laparoscopic or robot-assisted)partial nephrectomy(PN)between August 2017 and August 2021.
For inclusion in the study,patients were required to undergo four-phase(unenhanced corticomedullary,nephrographic,and urographic phase)contrast-enhanced CT.From each contrast-enhanced CT scan,the 3DVM of the kidney undergoing surgery was obtained(Fig.1),as described in previous studies[12,17].
Briefly,the reconstruction is focused on the kidney vasculature and collecting system,the kidney parenchyma,and the renal mass.Renal pedicle,comprehensive of both arterial and venous tree,is reconstructed using the dynamic region growing method.The urinary collecting system is reconstructed with the same method,using excretory phase of the urography CT scan.Renal parenchyma is segmented using a selective thresholding,separating different voxels and grouping them by a grayscale.The further step is the creation of the mathematical 3D model and the corresponding interactive 3D-PDF.After reconstructing the anatomy of both kidney and pathological renal mass,a careful evaluation of the renal vasculature and urinary collecting system is performed.The final product is a navigable PDF file.The whole process requires the involvement of a dedicated bioengineer with a production time of 24-48 h.The virtual navigation of the so-called“hyper-accuracy 3D model”(HA3D®)allows the surgeon to appreciate the anatomical details of the renal mass,focusing on its relationships with the vascular arterial and venous vessels,as well as with the intrarenal portion of the urinary collecting system.
A single minimally-invasive experienced surgeon performed all the interventions,planning the surgical strategy with the aid of 3DVMs before and during the whole procedure“on demand”,in a cognitive manner via tablet or integrated in the robotic console via Tile-Pro technology(Fig.2).A dedicated expert uro-pathologist performed all the histopathological evaluations of the specimens.
The study was conducted in accordance with good clinical practice guidelines,and informed consents were obtained from the patients.According to Italian law(Agenzia Italiana del Farmaco Guidelines for Observational Studies,20 March 2008),no formal institutional review board or ethics committee approval was needed.
To evaluate the baseline and postoperative renal function(ranging from 3rd to 5th day after surgery),lab measurements were used to assess serum creatinine and estimated glomerular filtration rate(eGFR).
2.2.Control group
For the purpose of the study,from our prospectively maintained database,we retrospectively selected all patients harboring complex renal masses(PADUA score≥10)who underwent minimally-invasive PN from August 2013 to August 2017,in which the surgical strategy was set on the basis of standard bidimensional CT scan images and in which pre-and post-operative functional assessment of serum creatinine and eGFR was collected.

Figure 1 Three-dimensional virtual model of a complex left renal mass.(A)Virtual image of the tumor and renal parenchyma;(B)Virtual image of the tumor hiding the renal cortex;(C)Virtual image of the renal parenchyma focused on the tumor bed;(D)Virtual image of the tumor bed hiding the cortical portion of the kidney.The direct interaction with the virtual tool allows the surgeon to focus the attention on some structures of the kidney increasing his/her knowledge on the specific case anatomy.
2.3.Measurements
For each patient considered,demographic data including age,body mass index,comorbidities classified according to Charlson Comorbidity Index(CCI),and American Society of Anesthesiologists score were collected[18,19].Preoperative data included clinical size and stage,as well as tumor surgical complexity according to PADUA score and R.E.N.A.L.nephrometry score[20,21].For the purpose of the study,only patients with PADUA score≥10(evaluated by expert uro-radiologist)have been included.Intraoperative data considered the type of minimallyinvasive approach,the operative time,the management of renal pedicle(global clamping,selective clamping,or clampless),the duration of ischemia,the estimated blood loss,the opening of urinary collecting system,and the intraoperative complications.
Postoperative and pathological data included the length of hospital stay,the 90-day postoperative complications,classified according to the modified Clavien system[22],the pathological size and stage,the histology,and grading,respectively.In addition,data on positive surgical margins(PSMs)were recorded,as well as the margins,ischemia,and complication(MIC)score[23].
2.4.Statistical analysis
Descriptive statistics included medians and interquartile ranges,as well as frequencies and proportions for continuous and categorical variables,respectively.The statistical significance of differences in medians and proportions was evaluated with the Kruskal-Wallis and Chi-square tests.We stratified all the analyses according to the 3DVM assistance availability during PN.We fitted multivariable logistic regression models predicting the MIC achievement.For this purpose,we relied on 163 patients(after the exclusion of patients harboring benign histology and with a clampless PN),in which the goal of ischemia time<20 min would have been reached by definition,as previously reported[23].Covariates consisted of age,CCI,preoperative eGFR,availability of 3DVM,type of clamping,and ischemia time.All statistical tests were two-sided with a level of significance set at p<0.05.Analyses were performed using the R software environment(version 3.4.1;https://www.r-project.org/)for statistical computing and graphics.
3.Results
The overall study population consisted of 222 patients;of those 79 patients represented the 3DVM group while 143 were considered as control group.Patients and tumor characteristics are reported in Table 1.
Overall median(interquartile range)age,body mass index,and CCI were 61(51-72)years,25.7(24.3-26.0)kg/m2,and 1(0-2),without differences between 3DVM group and control group.Similarly,tumor features were comparable between groups except for tumor size,with an overall median(IQR)PADUA score 11(10-11)and R.E.N.A.L.nephrometry score 9(8-10).Out of note,3DVM group harbored higher clinical tumor size(50 mm vs.45 mm,p=0.01)and higher rates of cT1b renal masses(70.9% vs.43.4%,p=0.0003).
As shown in Table 2,reporting the perioperative and pathological variables,a significant difference was recorded for the type of clamping,with higher rate of selective clamping in the 3DVM group and a higher rate of global clamping in the control group(selective clamping:41.8%vs.35.7% for 3DVM group and control group;global clamping:27.8% vs.34.3% for 3DVM group and control group;p=0.03),while concerning PSMs only two cases(2.5%)were recorded in the 3DVM group,while 4(2.8%)cases were found in the control group(p=0.89).
Postoperative complications rates were 16.5% vs.23.1%for 3DVM group versus control group(p=0.03).Specifically,major complications(Calvien Dindo>III)were higher for the control group(5.6%vs.2.5%,p=0.03).At last,a significantly higher proportion of patients from the 3DVM group reached the MIC score,if compared with the control group(65.8%vs.55.2%,p=0.01).All the other perioperative variables were comparable for 3DVM group and control group.
Concerning functional variables(Table 3),baseline and postoperative serum creatinine and eGFR were similar in 3DVM group and the control group.Conversely,in 3DVM group,baseline-weighted differential(b-WD)eGFR value was significantly lower than the control group(-22.2% vs.-17.7%,p=0.03).

Figure 2 Example of augmented reality technology during left robot-assisted partial nephrectomy.(A)Left kidney after its complete isolation from perirenal fat and vascular pedicle dissection;(B)Overlapping of virtual images over the real kidney enhancing the vascular structures and the collecting system;(C)Overlapping of virtual images focusing on the renal parenchyma.The intraoperative target was identified in order to overlap on the 3D virtual model comprehensive of all its components,which could be visualized separately on the basis of the surgeon’s preference.
3.1.Multivariable logistic regression models predicting MIC achievement
Overall,the achievements of MIC were 65.8%and 55.2%for 3DVM group and for the control group,respectively.The availability of 3DVMs predicted higher MIC rates(odds ratio[OR]=1.42,confidence interval:0.69-2.97,p=0.03),after adjustment for age,CCI,preoperative eGFR,and 3DVM availability(Table 4).Conversely,older age predicted lower MIC rates(OR=0.81,confidence interval:0.96-1.01,p=0.03).

Table 1 Preoperative characteristics of 222 patients with high complexity renal masses treated with minimally-invasive partial nephrectomy with(n=79)or without(n=143)the perioperative assistance of 3DVM.

Table 2(continued)

Table 2 Perioperative and pathological characteristics of 222 patients with renal masses treated with minimally-invasive partial nephrectomy with or without the perioperative assistance of 3DVM.
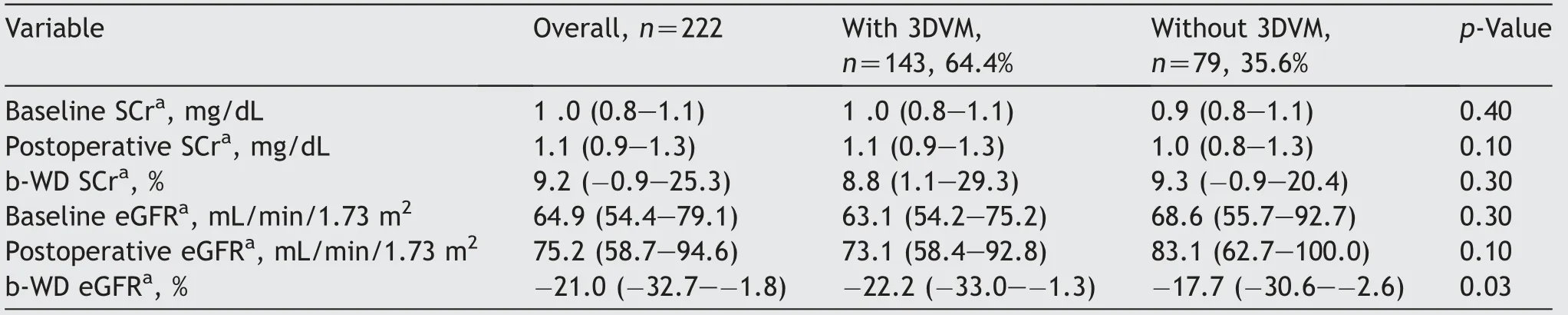
Table 3 Functional outcomes of 222 patients harboring renal masses treated with minimally-invasive partial nephrectomy with(n=79)or without(n=143)the perioperative assistance of 3DVM.
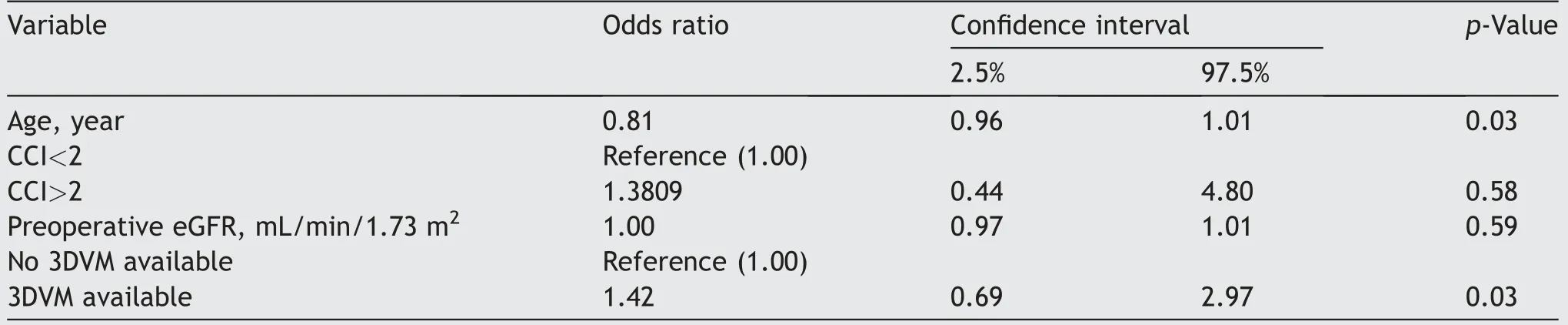
Table 4 Multivariate logistic regression model in 163 patients with renal masses treated with minimally-invasive partial nephrectomy with or without the perioperative assistance of 3DVMs,predicting the achievement of MIC defined as ischemia time<20 min,negative margins,and no significant complications(Clavien Dindo<3)a.
4.Discussion
Nephron-sparing surgery is particularly challenging in case of large or high complexity oncological masses where spatial representation of the tumor and its relationship with surrounding anatomy such as venous,arterial branches,and collecting system are of utmost importance[24-27].3DVMs face this need,avoiding the“building in mind”process that the surgeon should do to perceive the features of the organs in a real human body[28].
The role of 3DVMs has been recently evaluated by different authors in aiding the surgeon before and during robot-assisted partial nephrectomy,demonstrating how this technology is helpful in improving the preoperative evaluation of tumor complexity[29],in avoiding the global ischemia of the healthy renal remnant[30],and in reducing the loss of renal function(assessed with renal scan)[31].However,up to now,the role of 3DVMs for the achievement of MIC in high complexity renal masses has never been tested.Our results highlight some crucial points of discussion.
First,after stratification according to the availability of 3DVMs,the two groups resulted in comparable preoperative variables and PADUA score,except for a wide difference in cT1b rates distribution(70.9% for 3DVM group vs.43.4% for the control group,p=0.0003),with higher clinical tumor size distribution for the 3DVMs group(50 mm vs.45 mm,p=0.01).This can be justified by the fact that 3DVM assistance was more necessary during nephron-sparing surgery for larger renal masses,where the spatial anatomical representation was the most needed for performing a tailored surgery.
Second,concerning intraoperative and perioperative variables,3DVM group differed from the control group for the ischemia time,the type of clamping,and b-WD eGFR.Concerning the type of clamping,higher rate of selective clamping relative to global ischemia was observed in the 3DVM group(41.8% vs.35.7%,p=0.03).
The preference of selective clamping instead of global clamping can be explained considering the role of 3DVMs,providing a more precise and deeper knowledge of the vasculature and allowing the surgeon to optimize ischemic damage with clamping strategies alternative to globalclamping,as previously demonstrated[32-35].This is confirmed also by the fact that the b-WD eGFR was lower in 3DVM group(-22.2% vs.-17.7%,p=0.03).
Third,regarding the PSM rates,no differences were recorded between the two groups.Focusing on postoperative complications,the 3DVM group resulted in lower major complications rates(Clavien Dindo>III,5.6% vs.2.5%,p=0.03).
These granular differences resulted in higher MIC rates in favor of 3DVM group.This reads that the assistance of a 3DVM can be of aid in reducing ischemia,PSM,b-WD eGFR,and complications,thus optimizing the success of the procedure.
Finally,also multivariable logistic regression demonstrated a higher rate of MIC achievement(OR:1.42,p=0.03)with the 3DVM assistance during PN.
To date,no studies specifically have focused on the role of 3DVMs in optimizing the success of minimally-invasive PN,measured as Trifecta according to the currently available definitions(including MIC)[36].
Many authors focused their attention on the role of 3D volumetric assessment in evaluating renal function impairment[37,38],but without considering their impact in optimizing postoperative outcomes for defining the success of procedure.Likewise,some others assessed the role of CT scan features in predicting aggressiveness of renal masses[39]as well as 3D reconstructions in evaluating intraoperative surgeon orientation about tumor feeding arteries and tumor resection[40-42],but did not evaluated functional parameters.A recent study from our group[31]showed that the aid given by 3DVMs during different phases of the surgery leads to a lower functional drop of the operated kidney at the renal scan performed 3 months postoperatively,if compared with those cases performed without the assistance of a 3DVM.
To the best of our knowledge,the present study is the first showing the real impact of 3DVMs on MIC achievement after minimally-invasive PN in a selected cohort of highly complex renal masses.
It practically proves the clinical role of such new 3DVMs also in this setting,with perioperative and functional advantages.With these results,their introduction and diffusion in the daily practice are well justified.
Despite its strengths,this study has also some limitations.Indeed,3DVMs are not yet available in daily clinical practice,and their production requires close collaboration and support from biomedical engineers.Another issue related to the use of this technology concerns cost.Although cost-effectiveness was not one of the objectives of this study,it is to be assumed that production costs may initially limit the large-scale diffusion of this technology.Moreover,the variability in building time of 3DVMs could also restrain their adoption in clinical daily practice.This issue is mainly evident in case of low-quality CT-scan;in those cases,the segmentation to obtain 3DVMs could be longer than the usually 24-48 h and sometimes requires a multidisciplinary evaluation with expert radiologists.
The surgical procedures considered in this study were all performed by a single surgeon from a high-volume center;therefore,the outcomes and complications may not completely reflect those obtained by considering different settings.Moreover,both laparoscopic and robot-assisted approaches have been included for the study,even if no difference between the two groups(3D vs.standard techniques)was found in terms of type of approach.
Finally,also if 3DVM group was prospectively collected starting from 2019,its counterpart was retrospectively selected.However,our analyses showed that these two populations were comparable,even if these patients were diagnosed at a different time.Despite best efforts at statistical adjustment,these measures are not equivalent to a prospective randomized trial.
Notwithstanding these limitations,our study is the first showing the real impact of 3DVMs on postoperative outcomes after minimally-invasive PN in case of complex renal masses.The availability of 3DVMs opens a new chapter in the surgical planning of PN,since it increases anatomical details of high-complexity lesions,leading to a better definition of the surgical indication and better postoperative outcomes,with higher rates of successful PN.With these results,their introduction and diffusion in the daily practice is well justified.Obviously,to confirm and increase the strength of this evidence,a multi-institutional external validation study is being planned.
5.Conclusions
3D models represent an essential and useful tool to plan a tailored surgical approach in cases of surgically complex renal masses.They can be used in different ways,matching the surgeon’s needs from the planning phase to the pedicle management,tumor resection,and reconstructive phase,leading towards maximum safety and efficacy outcomes.
Author contributions
Protocol/project development:Daniele Amparore,Angela Pecoraro,Enrico Checcucci.
Data collection:Sabrina De Cillis,Alberto Piana,Mariano Burgio.
Manuscript writing/editing:Daniele Amparore,Angela Pecoraro,Federico Piramide,Paolo Verri,Michele Di Dio.
Supervision:Michele Di Dio,Matteo Manfredi,Cristian Fiori,Francesco Porpiglia.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
We would like to thank Dr.Andrea Bellin for his contribution to the work,producing the 3DVMs for the study.
 Asian Journal of Urology2022年3期
Asian Journal of Urology2022年3期
- Asian Journal of Urology的其它文章
- Burned-out testicular seminoma with retroperitoneal metastasis and contralateral sertoli cell-only syndrome
- Endoscopic management of adolescent closed Cowper’s gland syringocele with holmium:YAG laser
- Transcutaneous dorsal penile nerve stimulation for the treatment of premature ejaculation:A novel technique
- Bilateral calcified Macroplastique® after 12 years
- Culture-positive urinary tract infection following micturating cystourethrogram in children
- A phase II study of neoadjuvant chemotherapy followed by organ preservation in patients with muscle-invasive bladder cancer
