Impact of geographical and seasonal temperature on sperm parameters in Indian men who were partners in subfertile couples - A retrospective analysis
Yogita Dogra, Neeta Singh, Neena Malhotra, Reeta Mahey, Vanamail Perumal
1Department of Obstetrics and Gynaecology, Kamla Nehru State Hospital, Indira Gandhi Medical College, Shimla- Himachal Pradesh, India
2Department of Obstetrics and Gynaecology, AIIMS, New Delhi, India
3Statistics and Demography, Department of Obstetrics and Gynaecology, AIIMS, New Delhi, India
ABSTRACT
KEYWORDS: Infertility; Seasonal variations; Sperm;Progressive motility; Geographical temperature
1. Introduction
Infertility has become an ominous problem. On an average,about 10% of all the couples face difficulty in starting a family and this creates a feeling of great personal failure, particularly in India where religious and socio-economic traditions have made it almost imperative for everyone to have children[1]. The World Health Organization (WHO) task force on the diagnosis and treatment of infertility has shown that up to 15% of the population suffers with either primary or secondary infertility[2]. Most of the infertile couples have one of these three major causes including a male factor, ovulatory dysfunction, or tubal-peritoneal disease[3].Subfertility generally represents any form of infertility with longterm undesired contraception[4]. Semen analysis is frequently used to assess the parameters, including semen volume, pH, sperm concentration, sperm motility, and morphology. Various investigators have studied the increased testicular temperature as one of the primary causes of defective spermatogenesis. Even a single febrile episode of 39 ℃ has been demonstrated to affect semen parameters and sperm DNA integrity for one spermatogenic cycle[5]. A recent study in a flour beetle model system has demonstrated the deleterious effect of experimental heat waves on sperm production and viability[6].Seasonal variations in semen parameters with higher sperm concentrations in winter, and a more significant percentage of sperm with normal morphology in winter than in spring and summer have been reported by various authors[7-11]. However, studies comparing geographical temperature with sperm parameters are very scarce.The Intergovernmental Panel on Climate Change (IPCC) in its fifth assessment report briefed that warming of the global climate system is unequivocal and this warming has accelerated since the 1950s[12].Recent observations of the rapid rise in environmental temperature have caught attention if it could be contributing towards declining sperm parameters. Therefore, the present study was deliberated to evaluate not only the influence of rising temperature during various seasons but also the overall effect of geographical temperature on sperm parameters in Indian men who were partners in subfertile couples.
2. Materials and methods
2.1. Study settings
This retrospective cohort study was conducted at Andrology Laboratory of the Division of Reproductive Medicine at a tertiary care referral and research institute of Northern India from January 2009 to December 2017.
2.2. Materials
The retrospective review of records of semen analysis over nine years (January 2009 to December 2017) that was performed as a part of the necessary evaluation of infertile couple attending the Andrology Laboratory was undertaken. Each record contained the age of the patient, date of sample collection, number of days of abstinence and semen analysis results (volume, sperm concentration,total motility, progressive motility, non-progressive motility, nonmotile sperms and morphology). Azoospermic samples were excluded from the survey.
2.3. Methodology
Semen sample was collected by masturbation into a sterile, widemouthed polystyrene container in a private collection room near the laboratory. The recommended period of abstinence was a minimal of 48 h but not exceeding seven days.
2.4. Laboratory evaluation
Semen specimens were allowed to liquefy in an incubator at 37 ℃and were analyzed within 60 min after the samples were collected.The samples were well mixed, avoiding vigorous shaking, in the original container. The volume was measured using a disposable polycarbonate serologic pipette. Aliquots of semen samples were placed into a prewarmed (37 ℃) Makler counting chamber (Sefi Medical Instruments, Haifa, Israel). Evaluation of semen parameters was performed according to the WHO guidelines in terms of volume,sperm concentration, total motility, progressive and non-progressive motility, immotile sperms and morphology[13]. A minimum of 200 spermatozoa from no less than four different fields were analyzed from each specimen. During the study period, three technologists performed the semen analysis.
2.5. Mean temperature
The secondary data of mean temperature from 2009 to 2017 were taken from the published data of the Meteorological Department of the country[14].
The seasons were grouped as per Meteorological Department as below: winter: January to February, pre-monsoon: March to May,monsoon season: June to September, and autumn (post-monsoon):October to December.
2.6. Statistical analysis
Data were computerized using MS office Excel spreadsheet and analysis was carried out using statistical software SPSS IBM version 24.0. Continuous data were tested for normality assumptions using the Kolmogorov-Smirnov test. Descriptive statistics such as mean,standard deviation (SD) and range values were calculated. Mean values of various semen parameters were compared between seasons using one-way analysis of variance (ANOVA) test followed by Bonferroni pairwise post-hoc comparison test. Bivariate Pearson correlation coefficient (r) analysis was applied to measure the correlation between age, sperm parameters and annual temperature/seasons. Chi-square test was applied to compare the levels (normal vs. abnormal) of different sperm indices. Further, logistic regression analysis was carried out by stepwise procedure to find out significant variables contributing to progressive motility levels (<40% as abnormal and ≥40% as normal). The other study variables such as semen volume (<1.5 mL, ≥1.5 mL), sperm concentration (<15×106/mL, ≥15×106/mL), total motility (<40%, ≥40%), age groups (≤50 years and >50 years) and temperature (≤28 ℃ and >28 ℃) were considered as covariates. These cutoff values were followed based on the WHO criteria[13]. We presented adjusted odds ratio (OR) with 95% confidence interval (CI) for the significant variables. A P-value of less than 0.05 was considered statistically significant, and less than 0.01 was considered highly significant.
2.7. Ethical clearance and informed consent
The ethical approval was obtained from the Institute Ethics Committee (IECPG-616/28.11.2019, RT-06/19.12.2019) and the study had been performed in accordance with the ethical standards described in an appropriate version of the 1975 Declaration of Helsinki, as revised in 2000. The informed consent was obtained from all the patients before performing semen analysis and all the data included in the analysis were anonymized.
3. Results
3.1. Characteristics of the study subjects
A total of 3 433 reports of semen samples of infertile couples were analysed. Baseline characteristics are presented in Table 1. The mean age was (34.5±4.3) years with mean abstinence period of (4.2±1.3)days. About 75.5% of the samples were in the 31-40 years age group. The mean volume, sperm concentration, total motility and progressive motility were (2.7±1.1) mL, (40.8±23.4) million/mL,(55.1±16.7)% and (30.9±14.5)%, respectively. The percentage of samples with normal semen volume (≥1.5 mL), normal concentration(≥15×106/mL) and normal total motility (≥40%) were 93.6%, 85.4%and 89.6%, respectively. The semen analysis data of the study subjects are depicted in Table 2.
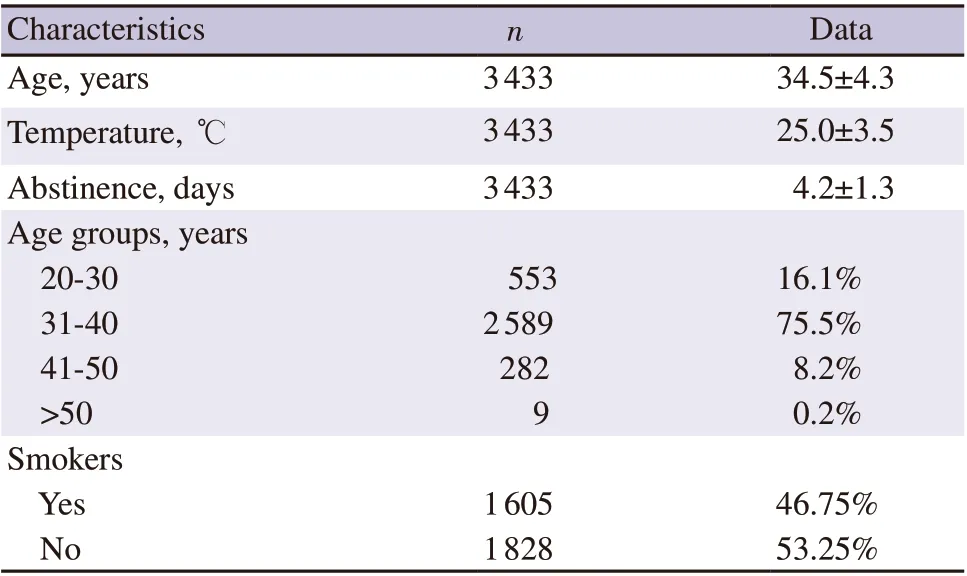
Table 1. Baseline characteristics of the study subjects.
3.2. Seasonal variation in semen parameters
We carried out ANOVA test to compare the mean values of semen parameters. While there was no significant variation in the mean volume, concentration, and total motility between seasons (P>0.05),there was a significant variation in the mean progressive motility(P<0.001). Pair-wise comparison showed that the mean values of progressive motility during pre-monsoon were significantly higher compared to the corresponding values during monsoon and autumn seasons (P<0.01). The significant decrease in progressive motility was noted from pre-monsoon (32.8±14.3)% to autumn (29.7±14.3)%with recovery towards winter (31.0±14.6)% (P<0.001) (Table 3).
Pearson product-moment correlation analysis (Table 4) shows that the age and abstinence had no bearing on semen parameters during the autumn season. During winter, pre-monsoon and monsoon,abstinence had significant negative correlation with total and progressive motility (P<0.05). Abstinence had positive correlation with semen volume in monsoon and sperm concentration in premonsoon period (P<0.05). In all the seasons, total motility had significant positive correlation with sperm concentration and progressive motility (P<0.05). Similar findings were observed for semen volume except in autumn season.
3.3. Significant factors for normal level of progressive motility
To assess significant variables for the normal level of progressive motility (≥40%), we carried out logistic regression analysis. Adjusted OR with 95% CI for the significant variables are presented in Table 5. While the sperm concentration was normal (≥15×106/mL), the likelihood of getting progressive motility increased (OR 3.74, 95%CI 2.81-4.96) compared to abnormal concentration (<15×106/mL).The chances of getting normal progressive motility reduced (OR 0.87, 95% CI 0.80-0.93) due to longer abstinence period. Similarly,when the temperature was more than 28 ℃, the likelihood of gettingnormal progressive motility decreased (OR 0.70, 95% CI 0.51-0.95). Overall, seasons played a significant role in predicting the normal progressive motility (P<0.001). Pre-monsoon emerged as the significant favourable season to get normal progressive motility level (OR 1.32, 95% CI 1.04-1.68) compared to winter. In contrast,autumn season emerged as the significant unfavourable season for normal progressive motility level (OR 0.79; 95% CI 0.63-0.99).

Table 2. Semen parameters of the study subjects.
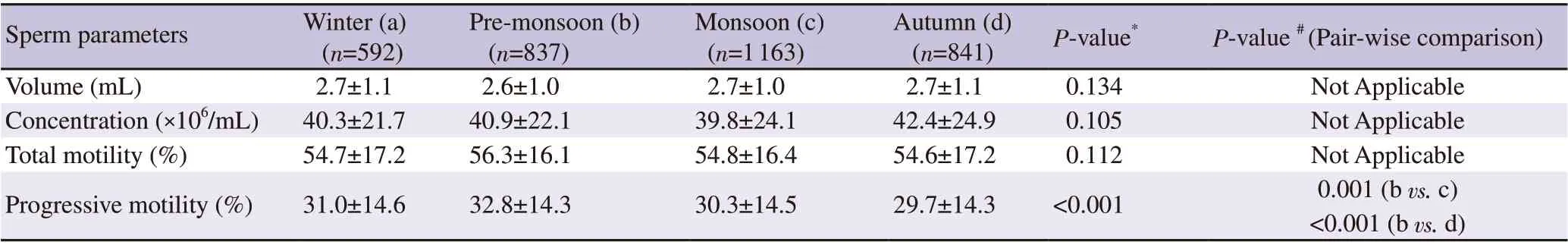
Table 3. Comparison of mean values of semen parameters between seasons.
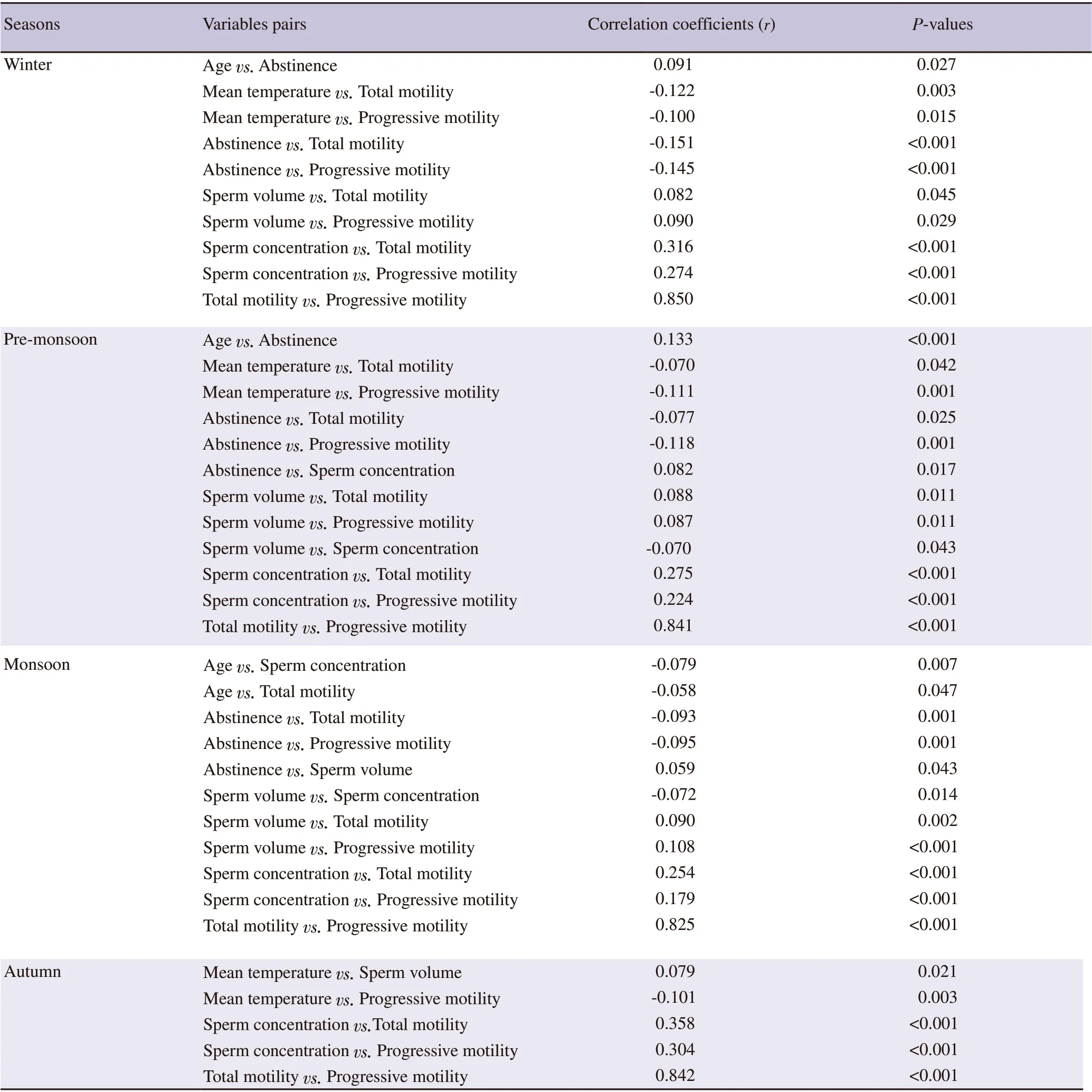
Table 4. Season-specific significant bivariate correlation coefficients.
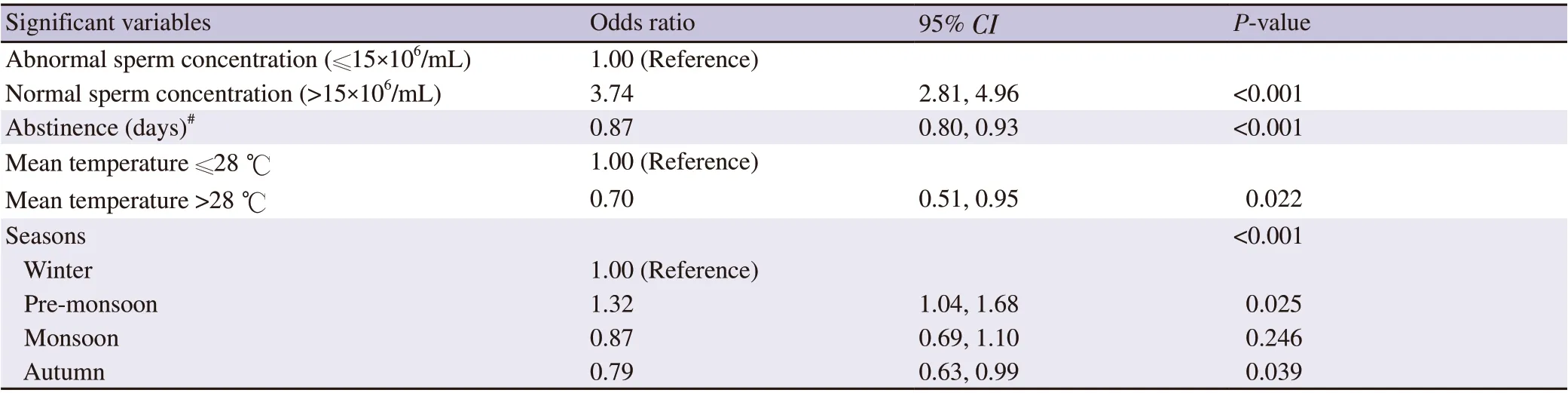
Table 5. Significant variables for progressive motility (≥40%) by logistic regression analysis (stepwise procedure).
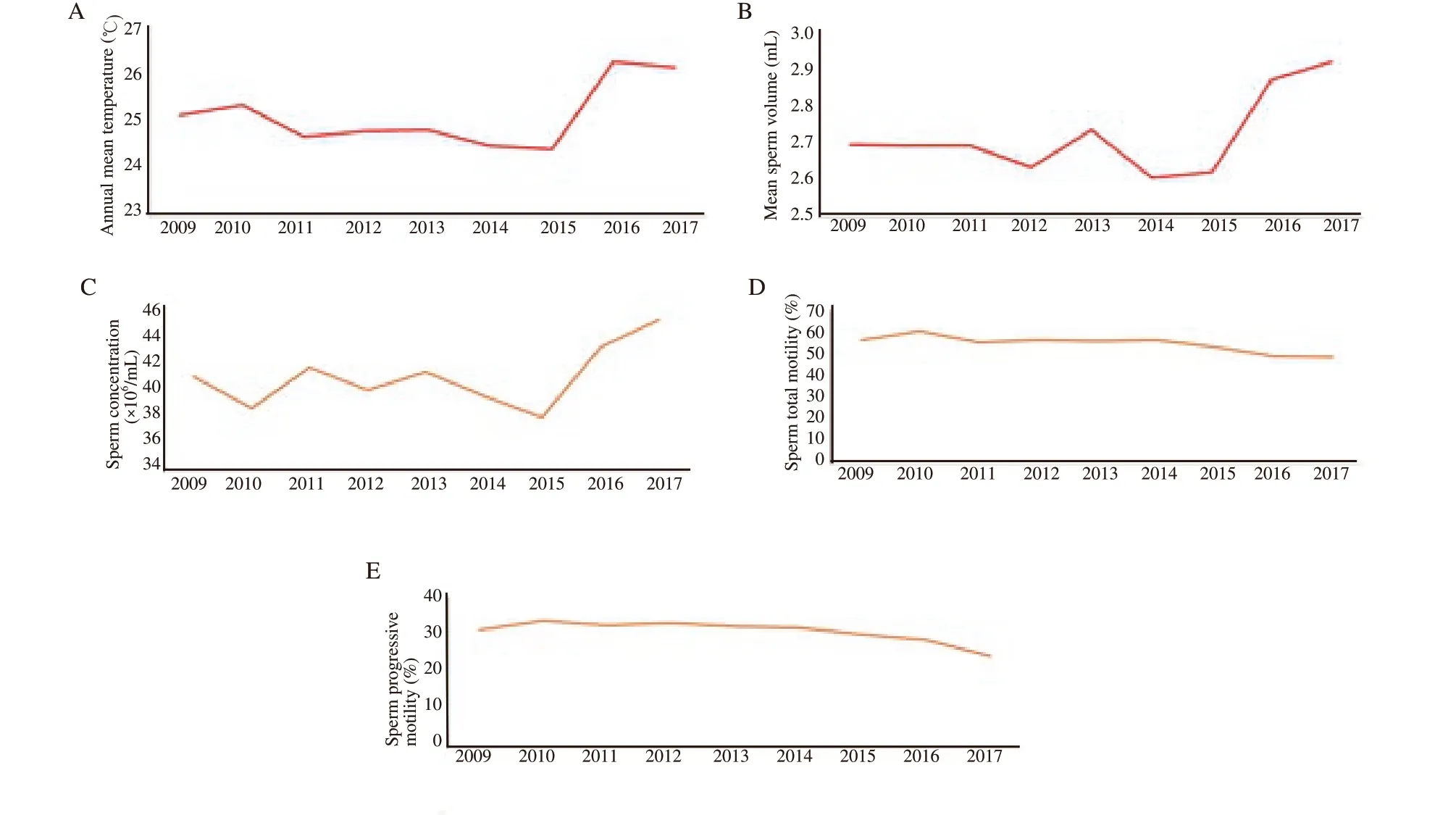
Figure 1. Year-specific mean values of temperature and sperm related parameters. A: annual mean temperature; B: annual mean sperm volume; C: annual mean sperm concentration; D: annual mean sperm total motility; E: annual mean sperm progressive motility.
3.4. Annual variation in semen parameters
The mean annual temperature of the country was on the rise over nine years (25.11 ℃ in 2009 to 26.29 ℃ in 2017) (Figure 1A). The annual variations in the semen parameters in terms of semen volume, sperm concentration, total motility and progressive motility are depicted in Figure 1B-E. On comparing the means of progressive motile percentage with a mean yearly temperature,there was a significant negative correlation (P<0.05). An analysis of variance showed that the effect of time on semen volume, semen concentration, total motility and progressive motility was significant(P<0.01). Our study results also depicted that the mean sperm motility was declining gradually from 56.55% in 2009 to 48.53%in 2017. Also, the progressive motility showed waning (31.16% in 2009 to 24.18% in 2017).
4. Discussion
The present study showed that while there was no significant variation in the mean semen volume, concentration, and total motility between seasons, there was a significant variation in the mean progressive motility. The significant decrease in progressive motility was noted from pre-monsoon to autumn with recovery towards winter. Normal sperm motility is likely to increase during pre-monsoon and monsoon. The increase in temperature and length of abstinence are likely to decrease the sperm motility. On comparing the means of progressive motile percentage with a mean yearly temperature, there was a significant negative correlation.
Male infertility is predominantly associated with deficiencies in sperm transport or spermatogenesis[9]. Several parameters that include semen volume, pH, sperm concentration, sperm motility,and morphology are assessed for evaluation of semen quality. It is well recognized that exposure of the testis to high temperatures negatively affects their function, leading to partial or total arrest of spermatogenesis[15]. The decline in spermatogenesis both qualitatively and quantitatively has already been demonstrated by slight increase (1 ℃-2 ℃) in testicular temperature consecutively for 15 hours/day in fertile men[16,17].
The uprising temperature influences human health directly or indirectly. Spermatogenesis depends upon the optimal temperature;therefore male fertility could be affected by the rising temperature.The testicular temperature is maintained at 3 ℃ lower than the core body temperature and is essential for effectual spermatogenesis.The testicular germ cells and Sertoli cells are highly sensitive to elevated temperature[18]. It has been established in earlier studies that certain males have an inherent flaw in scrotal thermoregulation and the majority of men with oligozoospermia had raised scrotal temperatures[19]. Febrile episodes, high summer temperatures,frequent hot baths and saunas are known to destroy germinal epithelium and also induce transient oligozoospermia, even in normal fertile males[20,21]. The cauda epididymis is sensitive to raised temperature. High scrotal temperature leads to swift disruption of absorptive and secretory function of cauda epithelium,thus changing the protein composition of cauda fluid with the resultant decline of its storage capacity[20]. The potential impact of artificial fever induced by steam baths or sauna on human fertility and spermatogenesis has been studied in few investigational study reports in the past[22-24]. The authors found that in healthy volunteers, a decline in total sperm count (>50%) occurred between 2-4 weeks after either one[23] or several sauna exposures[24], with complete recovery after 8 weeks. Dada et al concluded in their study that exposure of the testes to high environmental temperature leads to an elevation of intratesticular temperature with subsequent impaired spermatogenesis. They observed that out of 92 infertile males, 20 had exposure to high temperature at their workplace for a mean period of 8.5 years[25].
Tas et al observed that the sperm count has been dipping at a frightening rate of 2% per annum for the last 20 years. The yearly decline in sperm concentration, sperms with normal motility and sperms with normal morphology was also noticed. They attributed this to an increase in global temperature and environmental pollution[26]. The mean annual temperature of the country is on uphill for overthe nine years (25.11 ℃ in 2009 to 26.29 ℃ in 2017).The present study results suggest an association between the rising temperature and declining sperm motility.
Sengupta et al in their meta-analysis remarked the decreased sperm concentration in European male over the past 50 years. The lifestyle influences such as smoking and chewing tobacco, alcohol, high temperature and some modern electronic gadget, were revealed to affect reproduction adversely. These factors affect male fertility by interfering with spermatogenesis, spermiogenesis, motility, sperm DNA and chromatin integrity, hormonal regulation or by reducing the fertilizing capacity of spermatozoa[27]. Innumerable occupational factors and environmental agents have been proved to have a detrimental effect on male reproductive function. Diet, lifestyle,stress and socioeconomic status also affect semen quality[25].Therefore, the effect of rising temperature as a result of global warming on male fertility could not be doubted.
On analysing the seasonal variations in the results of semen samples, a significant decrease in progressive motility was noted from pre-monsoon to autumn with recovery towards winter. This affirms that motility of sperms decreases in pre-monsoon and monsoon period with recovery in winters.
In experiments with rhesus monkeys, the endogenous biological clock was found to reset annually by a change in the length of daylight which persuades such seasonal variation[28].
Ozelic et al in their retrospective study on 4 422 semen samples observed a steady decline in the rate of sperm with fast forward motility from spring to fall with a recovery noticed during the winter.The percentage of sperms with normal morphology was found to be statistically significantly higher in the spring samples compared with the summer samples[9]. Chen et al in their retrospective review observed seasonal variations in sperm concentration and morphology, with higher sperm concentrations in winter than in fall,and a higher percentage of sperm with normal morphology in winter than in spring and summer. The sperm percentage with normal morphology was observed the lowest in summer[8]. The above study results affirm our observations.
Zhang et al also observed significant effect of season on semen quality. The motility was found to be significantly higher in winter than other seasons. This is in contrast to the results of the present study which showed significantly higher progressive motility in premonsoon with falling trend and then recovery towards winter[11].Similarly, the study findings by Giorgi et al also corroborate with the present study[7].
The sperm count of an average Indian adult male is around 20 million/mL which was 60 million/mL three decades ago[29].The majority of men who were exposed to high temperature at their workplace, such as welders, dyers, blast furnace workers and those engaged in cement and steel factories are more prone to infertility.Besides the declining quantity of sperm production in males across the world, motility and morphology of the sperms are also abating. There has been a 2% decrease in the quality of male sperm annually[29].
A very recent study evaluated the likely association between ambient temperature and sperm quality in Wuhan, China, and also examined the interactive effect of particulate matter (PM 2.5) and temperature. It showed that exposure to ambient temperature has a threshold effect on sperm quality, and PM 2.5 enhances the effect of temperature on sperm quality when temperatures are above the threshold[30]. However, the sample size is very small as compared to the present study.
There are some strengths and limitations of the study. The present study has assessed the impact of seasonal variations as well as geographical temperature on semen parameters with a large sample size. The study subjects were men who were partners in infertile couples which included both fertile and infertile men. Azoospermic samples were also excluded from the analysis. The data on lifestyle factors were however lacking. The morphology data were not analyzed, as both WHO manuals, fourth and fifth editions, were used by the lab technicians during the study period.
In conclusion, the rising temperature seems to be detrimental to semen parameters, thereby adversely affecting the male fertility.Because of geographic variations in environmental temperature,studies should be conducted in various areas so as to reveal the impact of environmental temperature on sperm quality. This information would help clinicians to guide these subfertile couples to plan their infertility treatment. The present retrospective analysis suggests the possibility of an association between seasonal temperature and semen parameters. The significant decrease in progressive motility is noted from pre-monsoon to autumn with recovery towards winter. This affirms that motility of sperms decreases in pre-monsoon and monsoon with recovery in winter.The association of environmental factors with male fertility is therefore clinically relevant for counseling patients. Future analysis of prospectively collected dataset is needed to evaluate geographical variations affecting semen parameters after controlling the confounding factors.
Conflict of interest statement
The authors declare that they have no competing interests.
Funding
The study received no extramural funding.
Authors’contributions
Dr Yogita Dogra designed the study, collected all the data, and drafted the manuscript. Dr Neeta Singh, Dr Neena Malhotra and Dr Reeta Mahey drafted the manuscript. Dr Vanamail Perumal analysed and interpreted the scientific data/results. All authors read and approved the final manuscript.
 Asian Pacific Journal of Reproduction2022年4期
Asian Pacific Journal of Reproduction2022年4期
- Asian Pacific Journal of Reproduction的其它文章
- Reproductive health and rights in the COVID-19 era: Why and how are rights and choices still the answer?
- Assisted reproduction in the COVID-19 era: Dilemmas and conundrums
- Testicular vascularization at two locations in relation to hormonal levels, and pixel echotexture in bulls at different ages
- Season modulates endocrinological profiles and sex behavioural characteristics in indigenous male goats under tropical humid island ecosystem
- Iron supplementation for non-anaemic pregnant women and the incidence of hypertensive disorders in pregnancy: A systematic review and meta-analysis
- Conventional treatment options and herbal remedies for male infertility: An overview
