Season modulates endocrinological profiles and sex behavioural characteristics in indigenous male goats under tropical humid island ecosystem
Perumal Ponraj, Jai Sunder, Arun Kumar De, Rafeeque Rahman Alyethodi, Purna Chandra Mishra, Sneha Bhowmick, Debasis Bhattacharya
1ICAR-Central Island Agricultural Research Institute, Port Blair-744105, Andaman and Nicobar Islands, India
2Department of ARGO, OUAT-College of Veterinary Science, Bhubaneswar-751003, Odisha, India
ABSTRACT
KEYWORDS: Andaman and Nicobar Islands; Hormone; Goat;Libido; Season; Tropical humid island; Island ecosystem; Rain fall;Temperature humidity index; Light hour
1. Introduction
Teressa goat in Nicobar group and Andaman local goat in Andaman group of Islands are two meat goat breeds in humid tropical Andaman and Nicobar Islands, India. These goats survive and reproduce under harsh environmental condition, as they are highly heat resistant like other tropical goat breeds[1]; however,their productivity and reproduction rate are severely affected in extreme weather conditions. Goat population has been decreased(3.18%) gradually from 18th (2007) to 19th (2012) in Andaman and Nicobar Islands as per the livestock census of India. Goat population is decreased in Andaman and Nicobar Islands due to the fact that it suffers from intensive inbreeding, lack of suitable breeding bucks and breeding management. Goats are practicing the natural service as preferred breeding practice with the limitations of disease transmission, inbreeding depression, poor body confirmation of newborns of natural mating inbreeding parents and therefore loss of production and reproductive performances. These limitations would be overcome by following selection and artificial breeding programmes under the field condition.
Endocrinological profiles and libido are the key components in evaluation of breeding soundness and its related semen quality parameters of the breeding males[2]. Season influences the libido,testicular size and hormonal secretion either through photoperiod and/or through changes in temperature humidity index and rainfall in male animals[3]. The testes are highly sensitive to increasing ambient temperature; consequently, degenerative changes are observed as in the form of reduction in testicular weight, size and change in its consistency[4], which finally affects the testicular endocrine profiles, libido and semen production profiles. Heat stress decreases the secretion of reproductive hormones, of which,follicle stimulating hormone (FSH) and luteinizing hormone(LH) are essential for spermatogenesis and androgen synthesis[4].Testicular temperature should be at least 3 ℃-4 ℃ less than body temperature in goat to perform the optimum function, i.e. testicular temperature of 34.8 ℃-35.2 ℃ in caprine species is optimum for higher spermatogenesis and androgen synthesis[5]. Higher testicular temperature limits the blood flow into the testis, resulting in hypoxia and testicular degeneration which leads to reduction of fertility in breeding male[5]. Species variation and their inherent capability to adjust to the tropical or subtropical environments is also another variable that determines whether ambient temperature and/or relative humidity affect the buck reproduction and its fertility[6].
Reproductive seasonality in tropical regions like Andaman and Nicobar Islands is mainly controlled by neuro-endocrine mechanisms that are modified or influenced by external factors such as annual variations in the photoperiod, temperature humidity index, rainfall distribution and nutrition on reproductive physiology[7]. Day length and heat stress are two major factors determining the secretory pattern of hormones secreted by hypothalamus, pituitary, epiphysis and gonads[8]. Higher environmental temperature and longer day length decrease melatonin secretion (short day breeder) which in turn stimulates the prolactin secretion from adenohypophysis which in turn inhibits the secretion of gonadotrophin releasing hormone(GnRH) from hypothalamus and FSH & LH from adenohypophysis in summer than in winter season[8]. Prolactin secretion is higher during long days and low during short days and seems to be secreted in a pulsatile manner, which could be season-dependent; furthermore,a positive relationship existed between plasma prolactin and ambient temperature in male[8]. LH stimulates interstitial cells to produce androgen. FSH in mature males is associated with the stimulation and maintenance of spermatogenesis. Improper secretion of these hormones leads to poor sex libido, higher reaction time, poor sperm production and ultimately end up with infertility in male. Further,decrease in LH production has cascading effects on androgen production, secondary sex characteristics and libido profiles in breeding males[8]. Sexual behaviour of goats is an important factor for flock breeding efficiency and productivity in goat farming. Males with higher libido and shorter reaction time had higher ejaculation efficiency in caprine species[9]. Existence of a correlation between plasma testosterone, libido and scrotal circumference indicates that this can be used in evaluation of testicular function and breeding soundness of the male[10].
Hormonal profiles of different goat breeds have been investigated by several authors[11]. But there is a lack of similar information for indigenous goat breeds of Andaman and Nicobar Islands. Knowledge on reproductive variations in different seasons helps to improve the goat husbandry and breeding practices. Moreover, this will help to select the male: female ratio for breeding purpose and males with better libido and semen quality profiles in high temperature humidity index conditions can be selected for the development of breeds that are adapted to tropical humid climates. Therefore, the objective of the present study was to ascertain the seasonal variation in blood endocrinological profiles and libido pattern in Andaman local goat under humid tropical island ecosystem of Andaman and Nicobar Islands.
2. Materials and methods
2.1. Area of the study
The present study was conducted at Goat Breeding Farm, ICARCentral Island Agricultural Research Institute, Port Blair, Andaman and Nicobar Islands, India and is placed in between 6°45′ to 13°41′North Latitude and in between 92°12′ to 93°57′ East Longitude.The seasons were classified into rainy (April to November) and dry summer (December to March) as per the monsoon availability in Andaman and Nicobar Islands. Average sun light hours per day was differed significantly between rainy [(4.28±0.89) hours]and dry summer [(9.20±0.74) hours] seasons. Average rainfall differed significantly between rainy [(444.92±13.62) mm] and dry summer [(89.04±8.84) mm] seasons. Average temperature humidity index differed between rainy (84.92±1.59) and dry summer(85.59±1.15) seasons (Figure 1).
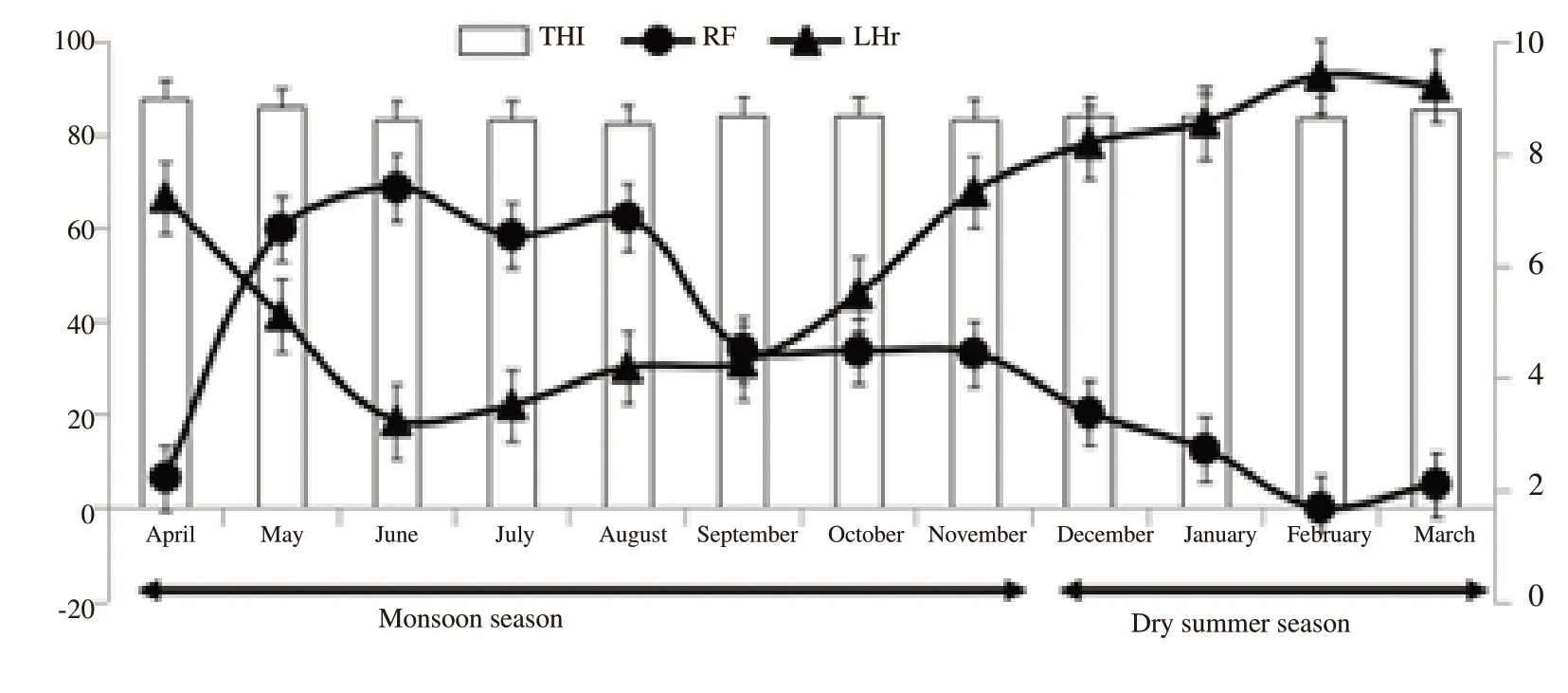
Figure 1. Month-wise climatological data during the experimental period in Andaman and Nicobar Islands, India (2019-2020). THI: temperature humidity index, RF: rainfall (mm), LHr: light hours.
2.2. Experimental animals
Ten apparently healthy (body condition score: 3.0-3.5 out of 5, classified as good) male Andaman local goats of 3-4 years of age with body weight of (32-37) kg were selected for the present study. The male Andaman local goats were maintained in the semiintensive system where they were allowed for grazing from 07:00 to 12:00 a.m. Experimental animals were maintained under uniform feeding, lighting, housing and other standard management practices as per the farm schedule. This experiment was conducted in peak of the respective seasons i.e. month of July (rainfall: 583.30 mm,temperature humidity index: 82.87, light hour: 3.50 h) and August(rainfall: 622.20 mm, temperature humidity index: 82.05, light hour: 4.17 h) for rainy season and January (rainfall: 125.80 mm,temperature humidity index: 84.16, light hour: 8.54 h) and February(rainfall: 13 mm, temperature humidity index: 83.73, light hour: 9.37 h) for dry summer season in the year of 2019 and 2020.Throughout this study, the nutrition of bucks remained uniform and constant.
2.3. Collection of blood and estimation of hormone profiles
Blood samples were collected from the experimental bucks by venipuncture of jugular vein into heparin tubes (20 IU of heparin per mL of blood) at 4-h intervals throughout the day (one day only; 6 collections) for rainy and dry seasons. Blood samples were collected at July and August for rainy season and at January and February for dry summer season for two years (4 replicates) for analysis of endocrinological profiles. Blood samples were centrifuged at 1 200×g for 15 min at 4 ℃. The plasma samples were separated, labelled and preserved at -80 ℃ for further analysis of hormone profiles.
FSH (analytical sensitivity: 0.6 mIU/mL; intra- and inter-assay coefficients of variation: 6.78% and 9.65%, respectively), LH(analytical sensitivity: 0.5 mIU/mL; intra- and inter-assay coefficients of variation: 6.55% and 9.87%, respectively), testosterone (analytical sensitivity: 6 pg/mL; intra- and inter-assay coefficients of variation:5.84% and 9.34%, respectively), cortisol (analytical sensitivity:35 pg/mL; intra- and inter-assay coefficients of variation: 5.33% and 7.89%, respectively) and prolactin (analytical sensitivity: 0.12 ng/mL;intra- and inter-assay coefficients of variation: 4.76% and 7.82%,respectively) were estimated using commercially available enzymelinked immunosorbent assay (ELISA) kits (500710, 500720,582701, 500360, 500730, Cayman Chemical Company, Ann Arbor,MI, USA, respectively) at optical density of 450, 450, 405-420, 405-420 and 450 nm, respectively. Thyroid stimulating hormone (TSH)(analytical sensitivity: 0.06 mIU/L; intra- and inter-assay coefficients of variation: 4.34% and 9.16%, respectively), triiodothyronine (T3)(analytical sensitivity: 0.1 mg/mL; intra- and inter-assay coefficients of variation: 5.65% and 6.87%, respectively) and thyroxine (T4)(analytical sensitivity: 8 nmol/L; intra- and inter-assay coefficients of variation: 5.28% and 8.76%, respectively) were estimated with commercially available ELISA kits (TF E-2000, TF E-2300,and TF E-2400, LDN Labor Diagnostika Nord GmbH & Co.KG,Germany, respectively) at optical density of 450. These hormones were estimated with use of 96-well clear polypropylene microplate using an Alere Microplate Reader (Alere Medical Pvt Ltd, India,AM 2100).
2.4. Sex behavioural score
Libido score, mating ability score and sexual behavioural score were measured as described by Anzar et al[12].
2.4.1. Libido score
Libido was scored on the basis of reaction time (in seconds), sexual aggressiveness and tactile stimulation. Reaction time, the time taken by a buck from exposure to the oestrus female until mounting,was recorded and the reaction time and corresponding scores were < 5 s: 6, 6-15 s: 5, 16-30 s: 4, 31-60 s: 3, 61-120 s: 2,121-300 s: 1 and >300 s: 0. Sexual aggressiveness, behaviour of a buck during approach toward the oestrus female was assessed visually and the bucks were classified as aggressive (uncontrollable,extremely eager to mount and approached oestrus female with full vigour; score: 4); active (approached oestrus female with less vigour and aggression; score: 3); dull (proceeded with a dull expression and took a longer time to mount than their counterparts; score: 2) or shy(exhibited mild sexual interest and was reluctant to mount; score: 1).Bucks exhibited certain behavioural characteristics after approaching the oestrus female. These were called tactile stimulations and they consisted of sniffing, bunting, licking, chin-resting, Flehmen and the like. The value of 0.2 was deducted from the total score obtained for reaction time and sexual aggressiveness for each and tactile stimulation. The libido score (%) was calculated as: [{(reaction time score + sexual aggressiveness score) - 0.2 per tactile stimulation}/10]×100.
2.4.2. Mating ability score
Mating ability was scored on a 10 point scale distributed among the different behavioural events displayed by bucks during ejaculation.Mating ability scores were marked as mounting (Mo): 1; penile erection (Er) as complete: 2 or partial: 1 or absent: 0; ejaculatory thrust (Th) as strong & rapid: 3, intermediate: 2 or weak & slow: 1;if semen ejaculate (Ej): 2; appears of concavity of back line (Bl):0.5; grasping of teaser at pelvic level (Gr): 0.5; penile movement to locate artificial vagina (Pm): 0.5 and buck’s perineum at the level of oestrus female’s perineum (Pr): 0.5. After mounting if a buck did not ejaculate, then 1 point was deducted from the total score for each futile attempt. If a buck did not ejaculate in 3 attempts, a refusal to ejaculate designation was noted and a 0 score was given. Mating ability score (%) was calculated as: [{(Mo + Er + Th + Ej + Bl + Gr+ Pm + Pr) - futile attempts}/10]×100.
2.4.3. Sexual behaviour score
Libido and mating ability scores (%) were averaged for first and second ejaculates separately, and net score (overall mean) was calculated by dividing their sums by 2. The sexual behaviour score(%) was calculated from the net score of libido and mating ability as follows: (Libido score + Mating ability score)/2.
2.5. Statistical analysis
SAS Software (SAS, Version 9.3.1; SAS Institute, Inc., Cary, NC,2011) was used for the data analysis. The data used in the study were tested for normality before analysis using Shapiro Wilk statistics.Paired t-test was applied to determine any possible differences in the observed experimental parameters with respect to between seasons. The mean values were expressed as mean±standard deviation (mean±SD). Differences were considered significant if P<0.05. Associations between the endocrinological as well as sexual behavioural profiles and meteorological parameters (rainfall,temperature humidity index, and light hour) of indigenous male goat (buck) of Andaman and Nicobar Islands both in rainy and dry summer seasons were analysed for statistical significance using Pearson’s correlation coefficient using SAS software.
2.6. Ethics statement
This study was approved by the Institutional Animal Ethics Committee of ICAR-Central Island Agricultural Research Institute,Port Blair, Andaman and Nicobar Islands, India with approval No:IXX14497 and project code: HORTCIARISIL201801300199 dated 23/08/2018. All animal experiments were performed according to the international guidelines on ethical use of animals.
3. Results
3.1. Endocrinological profiles
Endocrinological profiles such as FSH, LH, testosterone, TSH,T3, T4 and ratio of T3:T4 were significantly higher in rainy season than in dry summer season (P<0.05). On the other hand, cortisol and prolactin were significantly higher in dry summer season than in rainy season (P<0.05) (Figure 2).
3.2. Sex behavioural profiles
Sex behavioural profiles such as libido score, mating ability score and sex behavioural score were significantly higher in rainy season than in dry summer season (P<0.05) (Figure 3).
To sum up, endocrinological profiles and sex behavioural parameters differed significantly between rainy season and dry summer season (P<0.05).
3.3. Correlation analysis between hormone profiles as well as sexual behaviour score and climate variables
Endocrinological profiles and sexual behavioural scores significantly were correlated with meteorological parameters in indigenous goat buck of Andaman and Nicobar Islands in rainy and dry summer seasons (P<0.05) (Table 1). Rainfall had significant positive correlation with FSH, LH, testosterone, TSH, T3, T4, libido score, mating ability score and sex behavioural score (P<0.05) and had significant negative correlation with cortisol and prolactin in both rainy and dry summer seasons (P<0.05). On the other hand,THI and light hour had significant negative correlation with FSH,LH, testosterone, TSH, T3, T4, libido score, mating ability score and sex behavioural score (P<0.05) and significant positive correlation with cortisol and prolactin in both rainy and dry summer seasons in indigenous goat bucks under humid tropical island ecosystem of Andaman and Nicobar Islands (P<0.05).
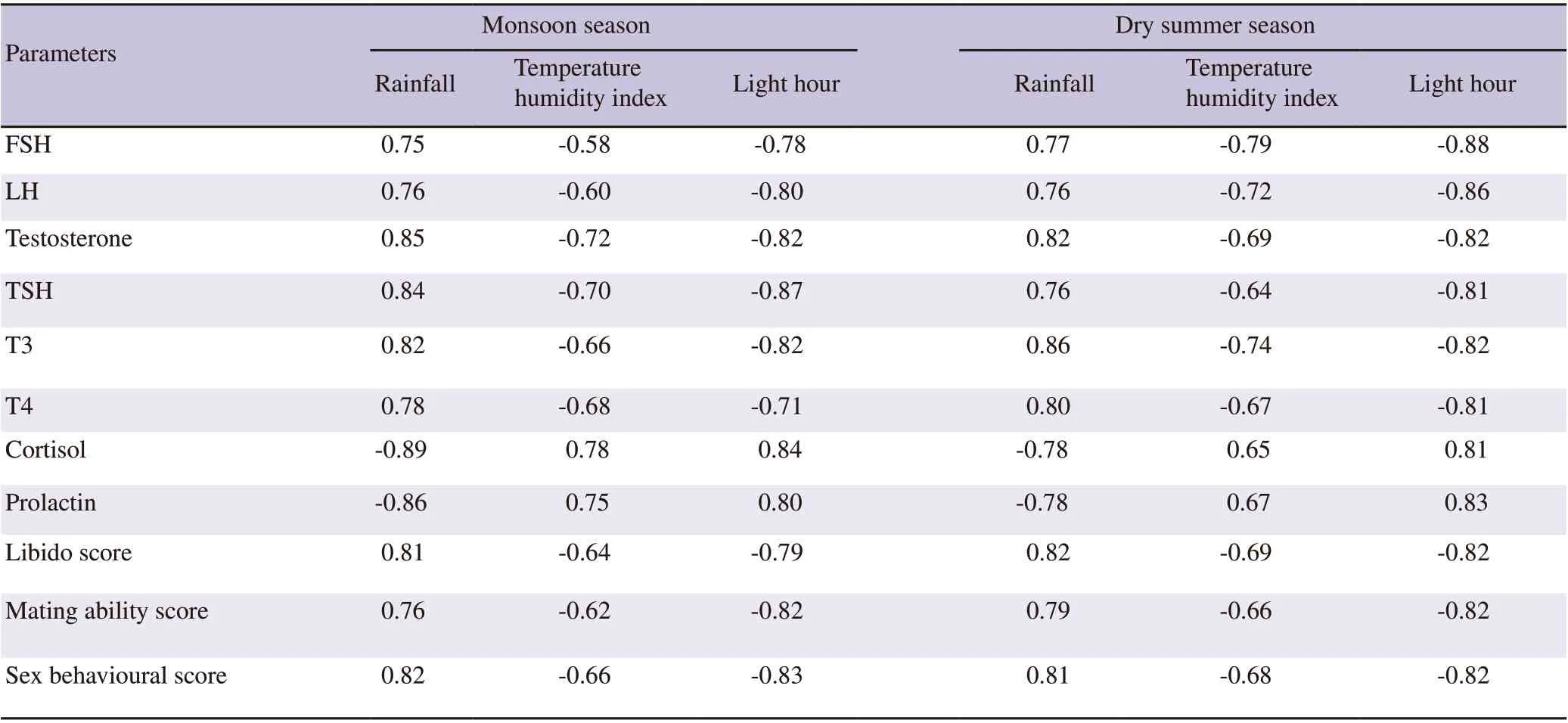
Table 1. Correlation (r) between the endocrinological as well as sexual behavioural profiles and meteorological parameters of indigenous goat (buck) of Andaman and Nicobar Islands in rainy and dry summer seasons.

Figure 2. Effect of season on serum endocrinological profiles of indigenous buck of Andaman and Nicobar Islands, India. A: FSH: follicle stimulating hormone (mIU/mL), LH: luteinizing hormone (mIU/mL), testosterone (ng/mL); B: TSH: thyroid stimulating hormone (µIU/mL), T3: triiodothyronine (ng/mL);C: T4: thyroxine (ng/mL), cortisol (ng/mL) and prolactin (ng/mL). D: T3:T4 ratio in monsoon and dry seasons. Vertical bar on each point represents standard deviation of mean. Vertical bar with small letters (a, b) indicates significant difference between monsoon and dry seasons (P<0.05). n=10 bucks. Monsoon:April to November; Dry: December to March.

Figure 3. Effect of season on sex behavioural profiles of indigenous buck of Andaman and Nicobar Islands, India. Monsoon: April to November; Dry:December to March. Vertical bar on each point represents standard deviation of mean. Vertical bar with small letters (a, b) indicates significant difference between monsoon and dry seasons (P<0.05). n=10 bucks.
4. Discussion
Season has significant effect on endocrinological profiles and libido scores in male Andaman local goats, which in turn induces significant effects on the production and reproduction performances.Goats in Andaman and Nicobar Islands are perennial breeder;however, available literature speculates some sort of seasonality is prevailed as similar to other tropical goat breeds[13]. Here in Andaman and Nicobar Islands, stress is due to combination of higher temperature humidity index (85.59), longer photoperiod(9.20 h), reduced rainfall (89.04 mm), higher solar direct irradiance[(6.24±0.56) kWh/m2/day] and higher sea surface temperature[(29.94±1.30) ℃] in summer season. With the short width of Andaman group of Islands (average: 24 km and maximum: 52 km),effect of these stress factors can cause severe adverse effect on livestock health and well-beings in Andaman and Nicobar Islands.
Heat stress induces negative impact on animal’s ability to maintain their energy, hormone and mineral balances and it is a major limiting factor in livestock production under humid tropical climate of Andaman and Nicobar Islands. Serum endocrinological profiles were consistent with the seasonal trend of rainfall and feed availability, indicating that these profiles are sensitive to seasonal variations as in feed availability and protein intake. Seasonal effect on the reproductive characteristics of several goat breeds has been reported[13]. Thus, measurement of endocrinological profiles in different seasons in Andaman local goat will help to the goat rearers or farmers to adopt the suitable protecting mechanisms in the adverse summer season. Moreover, Andaman local goat was reared in semi-intensive system and exposed to different photoperiods and temperatures in summer and rainy seasons in Andaman and Nicobar Islands. There was paucity of information on seasonal effect on the endocrinological profiles and sexual characteristics in Andaman local goat and to the best of our knowledge, this is the first study on effect of season on these parameters. However, many authors reported that summer season had severe adverse effects on the production and reproduction performances in different animal species[14].
The testes are highly sensitive to increasing ambient temperature and heat stress affects the scrotum and testicular structures which in turn decreases the secretion of gonadal hormones, particularly androgen[5]. Scrotal temperature was significantly higher in summer season than in rainy season in male Andaman local goat (33.75 ℃ vs.30.79 ℃). Scrotal temperature (33.75 ℃ and 30.79 ℃) was less than the rectal (39.78 ℃ and 37.64 ℃) and skin (37.86 ℃ and 35.74 ℃)temperatures in summer and rainy seasons in goat[5]. However, these temperatures were slightly higher than the temperature of thermal comfort range, recommended for small ruminants (20 ℃-30 ℃)[15];still these goats reproduce normally, which indicates that Andaman local goat has the testicles with higher thermoregulatory capacity and has adapted to the existing environmental conditions. Solar radiation has a major effect on the thermoregulation of grazing ruminants, as dry summer season had significantly higher solar irradiance (kWh/m2/day) than rainy season [(6.24±0.56) kWh/m2/day vs. (3.47±0.95) kWh/m2/day]. Therefore, dry summer season had significantly higher scrotal temperature than rainy season in goats of Andaman and Nicobar Islands. However, Andaman local goat breed of Andaman and Nicobar Islands is tolerant to survive and reproduce under higher temperature humidity index, day length and solar radiation in humid tropical island ecosystem. Heat stress reduces the blood flow to the testis which in turn affects the blood level of testosterone[16]. Here, it is also informed that goat breeds other than Teressa and Andaman local goat such as Malabari and its crosses or Boer crossbred were not survived and not reproduced successfully.
Longer day length, higher temperature humidity index and shorter rainfall adversely influenced the hypothalamic-hypophysealgonadal axis activity, subsequently decreased GnRH production,and reduced the volume of seminiferous tubule, causing reduction of testicular testosterone[4]. Photoperiod is well known to activate this seasonal cycle by working on the neuro-reproductive-endocrine system. Seasonal production models are generally parallel for most of the goat breeds; changes in the quantity of the hormone production happen mostly due to the latitude and longitude[17]. Level of the male or female hormones (hypothalamus to pituitary and subsequently to gonad axis) goes through changes depending on the basis of photoperiod[8]. Further availability of green fodder with rich minerals and vitamin A improved the activation of hypothalamicpituitary-gonadal axis and increased the plasma testosterone levels in rainy season[18]. Similarly, negative energy balance in summer season depresses the activity of the GnRH pulse generator, reduction in gonadotrophins production, reduction of scrotal circumference and semen production. It was observed in caprine species that lower plasma testosterone was detected during summer seasons[19].Concentration of prolactin was elevated during the summer season and hyperprolactinemia inhibited GnRH secretion which in turn impaired the LH release from the pituitary gland, and thus disturbed the hypothalamic-pituitary-gonadal axis[20]. Perumal et al[4] reported in bovine species that LH and testosterone were significantly higher in winter than summer season; LH and testosterone and LH and FSH were positively correlated. Daily temperature and sunshine hours were negatively correlated with LH and testosterone concentration[4],as summer heat stress and longer day length had reduced the secretion of LH and testosterone in Andaman local goat in Andaman and Nicobar Islands. This in turn creates hormonal imbalance.Improper secretion of these hormones leads to reduced production of testicular testosterone, poor libido, and increased reaction time,poor semen quality and ultimately end up with infertility in breeding male[4]. Similar results were reported by Barkawi et al[11]. Similarly,Vérata and Malguena bucks were reported that plasma testosterone concentration was the highest when photoperiod was decreased and the lowest when the photoperiod was increased[8]. Thus, the frequency and the amplitude of the LH peaks and the testosterone concentration evolved with season. Variation in the endocrine profiles in the study with studies of others was most probably due to large differences in the local experimental conditions (rainfall,intensity and duration of light, ambient temperature and relative humidity). It may be pertinent to inform here that rhythmicity in endocrine profiles is determined by many different factors including nutrition, metabolic rate and climatological and environmental conditions. Thus, it is well established that serum testosterone and sexual activity vary depending on season. During the short-day photoperiod and reduced temperature (rainy season), concentration of melatonin is increased and protected the testicular tissue as an antioxidant which in turn increased the testosterone production in Andaman local goat[7]. In goats, melatonin induces the hypothalamus to release the GnRH via pulsatile secretion, which acts on the anterior pituitary and leads to the release of greater amounts of the gonadotropins FSH and LH[21]. Variation in concentration of melatonin in different seasons may be the causes of greater values of FSH, LH, T4 and testosterone obtained in rainy season as compared to summer season. It is probable that this seasonal variation in testicular responsiveness to LH is associated with seasonal changes in LH receptor populations in the testis[4].
Cortisol is a stress hormone and increases in environmental and climatological stresses. As reported in porcine species[22], plasma cortisol concentrations have been increased in Andaman local goat in summer season in Andaman and Nicobar Islands. Seasonal changes of cortisol might be associated with remarkable alterations in secretion of reproductive hormones in male goats[23]. Dry summer season had higher concentration of cortisol in the Andaman local goat which might be due to hypothalamic-pituitary-adrenal cortical axis activation and sympatho-adrenal medullary axis activation which in turn stimulates the circulatory cortisol and corticosterone concentrations[24]. Cortisol concentration is not only influenced by acute or chronic heat stress but also alteration in the photoperiod[25].Similar results were obtained in our study in Andaman local goat.Similar reports were also observed in Balady and Damascus goats[26]and Saanen bucks[27]; in which study, higher concentrations of plasma cortisol was reported in summer season than winter season.
Prolactin is synthesised in anterior pituitary lactotrophs and its exact function is not known or yet to be understood in male reproductive system, although it plays a significant role in male infertility[28]. Prolactin controls the central nerve system centres for regulation of the sexual behaviour and sexual arousal. Prolactin inhibits the hypothalamic-pituitary-adrenal axis activity and the neurohypophysial oxytocin system[29]. Dominant seasonal factors affecting the annual prolactin release are photoperiod and environmental temperature. Higher ambient temperature and longer photoperiod in summer increased the plasma prolactin concentration in bubaline species[30]. Furthermore, temperature higher than 27 ℃ markedly increases the plasma prolactin in goats irrespective of photoperiodic variations[31]. Similarly, in this study,prolactin concentration increased with the increase of photoperiod and temperature values in Andaman local goat of Andaman and Nicobar Islands. Similar results were reported in Angora goats[32]. Photoperiodic effect on prolactin release is transmitted through melatonin hormone; increase of day length declines the melatonin release which in turn stimulates the release of prolactin.Hyperprolactinemia stimulates hypogonadism in male by inhibiting the GnRH pulsatile secretion[20] and subsequently inhibiting the production and release of FSH, LH and testosterone. Thus, an inverse relationship was existed between prolactin and FSH, LH and testosterone in buck as similar to the ram[33]. Further, prolactin triggers the adrenal corticoid’s hyper secretion and inhibits the GnRH secretion through its receptors on dopaminergic neurons which in turn causes hyperprolactinemia, which is one of the major important causes of male infertility. Similar observation was reported in the present study that goats in rainy season had significantly lower level of prolactin than summer season.
Thyroid hormones play important roles in maintenance of basal metabolic rate and are essential for somatic cell growth. T4 concentration was significantly increased in rainy season whereas it was decreased in summer season in Andaman local goat.Similar reports were observed in bubaline species[34]; lower T4 concentration in summer season was due to heat stress and longer day length which have direct effect on thyroid gland structure and its functions[35]. A major exogenous regulator of thyroid gland activity is the environmental temperature, so an inverse relationship between ambient temperature and blood thyroid hormones concentrations has been found in sheep[36]. During heat stress, blood T3 and T4 concentrations as well as metabolic rate were decreased[37].Temperature has reduced the thyroid hormones in two different ways; it induces direct effect on thyrotropin releasing hormone which consequently reduces plasma T4 concentration and on indirect effect,it decreases the appetite which in turn reduces the thyroid hormone concentration[8]. The seasonal pattern of blood thyroid hormone levels often showed maximal values during winter (cold months)and minimal values during summer (hot months)[38]. Furthermore,T3 induces the synthesis of soluble protein in Leydig cells, which stimulates androgen release[39]. Thus, the deficiency of the thyroid hormones affects the androgen synthesis and release. Thyroid hormones are required for long-term expression and maintenance of the endogenous seasonal reproductive rhythm in small ruminants[40].Seasonal effect of thyroid hormones is due to the presence of thyroid hormone receptors in GnRH and other neurotransmitters-containing neurons[41]. Similarly, photoperiod regulates the expression of typeⅡ deiodinase gene in the mediobasal hypothalamus of the goat;hence, season affects the bioavailability of thyroid hormones for the reproductive neuroendocrine axis[41].
Sexual behaviour scores were higher in rainy season than in summer season in Andaman local goat. Buck fertility is dependent on both the semen quality and libido. The real reproductive ability of males can be indirectly determined by libido, which is the degree of interest of the male to copulate[42]. Season had a significant effect on different sexual behaviour scores. According to Azevêdo et al[43],in tropical climate regions like Andaman and Nicobar Islands, the libido of small ruminants generally reveals some variation, although it can be influenced by ambient variables such as temperature humidity index, rainfall, insulation, or by a change in the feed offered. However, the sexual activity is greater, when there is a decrease in the amount of light, as it stimulates males to synthesize androgens and produce pheromones[11]. In our study, short-day length enhanced melatonin secretion which in turn improved the endocrinological profiles particularly testosterone, which in turn enhanced the libido. This was confirmed by presence of a positive correlation between libido score and testosterone concentration in goat bucks. Earlier studies reported that increased testosterone concentrations which in turn reduced the reaction time and elevated testosterone concentrations tended to be associated with enhanced courtship behaviours[44]. Again, the smelling of bucks is more pronounced during the breeding season than in non-breeding season which is due to the action of testosterone on the sebum glands in the skin, the head and the neck[11]. Karaca et al[44] reported the testosterone and reaction time were negatively correlated (r = -0.54).
However, the present study has some limitations. In this study,we examined the seasonal effect on endocrinological profiles and sex behavioural parameters to select the suitable season to improve the reproductive performances. We used limited parameters such as hormone and libido profiles and the results indicated that rainy season is suitable for breeding related programmes for Andaman local goat. Therefore, we need further studies that effect of seasons on scrotal & testicular profiles, blood antioxidant & oxidative stress profiles, semen quality profiles, in-vitro & in-vivo fertility profiles in goat bucks are warranted to confirm the present findings.
In conclusion, endocrinological profiles and sex libido behaviours of goat differ significantly between rainy and dry summer seasons.Significantly higher levels of FSH, LH, testosterone, TSH, T3, and T4 are observed in rainy season than in dry summer season, whereas cortisol and prolactin are significantly higher in dry summer season than in rainy season. Similarly, libido score, mating ability score and sex behavioural score are significantly higher in rainy season than in dry summer season. Based on the results, the study suggested that rainy season has greater beneficial effects than dry summer season on reproduction and artificial breeding programmes in the semiintensive management of goat in the present location.
Conflict of interest statementThe authors declare that there is no conflict of interest involved in the present work.
FundingThis research was funded by a Grant from All India Coordinated Research Project on Goat Improvement (ICAR-AICRP on Goat Improvement), Indian Council of Agricultural Research, New Delhi,India with Grant number AICRP-Goat Improvement/ICAR-CIARI.
Authors’contributionsPerumal Ponraj contributed to conceptualization; Perumal Ponraj,Arun Kumar De and Jai Sunder contributed to data curation;Perumal Ponraj and Debasis Bhattacharya contributed to formal analysis; Perumal Ponraj and Sneha Bhowmick contributed to investigation; Perumal Ponraj, Jai Sunder and Purna Chandra Mishra contributed to methodology; Debasis Bhattacharya, Jai Sunder and Perumal Ponraj contributed to project administration;Perumal Ponraj, Arun Kumar De and Rafeeque Rahman Alyethodi contributed to writing original draft; Perumal Ponraj, Arun Kumar De and Rafeeque Rahman Alyethodi contributed to writing,reviewing and editing.
 Asian Pacific Journal of Reproduction2022年4期
Asian Pacific Journal of Reproduction2022年4期
- Asian Pacific Journal of Reproduction的其它文章
- Conventional treatment options and herbal remedies for male infertility: An overview
- Iron supplementation for non-anaemic pregnant women and the incidence of hypertensive disorders in pregnancy: A systematic review and meta-analysis
- Impact of geographical and seasonal temperature on sperm parameters in Indian men who were partners in subfertile couples - A retrospective analysis
- Testicular vascularization at two locations in relation to hormonal levels, and pixel echotexture in bulls at different ages
