Vicinal hydroxyl group-inspired selective oxidation of glycerol to glyceric acid on hydroxyapatite supported Pd catalyst
Difan Li,Xiuge Zhao,Qingqing Zhou,Bingjie Ding,Anna Zheng,Qingpo Peng,Zhenshan Hou*
Key Laboratory for Advanced Materials,Research Institute of Industrial Catalysis,School of Chemistry and Molecular Engineering,East China University of Science and Technology,Shanghai,200237,China
Abstract Selective oxidation of glycerol provides a feasible route towards the sustainable synthesis of high value-added chemicals.Herein,the hydroxyapatite (HAP) supported palladium (Pd) species were fabricated by impregnation and subsequent calcination.The as-obtained heterogeneous Pd catalyst afforded not only excellent selectivity to glyceric acid (GLA) up to 90% with 59% conversion of glycerol but also good recyclability by using molecular oxygen as an oxidant under mild conditions.The characterization of catalysts indicated that both the surface basicity and Pd sites on the catalyst played a crucial role in promoting glycerol oxidation.Notably,it demonstrated that the presence of the vicinal hydroxyl group of glycerol molecule can assist the oxidation reaction via forming a coordination between the vicinal hydroxyl group and Ca2+sites on HAP-derived catalysts.In this catalytic process,the secondary hydroxyl of glycerol kept untouched and the primary hydroxyl of glycerol was converted into carboxyl group,while the Pd species acted as active centers for cooperatively promoting the subsequent oxidation to generate GLA.Additionally,this catalytic system can be extended widely for the oxidative conversion of other vicinal diols into the cor111responding α-hydroxycarboxylic acids selectively.Isotope labeling experiment using O confirmed that H2O not only acted as solvent but also was involved in the catalytic cycles.On the basis of the results,a possible reaction mechanism has been proposed.The HAP-supported Pd catalytic system has been shown to serve as an effective approach for the upgrading of bio-derived vicinal diols to high value-added chemicals.© 2020 Institute of Process Engineering,Chinese Academy of Sciences.Publishing services by Elsevier B.V.on behalf of KeAi Communications Co.,Ltd.This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Keywords: Hydroxyapatite;Palladium;Glycerol oxidation;Glyceric acid;Vicinal hydroxyl group
1.Introduction
Developing renewable non-petrochemical resources,sustainable biofuels and alternative value-added chemicals derived from biomass have attracted a growing interest in recent years.In these processes,the utilization and production of biodiesel come into being due to the depletion of fossil fuels,human health hazards and climate change [1-3].Biodiesel,which comprises a mixture of mono-alkyl esters of long-chain fatty acids is gaining popularity as a fungible fuel because of its availability,eco-friendly,non-toxicity and biodegradability [4].It deems to be a promising and sustainable biofuel compared to petroleum-derived fuels,which can be produced by the procedure of either transesterification of triglycerides or esterification of free fatty acids,alongside producing a large number of glycerol as a potentially valuable by-product in this process [5].Additionally,other bio-derived polyols,such as 1,2-propanediol and ethylene glycol are known as important platform chemicals and they are produced by an alternative route involving selective dehydroxylation of glycerol through chemical hydrogenolysis or biocatalytic reduction [6,7].Therefore,upgrading of bio-based polyols to commercial products improves the economic feasibility of biodiesel industry and provides sustainable path for development and utilization of renewable biomass resource [8,9].
Different strategies including microbiology,fermentation,and chemistry have already been spread to transform glycerol into different valuable derivatives[10].Over the past decades,researchers have been trying to find new applications of glycerol as a low-cost feedstock for its functionalization which led to the introduction of a number of selective processes for converting glycerol into commercially valued products.The selective oxidation of glycerol is one of great importance transformation routes,which has attracted growing attentions in recent years.Nevertheless,the pathway of glycerol selective oxidation is extremely complex and challenging,involving the issues of oxidation position for primary versus secondary hydroxyl group,and the partial oxidation to aldehydes and ketones or deep oxidation to carboxylic acids,or even C-C bond cleavage,leading to the formation of small molecules[11-13],including dihydroxyacetone (DHA),glyceraldehyde(GALD),glyceric acid(GLA),tartronic acid(TA),oxalic acid(OA),glycolic acid (GA) and formic acid (FA),etc.These products can be flexibly converted into various market products,and widely used in the field of cosmetics,food,medicine and other industries.Among these potential products,GLA is an interesting compound owing to its various applications in different domains such as treatment of skin disorders,anionic monomer of packaging materials for volatile agent and biodegradable fabric softener [14].However,due to the diversity of oxidation products,it is still an enormous challenge to oxidize the glycerol with a superior selectivity toward a single desired product.
The homogeneous base catalyst such as sodium hydroxide is usually applied in selective oxidation of alcohols,but inherent homogeneous property and difficulty in catalyst separation limit further applications.These catalytic processes are severely energy-consuming and economical drawbacks because it is either usually conducted under high temperature or often leads to low catalytic activity[15,16].Alternatively,in order to overcome this problem,a lot of efforts were focused on heterogeneous catalytic systems by using monometallic Au/CMK-3,Pt/CNTs,Au/N-CNFs,Pt/K-AMC,Pt/MCN and Pt/MgO/SBA-15 [17-22],or bimetallic AuAg/Al2O3,AuPd/Mg(OH)2,AuPd/MgOnano,AuCu/ZnO and Ni1Co1Ox[23-27]catalysts with oxygen as oxidant.It was demonstrated that the reaction conditions (pH,temperature and substrate to metal ratio)and the structure of the catalyst(metal,particle size and support) had a significant impact on the catalytic activity and selectivity of products [28],but the intrinsic relationship between the support structure and the oxidation behavior of the glycerol molecule retained phenomenological.Besides,these expensive catalytic systems were still subjected to low selectivity toward GLA or the diversified products with harsh reaction conditions.Consequently,the development of efficient heterogeneous catalytic system for glycerol oxidation under mild reaction conditions via tailoring metal-basic active sites on the catalysts is highly promising.
Hydroxyapatite (HAP) belongs to a large family of isomorphic compounds and is one of the most common forms of calcium phosphate with apatite structure,is known that the main component of bones and teeth [29].HAP has good bioactivity,biocompatibility and strong osteoinduction properties,thus extensively applying in biomedical field contains bone substitute material,biopolymer composite,and as a drug carrier in drug and gene delivery systems [30-33].Owing to its structure flexibility,rich surface chemistry,interesting characteristics and catalytic properties,together with Ca2+sites surrounded by a phosphate group tetrahedron parallel to the hexagonal axis,the HAP possesses a very high ion-exchange ability,great adsorption capacity,and acid-base properties and good hydrothermal stability,and it is known that it starts to decompose into other phases such as tricalcium phosphate (TCP;Ca3(PO4)2) at temperatures higher than 800°C[34].Especially,HAP has been widely used as catalyst support and even a catalyst,which plays an important role in catalytic field by synergistic interactions with a variety of metals.Thus HAP or HAP-supported metal catalysts have been employed for cross-coupling,condensation,oxidation,dehydrogenation,photocatalytic and other miscellaneous reactions [35-39].
As discussed above,although HAP-supported metal (Pd)catalysts have been employed for the oxidation of alcohols,they were never tested in the oxidation utilization of vicinal diols,being important biomass-derived platform molecules.In order to address this issue,herein,we report a promising heterogeneous HAP-derived Pd catalyst,which was prepared conveniently through an incipient wetness impregnation process with Pd(II)precursor,followed by drying and calcination.The resulting Pd catalysts were firstly employed for glycerol oxidation and it was found that the catalysts were highly active and stable in oxidation of glycerol under relatively low oxygen pressure,and meanwhile they exhibited the high selectivity to GLA (90%).Systematic activity evaluations and catalyst characterizations have demonstrated that the surface Pd(II)species were highly relevant to catalytic oxidation activity and Ca2+sites on HAP related to selectivity toward GLA by oxidation of primary hydroxyl group,but the secondary hydroxyl group remains untouched via a coordination of the vicinal hydroxyl groups to Ca2+sites on the HAP-supported Pd catalysts.Notably,the other vicinal diols were also preferentially converted into cor111responding α-hydroxycarboxylic acids.On the basis of the results above,the plausible reaction mechanism for the oxidative conversion of glycerol to GLA has been proposed.
2.Experimental
2.1.Chemicals
All chemicals and solvents were commercially available and used as received without further purification.Calcium nitrate tetrahydrate (Ca(NO3)2·4H2O,99%),diammonium hydrogen phosphate ((NH4)2HPO4,98.5%),ammonium hydroxide (25 wt%),sodium hydroxide (NaOH,98%),1-propanol (AR),1-butanol (AR),ethylene glycol (AR),1,2-propanediol (AR),1,3-propanediol (AR),ferrous sulfate(FeSO4·7H2O,99%),concentrated sulphuric acid (H2SO4,98 wt%) and hydrochloric acid (HCl,37 wt%) were obtained from Lingfeng.Glycerol (AR) was purchased from Sinopharm.Glycolic acid (GA,99%) and formic acid (FA) were provided by Macklin.Glyceric acid (GLA) was provided by Tokyo Chemical Industry.Cerous sulfate (Ce2(SO4)3·4H2O,98%) and 1,10-phenanthroline (99%) were acquired from Aladdin.Tetraammine palladium nitrate (Pd(NH3)4(NO3)2,35%),tetraammine palladium chloride (Pd(NH3)4Cl2,40%)and tetraammine palladium acetate (Pd(NH3)4(OAc)2,35%)were purchased from Kunming Boren Precious Metals Co.Ltd.High purity O2(99.9%) was supplied by Shangnong Gas Factory.O was obtained from Shanghai Yuanneng Biotechnology Co.Ltd.
2.2.Catalyst preparation
2.2.1.Synthesis of hydroxyapatite (HAP)
The support HAP was synthesized by a chemical precipitation method according to the literature [40].Typically,0.6 mol L-1(NH4)2HPO4aqueous solution (50 mL) was added dropwise to 1.0 mol L-1Ca(NO3)2·4H2O aqueous solution (50 mL) (Ca:P molar ratio=1.67) and then the pH of the mixture was adjusted to 10 by adding a certain amount of ammonium hydroxide under vigorous stirring at room temperature.The resultant milky like suspension was heated to 80°C immediately and stirred continuously for 3 h,followed by filtered and washed repeatedly with deionized water till neutrality.The resulting material was dried at 80°C for 12 h and calcined at 500°C for 3 h then the obtained white solid powder was denoted as HAP.
2.2.2.Preparation of Pd/HAP-x catalysts (x=NO,Cl and Ac)
The HAP-supported Pd catalysts were prepared by the conventional incipient wetness impregnation.Briefly,0.5 g HAP was impregnated with an aqueous solution of 0.0143 g Pd(NH3)4(NO3)2to achieve incipient wetness under vigorous stirring.After continuous stirring vigorously at 50°C about 12 h,the sample was dried and further calcined at 500°C for 3 h.The sample obtained was assigned as Pd/HAPNO.For comparison,the other HAP-supported palladium catalysts were also prepared in a similar method,starting from different palladium precursors Pd(NH3)4Cl2and Pd(NH3)4(OAc)2.The as-resultant samples were assigned as Pd/HAP-Cl and Pd/HAP-Ac,respectively.The actual Pd contents (around ca.1.0 wt%) of all the catalysts (Pd/HAP-x)were determined by ICP-AES.Additionally,different Pd loadings of catalysts were synthesized in a similar method with Pd(NH3)4(NO3)2as palladium precursor.
2.3.Characterizations
Powder X-ray diffraction(XRD)patterns were collected on a SmartLab diffractometer from Rigaku equipped with a 9 kW rotating anode Cu source at 45 kV and 100 mA (5-80°,0.2°s-1).The morphologies were tested with a field emission scanning electron microscope (GeminiSEM 500;accelerated voltage: 3 kV).High resolution transmission electron microscopy (HRTEM) was performed in a JEOL JEM 2100 transmission electron microscope operating at 200 kV with a nominal resolution of 0.25 nm.The samples for HRTEM were prepared by dropping the aqueous solutions containing the NPs onto the carbon-coated Cu grids.Nitrogen (N2) adsorption isotherms and Brunauer-Emmett-Teller (BET) surface areas were measured at-196°C by using a BELSORP-MINI analyzer.The samples were degassed at 200°C for 2 h to a vacuum of 10-3Torr before analysis,and pore size distribution curves were calculated using the Barrett-Joyner-Halenda(BJH)model.X-ray photoelectron spectroscopy(XPS)spectra were performed on an ESCALAB 250Xi spectrometer equipped with Al Kα radiation (1486.6 eV),with the calibration peak being the C 1s peak at 284.6 eV.Metal contents were detected by an Agilent 725 inductively coupling plasma atomic emission spectrometer (ICP-AES).Basic sites were measured by carbon dioxide temperature programmed desorption(CO2-TPD)using a Catalyst Analyzer BELCAT-B.The samples were pretreated at 300°C for 1 h,and then cooled to 50°C under helium (He) gas.CO2adsorption was carried out at 50°C for 1 h under CO2/He gas.After samples were purged under He gas for 1 h,the temperature was heated to 800°C (10°C min-1).The desorbed gas was determined by using a Gow-Mac thermal conductivity detector (TCD).Hydrogen temperature programmed reduction (H2-TPR) was carried out on the VDsorb-91i chemisorption analyzer.TPR runs were performed under the flowing 10% H2/Ar,ramping the temperature at 10°C min-1and using a Gow-Mac TCD.The adsorption states of glycerol on catalysts were studied on a Nicolet Magna 550 FT-IR spectrometer.The spectrum of sample without absorbed glycerol was recorded as background.The samples were impregnated with glycerol aqueous solution via ex-situ wet impregnation method.25 mg of sample was saturated with 2 wt% glycerol aqueous solution(2 mL),followed by drying under the vacuum at 60°C for 30 min.For the preparation of oxalic acid-poisoning catalyst,5 mL of 0.3 mol L-1oxalic acid aqueous solution was added to 50 mg Pd/HAP-NO catalyst.The resulting suspension was stirred at room temperature for 2 h,and then water was evaporated at 70°C under the vacuum to afford 4%OA-Pd/HAP-NO catalyst.The sample was used for activity test and FT-IR measurement.
2.4.Catalytic reaction
The selective oxidation of glycerol was carried out in a high-pressure batch autoclave of stainless steel with a 25 mL polytetrafluoroethylene inlet equipped with a heating block and magnetic stirrer.In a typical reaction,5.5 mmol glycerol(0.5 g),4.5 mL H2O,1 eq.NaOH(0.23 g),and certain amount of catalyst were added into the reactor,which was sealed and flushed by O2thrice.Then the reactor was charged with 1 MPa O2at room temperature,the reaction was conducted at 80°C for 5 h with vigorous stirring in a heating jacket.After a specific time,the reactor was quenched in a cold-water bath and the reaction mixture was centrifuged and diluted with deionized water then ion exchanged and filtered with a 0.22 mm filter membrane for product analysis.The liquid product was analyzed by a high performance liquid chromatography (Agilent 1260) equipped with an ultraviolet detector and a refractive index detector in series.A SilGreen H column(300 mm × 7.8 mm) was employed for product separation with 5 mmol L-1H2SO4aqueous solution as the mobile phase and had a flow rate of 0.5 mL min-1.The column oven temperature was 50°C and 20 μL of each sample was injected.An external calibration method was used for the quantification of the reactants consumed and products generated.The identification and quantification of the oxidation products were carried out by comparison with pure standards using calibration curves.All values determined for selectivity and carbon mass balance were provided by HPLC analysis.The conversion of glycerol and selectivity towards products were calculated as follows:

The reusability was assessed by a recycling test.After the reaction,the catalyst can be easily separated from the reaction mixture by centrifugation,simply washing with deionized water,and then these adsorbed species can be facilely removed and dried in vacuum.The spent catalyst was charged into the next run for reuse under the identical reaction conditions.The kinetic isotope effect (KIE) was studied on the oxidation of glycerol over the Pd/HAP-NO catalyst using H2O/D2O as solvent for the reaction.Reaction conditions for the KIE experiment: 5.5 mmol glycerol,4.5 mL H2O/D2O,1 eq.NaOH,0.03 g Pd/HAP-NO,80°C,1 MPa O2.The rate constants were determined from the experimental data based on the glycerol concentration by first order reaction kinetics.
The existence of peroxide species in the liquid phase were examined by potential difference titration of Ce3+/Ce4+.After the reaction,the reaction liquid was separated by centrifugation and acidification under ice bath,and then one drop of the mixed aqueous solution of 1,10-phenanthroline(0.075 mol L-1) and FeSO4(0.025 mol L-1) (indicator) and two drop of the concentrated HCl were added into the reaction mixture.The end point of titration was determined until the solution turned blue and did not change within 30 s.The blank sample was also tested in the absence of catalyst.
Isotope labeling experiment was performed usingO as solvent.The oxidation reaction was carried out with 1 mmol glycerol,1 mLO,1 eq.NaOH and 0.01 g Pd/HAP-NO.A Q-Exactive plus liquid chromatograph mass spectrometry was used to identify the formed labeled products.
3.Results and discussion
3.1.Characterization of the materials
The XRD patterns of a series of support HAP and Pd/HAPx samples are shown in Fig.1.The patterns of all the samples are in good agreement with the hexagonal structure of crystalline hydroxyapatite(JCPDS No.09-0432)[41],and there is no other impurities phase formation,indicating that the HAP support has been successfully synthesized.After Pd species are loaded onto HAP,the crystalline structure of the different Pd/HAP-x catalysts are maintained,that is,no obvious diffraction peaks of the supported metal or metal oxide phase are observed in any Pd/HAP-x catalysts,indicating that the possible Pd species are highly dispersed on the support of HAP under calcination treatment,as well as the low metal loading amount.
Fig.2 shows the morphology of HAP-derived catalysts revealed by the SEM images.Pristine HAP reveals its crystal structure that are arranged almost anomalous morphology in large domains with smooth surface.When the Pd species are supported on the HAP surface,the nanostructure remains,indicating the good crystallinity of the HAP in line with that of XRD patterns.In order to identify the properties of Pd species,the samples are further investigated by HRTEM.The cor111responding HRTEM images and the particle size distribution diagrams are shown in Fig.3,indicating that the mean sizes of Pd particles on the catalysts is in the range of 2-3 nm (Table 1).It can be seen that Pd/HAP-Ac catalyst seems to give the smallest Pd nanoparticles.Especially,all the Pd/HAP-x catalysts show typical irregular and elongated nanosheets morphology as the original HAP,suggesting that supporting Pd species do not change the structure of HAP.The Pd species are characterized by irregular spherical-shaped morphology and uniformly disperse on the support surface.The crystalline nature of the Pd species can also be confirmed from the clear lattice fringes observed in HRTEM image (Fig.3d).The interfringe spacing for the lattice fringes are calculated to be 0.234 nm and 0.262 nm that correspond to the (111) lattice planes of the metallic Pd and (101) lattice planes of the tetragonal PdO respectively[42].Fig.3e is a SAED pattern of the Pd species,which exhibits clear diffraction rings and diffraction spots distributed as ring,suggesting low crystallization of the as-synthesized Pd/HAP-NO catalyst.It can be indexed to (111) of metallic Pd and (101) of PdO in good agreement with the lattice fringes,respectively.This indicates that metallic Pd species are still existed even the catalysts are subjected to the calcination process.
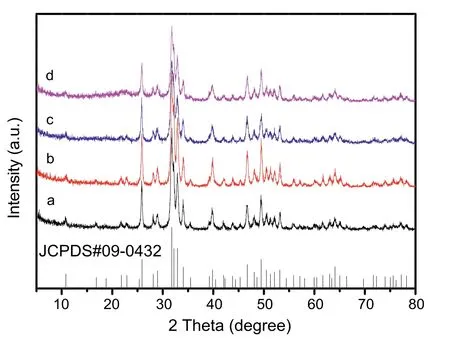
Fig.1.XRD patterns of(a)HAP,(b)Pd/HAP-NO,(c)Pd/HAP-Cl and(d)Pd/HAP-Ac.
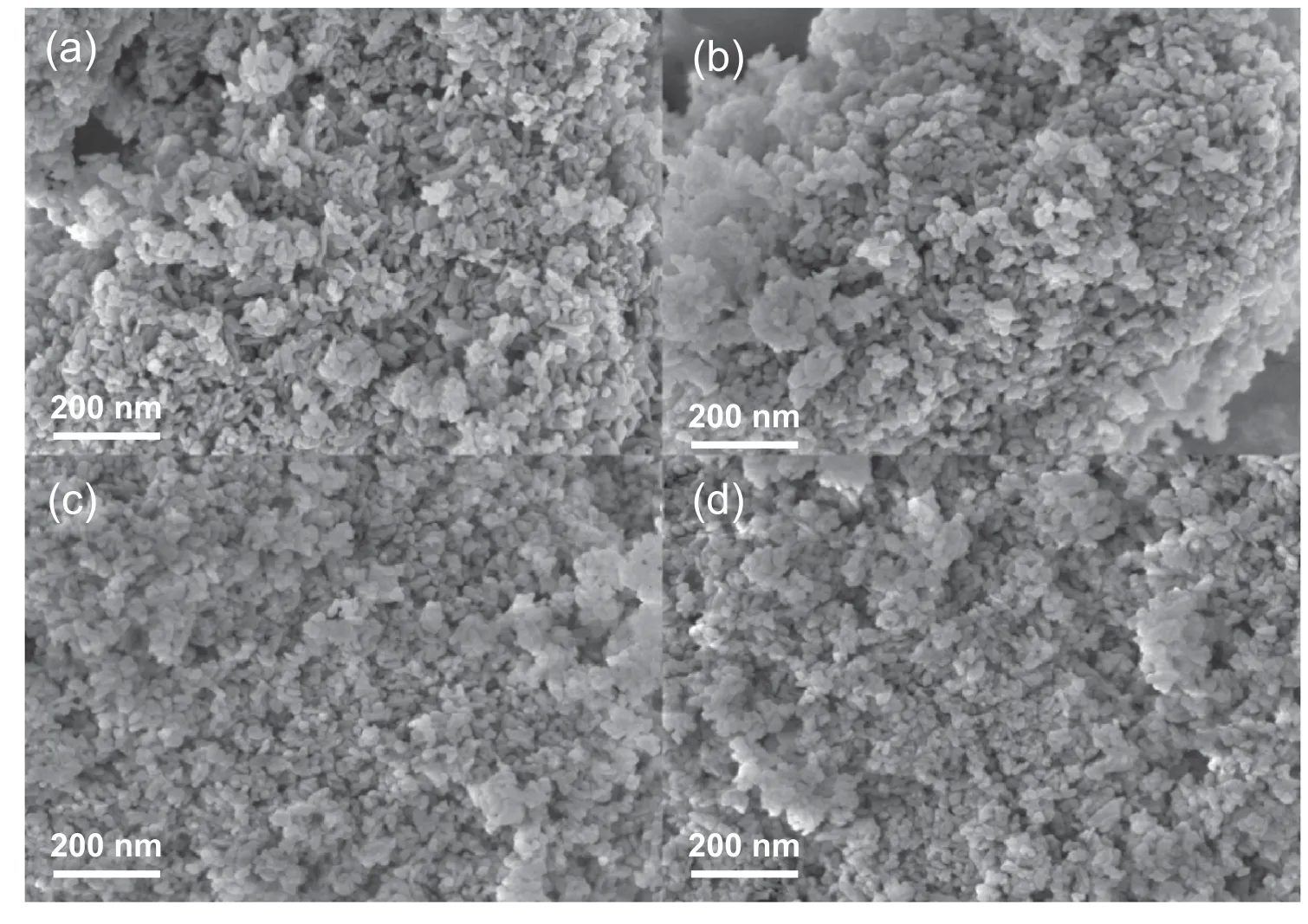
Fig.2.SEM images of (a) HAP,(b) Pd/HAP-NO,(c) Pd/HAP-Cl and (d) Pd/HAP-Ac.
The N2adsorption-desorption isotherms are determined to inspect the textural properties for the various Pd/HAP-x samples.As presented in Fig.4a,all the samples exhibit typical type IV curves with a H3 hysteresis loop characteristic and the isotherms for the pure HAP and Pd/HAP-x catalysts are extremely similar,revealing that the loading of a small amount of Pd species has a little effect on the porous structure of the pristine HAP.When the relative pressure is higher than 0.9 of P/P0,the sharp change of nitrogen adsorption volume can be observed,which is due to the large number of macropores formed by the accumulation of particles [43].Moreover,the introduction of Pd species into the HAP would decrease slightly the specific surface area in comparison with bare HAP,which may be due to the penetration of the partial Pd active species into the pores of HAP.In addition,the pore sizes of all the catalysts calculated from the BJH desorption are listed in Table 1.Interestingly,the pore size distribution of the HAP bare support consists of a broad peak centered at ca.25 nm,and a slight decrease of pore size can be observed for Pd catalysts.Meantime,the pore size distribution peaks become broad,stemming from the existence of Pd species in large pore (Fig.4b).
The reduction properties of all the Pd/HAP-x catalysts are examined by H2-TPR and the results are shown in Fig.5a.It is observed that the sole HAP has no obvious reduction signal at low temperature region,but an additional less intense peak can be observed at higher reduction region around 550-700°C,being probably owing to the consumption of surface oxygen anions species in HAP support [44-47].With respect to the Pd/HAP-x catalysts,there are two obvious reduction peaks in the temperature region of 50-800°C.The strong peak at low temperature belongs to the reduction of PdO species on the surface of the support [48] and the high temperature can be also assigned to the removal of surface oxygen in HAP support itself as shown in the sole HAP.It can be seen that Pd/HAPAc,Pd/HAP-NO and Pd/HAP-Cl catalysts show the reduction peaks of Pd(II) species appearing at 178,189,and 200°C respectively,which means that Pd(II) species on Pd/HAP-Ac are most easily reduced among three catalysts,revealing that very small and easily reducible smaller Pd(II) species can be formed after calcination as Pd(NH3)4(OAc)2is used as precursor (Fig.3a-c) [49].
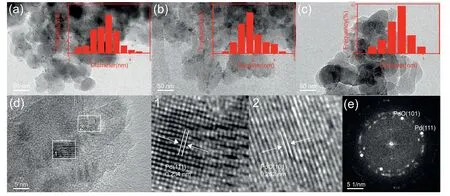
Fig.3.HRTEM images and particle size distribution diagrams of (a) Pd/HAP-NO,(b) Pd/HAP-Cl and (c) Pd/HAP-Ac.(d) Different magnification/interplanar spacings of Pd/HAP-NO and (e) SAED ring patterns of Pd species.
To investigate the surface basicity of the HAP support and Pd/HAP-x catalysts,the CO2-TPD are carried out and shown in Fig.5b.It is seen that all the catalysts show similar broad CO2desorption peaks,indicating that the same basic sites with different strengths are present on the catalyst surface.The desorption peaks of CO2are classified into three regions ranged around 100-350°C,350-600°C and 600-750°C,cor111responding to the weak,medium and strong basic sites,respectively [50].The weak and medium basic sites can be separately attributed to the contribution of phosphate anions bound to Ca2+and CaO species (i.e.,O2-anions),while the strong basic sites can be attributed to OH-anion[51,52].The cor111responding peak areas have been normalized and the amounts of basic sites in the catalysts are calculated and listed in Table 2.It can be seen that the amounts of the weak basic sites are dominant on all samples.Besides,although the CO2desorption peaks due to the strong basic sites become broad after the modification of Pd species,the amounts of total basic sites and the strong basic sites remain almost unchanged.Meantime,the medium basic sites show only a slight increase of amount after Pd modification likely due to CO2absorption on Pd species (Table 2).Conversely,all the Pd/HAP-x catalysts have shown a considerable decrease of the weak basic sites(183,180,174 μmol g-1)in contrast with that of the sole HAP (204 μmol g-1),suggesting that a Pd(II)-phosphate complex is formed and Pd sites may be coordinated by oxygen atom of P=O bonds,leading to a decrease of the basicity of HAP [53].
In order to understand the surface chemical states and ensure the role of metallic species,which is a prerequisite for the selective oxidation of glycerol,the Pd/HAP-x samples in the form of the oxidation state are subjected to XPS analysis.As shown in Fig.6a,the survey XPS spectra of Pd/HAP-x contain similar element peaks for O 1s,Ca 2p,and P 2p.The O 1s peak(531 eV)and the P 2p peak(133.6 eV)are attributed to the binding energy of P-O bond in apatite,whereas the Ca 2p peak (346 eV) is deconvoluted into Ca-O bond,these of which are linked to the HAP structure [54].In addition,the binding energy of Ca 2p and Pd 3d are close to each other and their peaks are partly overlapped,special for Pd 3d3/2.Thus the Pd 3d5/2spectra can be deconvoluted into two doublet peaks,in which the relatively lower binding energy peak is assigned to metallic Pd species and the other peak results from the oxidized Pd(II) species on the surface region of the catalysts(Fig.6b-d)[55].As shown in Fig.6b-d and Table 2,the Pd/HAP-NO sample contains the largest amounts of these Pd(II) species (71.3%),while the other analogues such as Pd/HAP-Cl and Pd/HAP-Ac afford the 60.5% and 53.8% of the Pd(II) species respectively,in line with H2-TPR results that indicate the easy reducibility of Pd/HAP-Ac.This reveals that the Pd(II)is formed preferentially on the surface of HAP when Pd(NH3)4(NO3)2is used as Pd precursor likely due to the strong oxidizing ability of nitrate anion.Moreover,the XPS characterization suggests that all the HAP-supported Pd catalysts contain both Pd (0) and Pd(II) species,which plays a crucial role in molecular oxygen activation and the sequential glycerol oxidation.
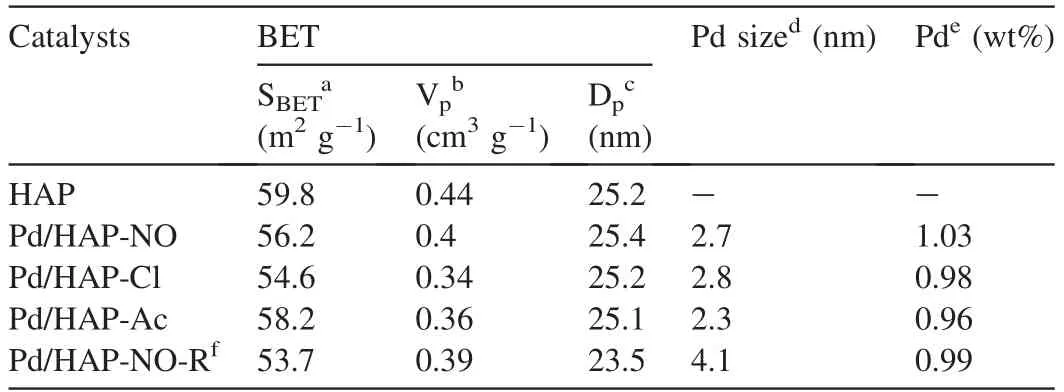
Table 1 Physicochemical properties of HAP and Pd/HAP-x catalysts.
3.2.Catalytic activity
The Pd/HAP-x and the other control samples are employed as catalysts for the selective oxidation of glycerol to GLA with O2as the oxidant,and the catalysis evaluation starts under relatively mild conditions and the results are shown in Table 3.The reaction does not proceed in the absence of a catalyst(entry 1).Either a sole HAP or NaOH solution shows a very poor activity for glycerol oxidation due to the lack of oxidative sites under the present mild conditions,suggesting the oxidative sites on the catalysts are required for the reaction(entries 2 and 3).In addition,no product or low activity is detected over the Pd/HAP-NO under nitrogen atmosphere or in absence of base(entries 4 and 5),indicating that an aerobic process and the alkaline medium are required to promote the oxidation reaction under the used conditions.

Fig.4.(a) N2 adsorption-desorption isotherms and (b) pore size distribution curves of HAP and Pd/HAP-x.
For comparison,the different palladium precursors-derived Pd/HAP-x catalysts are tested for the selective oxidation of glycerol.Interestingly,the Pd modified HAP catalysts show moderate to good catalytic activity (entries 6-8),which reveals that the oxidizing sites (Pd) could be important for oxidizing glycerol to GLA selectively.Notably,the Pd/HAPNO catalyst affords the best performance for this reaction,which 59% of glycerol is consumed along with 90% selectivity to GLA,and only a small amount of C-C bond cleavage products like GA and FA were detected(entry 8).It should be also noted that the reaction hardly occurs under solvent-free conditions,indicating that water could be very crucial for the oxidation reaction (entry 9).Additionally,a commercially available Pd/C catalyst is also utilized for selective oxidation of glycerol and only affords 30.8% yield of GLA (entry 10),likely resulting from the absence of the appropriate basic sites.The superior activity of Pd/HAP-NO is attributed to the follow reasons.On one hand,the Pd(II)species are highly relevant to the catalytic performance for glycerol oxidation (Fig.S1),while more high valence Pd(II)species were distributed on the Pd/HAP-NO catalyst (Table 2).On the other hand,the basic sites on the HAP are crucial and were conducive to the adsorption of glycerol on the catalyst surface,which are responsible for the superior catalytic performance on Pd/HAPNO catalyst,as compared with Pd/C catalysts.Furthermore,the Pd species are highly dispersed on the Pd/HAP-NO catalyst (Table 1 and Fig.3),which would favor for molecular oxygen activation and sequentially glycerol oxidation.On the basis of the above results,Pd/HAP-NO is employed as the catalyst for subsequent investigation.
Further catalytic assessment of Pd/HAP-NO is performed for aerobic oxidation of glycerol with dioxygen by systematically exploring the influence of various reaction parameters,as shown in Fig.7.It can be seen that as the reaction temperature varies from 40 to 120°C,the conversion of glycerol and the GLA selectivity increases significantly,accompanying with the formation of a trace GA and FA (Fig.7a).However,the GLA selectivity affords an optimum around 80°C,and then decreases remarkably with higher temperature.This phenomenon can be interpreted that the appropriate reaction temperature is crucial for the formation of major product GLA,and otherwise the product could decompose to low carbon by-products (GA and FA) under the higher temperature.Subsequently,the effect of reaction time on conversion of glycerol has been examined.As shown in Fig.7b,the conversion increases with reaction time,and 59.3% conversion and 90.3% selectivity of GLA were obtained after 5 h.However,the GLA selectivity declines slightly with the prolonged reaction time due to the slight decomposition of GLA.Noticeably,the oxygen pressure has a positive effect on conversion of glycerol then reaches a plateau at 1 MPa,and GLA selectivity shows a distinct increase but level off if oxygen pressure is more than 1 MPa (Fig.7c).Additionally,the amount of NaOH also affects the oxidation activity of glycerol.As shown in Fig.7d,the conversion of glycerol increases gradually but the selectivity to GLA drops down when the dosage of NaOH was more than 1 eq.It should be noting that glycerol would be subjected to lower conversion if less amount of NaOH was used.It indicates that the presence of base significantly improves the catalytic activity,but excessive alkali can result in C-C bond cleavage via retro-aldol reaction[56].Additionally,the influence of the amount of catalyst is also tested(Fig.S2).The enhancement of glycerol conversion as the amount of catalyst increases.When the amount of catalyst increases to 0.03 g,the maximum glycerol conversion and higher selectivity towards GLA are observed.With a further increase of the catalyst amount,the conversion of glycerol is almost unchanged and but the selectivity towards GLA decreases slightly due to the oxidative cleavage of C-C bond.On the other hand,as shown in Fig.S3,1%Pd/HAP-NO catalyst shows higher GLA yield than that of 0.5%Pd/HAPNO,mostly due to its higher density of Pd sites.However,further increasing the Pd loadings would lead to a minor decrease of GLA yield attributing to the over-oxidation.Finally,under the optimal reaction condition as shown above,the different Pd-derived catalysts are compared for glycerol oxidation.It can be seen that Pd/HAP-NO indeed proves its superiority among the used catalysts (Fig.S4).
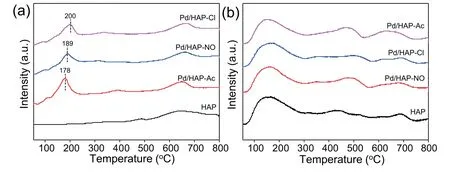
Fig.5.(a) H2-TPR and (b) CO2-TPD profiles of HAP and Pd/HAP-x catalysts.
3.3.The crucial role of vicinal hydroxyl group in the oxidation of glycerol
Next,the different alcohols including 1-propanol,1-butanol,1,3-propanediol,ethylene glycol and 1,2-propanediol as substrates are subjected to the similar oxidation condition to identify the key role of the vicinal hydroxyl group (Table 4).Unfortunately,either monohydroxy alcohols (1-propanol,1-butanol) or dihydroxy alcohol (1,3-propanediol) proves to be poor substrates,and no oxidation reaction occurs(entries 1-3),which is actually consistent with the previous studies [57].Next,the catalyst is applicable to the oxidation of the other vicinal diols like ethylene glycol and 1,2-propanediol.Notably,it is found that the ethylene glycol is a reactive substrate and oxidized selectively into the cor111responding glycolic acid with selectivity of 86.3% (entry 4).The catalyst also affords lactic acid as the major product with 75.3%selectivity for oxidation of 1,2-propanediol along with a small amount of GA and FA,arising from C-C bond scission(entry 5).

Table 2 The basicity and chemical state of Pd species on HAP and Pd/HAP-x catalysts.
Based on the results above,it can be seen clearly that the vicinal diols help to be activated on the catalyst surface,thereby promoting the selective oxidation reaction to produce cor111responding acids.The adsorption structure of reactant on the surface is crucial for the reaction route and product selectivity.To identify the adsorption state of the hydroxyl group on catalyst,FT-IR spectra of glycerol adsorbed over different catalysts are recorded and the results are shown in Fig.8.For pure glycerol,two intensive peaks at 1088 and 1048 cm-1can be attributed to the C-O vibrations of secondary and primary hydroxyl groups of glycerol,respectively(Fig.8a) [58].After the glycerol adsorbed on HAP,the cor111responding C-O band are shifted to 1127 and 1070 cm-1,which are assignable to the strong interaction between both the hydroxyl groups of glycerol and surface Ca2+sites on the HAP support,indicating strong surface adsorption of glycerol(Fig.8c).However,a series of Pd supported catalysts only weakly change the adsorption state of primary C-O band but have no effect on the absorption of secondary C-O band(Fig.8d-f),in comparison with that of HAP.However,only a weak blue shift of glycerol absorbed on the 4%OA-Pd/HAONO is observed (Fig.8a and b),suggesting clearly that the poisoning of Ca2+sites by oxalic acid would decrease obviously the interaction of glycerol with Ca2+sites.Furthermore,the poisoning of Ca2+sites also results in a sharp decrease of the catalytic activity(Table 3,entry 8 vs.11).The above results suggest that surface Ca2+sites on the catalyst are extremely important and can greatly promote the adsorption of glycerol and activate hydroxyl group for the oxidation of glycerol.
According to the findings in our present work,the possible reaction mechanism over the present Pd catalyst in an alkaline solution is proposed as follows,involving molecular oxygen activation,oxidative dehydrogenation of glycerol and the removal of the protons by active oxygen species to generate GLA as a product (Scheme 1).In the first step,as discussed above,glycerol is absorbed on the surface of catalyst (species I),along with the coordination of the primary and secondary hydroxyl group with Ca2+sites as reflecting from glycerol absorbed FT-IR spectra on the catalyst,in which the O-H bond of the hydroxyl group becomes lengthened and weakened.Meantime,the primary hydroxyl group of glycerol molecule is deprotonated with the help of base (OH-) and form alkoxide (II),then a H2O molecule is released simultaneously.
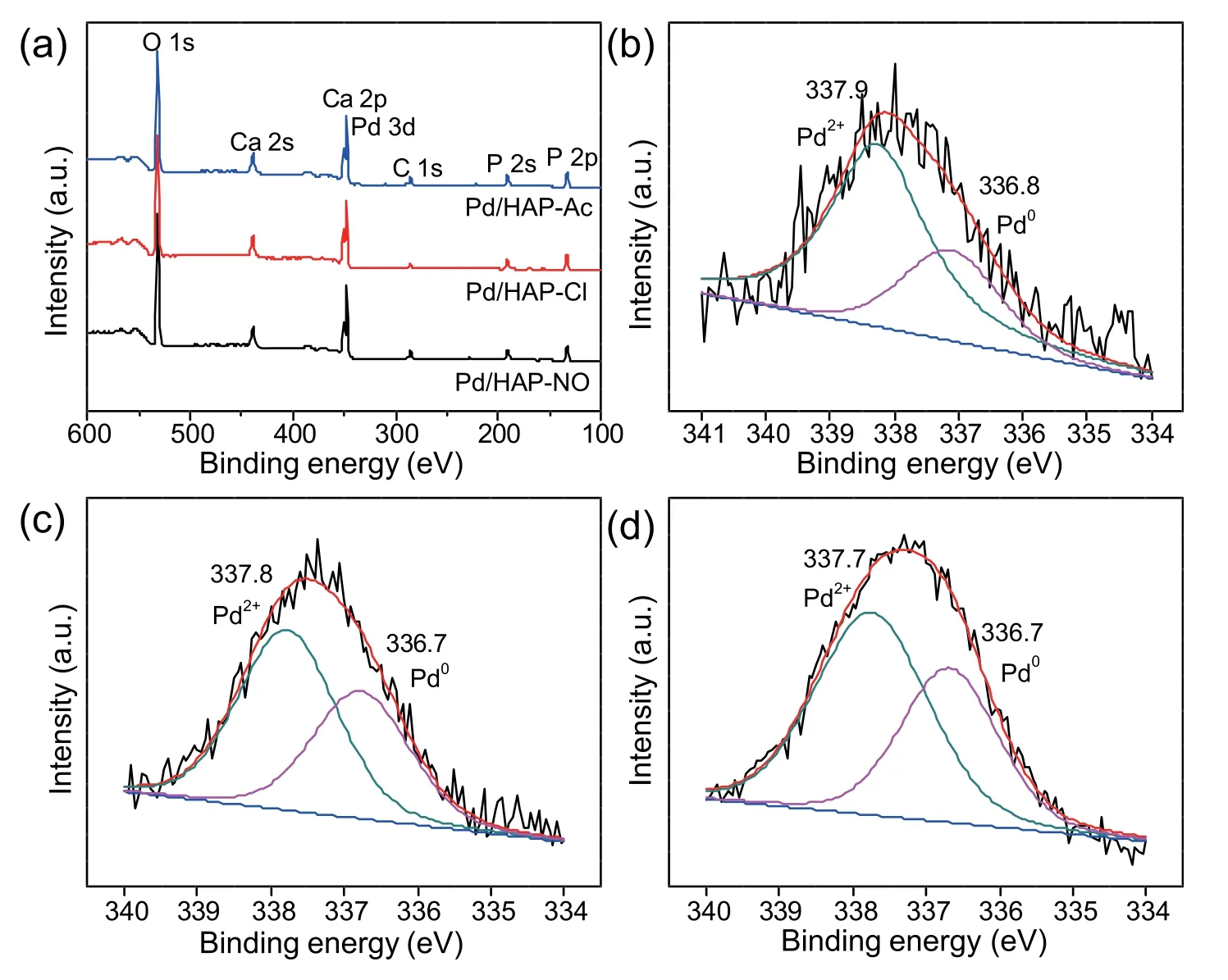
Fig.6.(a) Survey and (b) Pd 3d5/2 XPS spectra of Pd/HAP-NO,(c) Pd/HAP-Cl and (d) Pd/HAP-Ac.
As discussed above (Table 3),O2is an indispensable component in this reaction (Table 3,entries 3,5 and 8),suggesting that molecular oxygen could be activated by Pd (0)species to afford peroxypalladacycle intermediate species(III)via oxidative addition [59].Meantime,Pd(II) species are responsible for the deprotonation of β-hydrogen in the oxidation of alcohols,which may be a rate determining step.Here,the electron transfer from the Pd(0)species to adsorbed oxygen can not only promote the activation of molecular oxygen but also facilitate the formation of metal-hydride because of the nature of β-hydrogen with partial negative charge in the alkoxy intermediate[60].Thus,the generation of intermediate(III) is suggested in our Pd/HAP-NO catalytic system.Then,eliminating β-hydrogen in metal-hydride may produce glyceraldehyde intermediate (IV),while the eliminated hydrogen can be captured by surface active oxygen species -O-O-get OOH-intermediate,then react with H2O to produce H2O2species [15],which have been confirmed to be present by the immediate potential difference titration of Ce3+/Ce4+after reaction.
Next,after the forming glyceraldehyde is attacked by H2O to afford intermediate geminal diol (species V),which interacts further with adjacent Pd(II)species to generate a metalhydride through the H-metal bond,while molecular oxygen can be activated on Pd species in a similar way as the last step.Finally,active oxygen species can couple with hydrogen adsorbed on the surface of catalyst and basic sites (such as O2-,as shown in the intermediate IV species) to produce H2O2and generate GLA product.To understand this step more clearly,the study on kinetic isotope effect (KIE) is further carried out for the oxidation of glycerol in different solvents(H2O and D2O).As shown in Fig.S5,a linear relationship is obtained between ln (CA0/CA) and reaction time,where CArepresents the concentration of glycerol at a set time and CA0is the initial concentration,respectively.The results demonstrate the first order reaction with respect to glycerol consumption.The rate constants measured with H2O and D2O are kH=0.1144 h-1and kD=0.0922 h-1,respectively,giving a KIE of kH/kD=1.24.It is likely that hydrogen and deuterium may be exchanged between water and the hydroxyl group that cannot be eliminated,but it remains plausible that the O-H activation energy could affect the rate.This indicates that the H2O involved reaction could not the rate-determining over Pd/HAP-NO catalyst,and the β-hydrogen abstraction (III to IV,V to GLA in Scheme 1)could be proposed as the key step for glycerol oxidation [61,62].
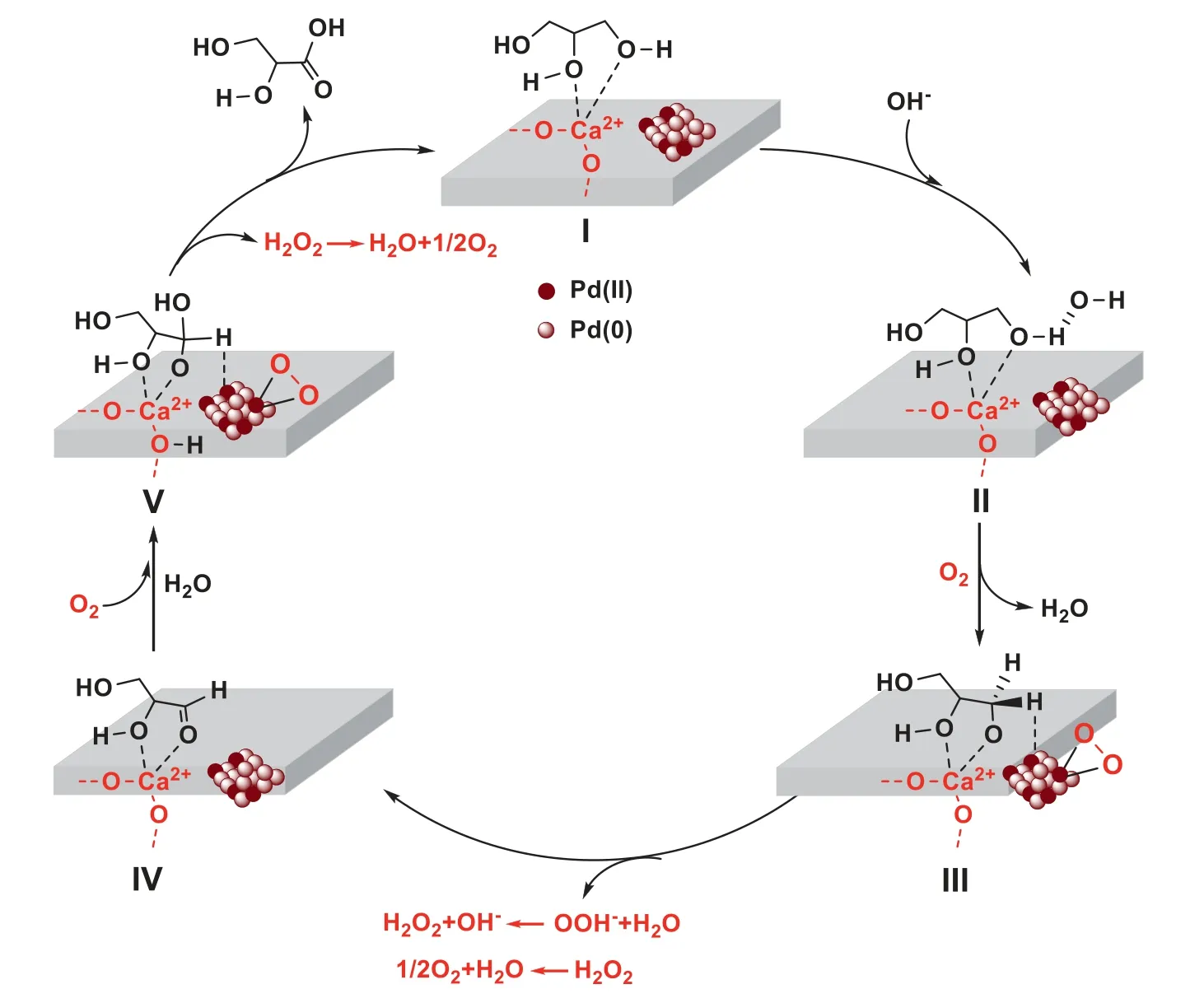
Scheme 1.Possible reaction mechanism for selective oxidation of glycerol to glyceric acid.
To identify the role of H2O in the reaction,the oxidation of glycerol over Pd/HAP-NO is performed usingO as solvent.In the presence ofO,after reaction,the mass spectrum of the products shows that one or two18O atoms are incorporated into the molecules of glyceric acid (Fig.S6).Based on the above labeling experiments and the poor oxidation activity under solvent-free condition (Table 3,entry 9),H2O not only acts as solvent but involves in the catalytic process.
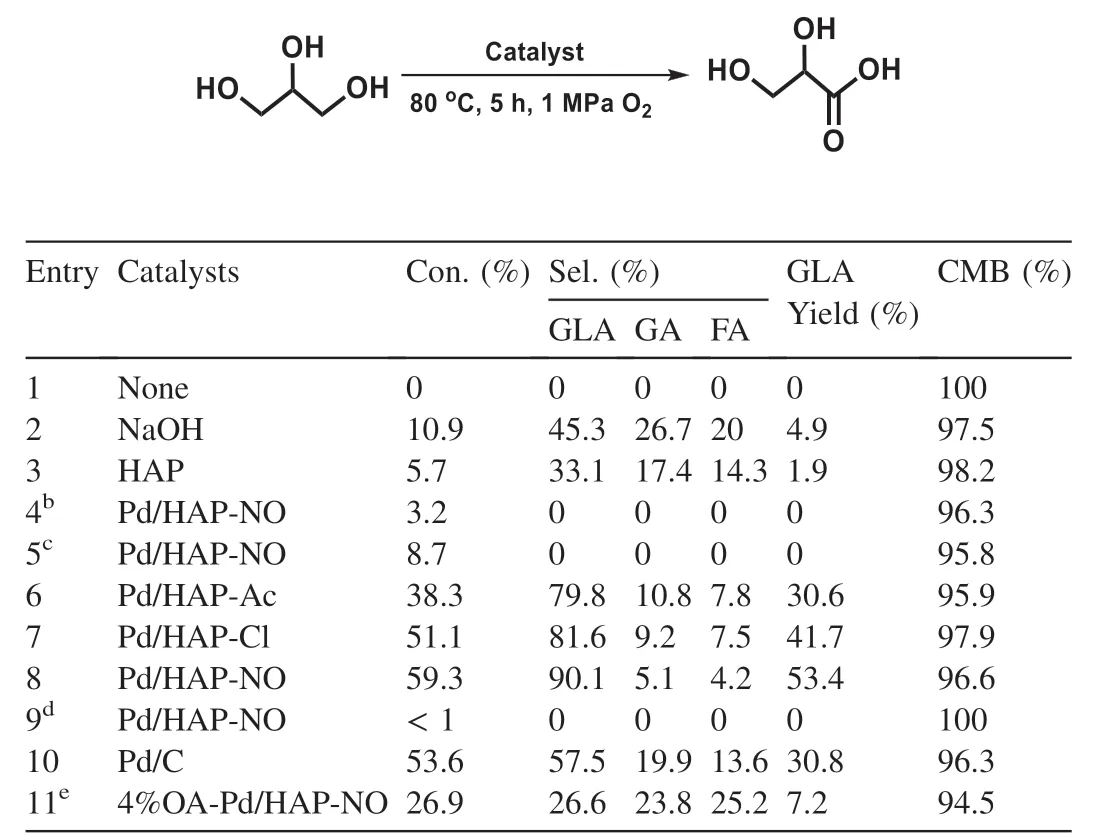
Table 3 Catalytic performance of various catalysts for selective oxidation of glycerol.a
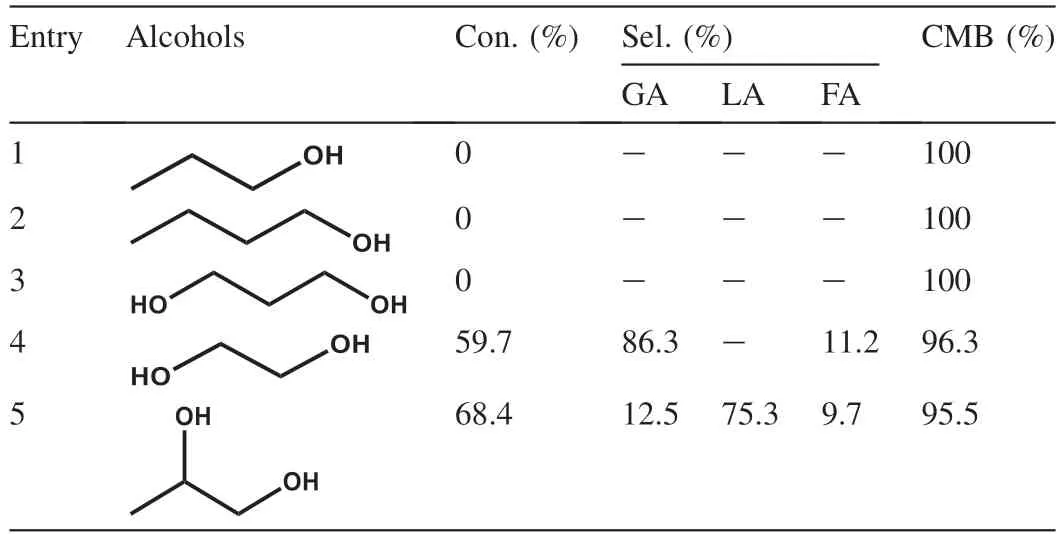
Table 4 Catalytic activity for selective oxidation of various alcohols by using Pd/HAPNO.a
In the selective oxidation of glycerol,a trace of GA and FA has been detected likely originating from retro-aldol condensation reaction under alkaline conditions.The whole process demonstrates no substantial accumulation of the intermediates,which accounts for their absence in the course of reaction,especially for glyceraldehyde.In the mechanism,Pd (0)species are responsible for molecular O2activation to afford active peroxy palladacycle species,while Pd(II)species play a crucial role in facilitating hydrogen abstraction by the β-carbon atom owing to the partial negative charge β-hydrogen,and thus the Pd(II) species are strongly related to glycerol oxidation on the present catalyst.
3.4.Catalyst recycling
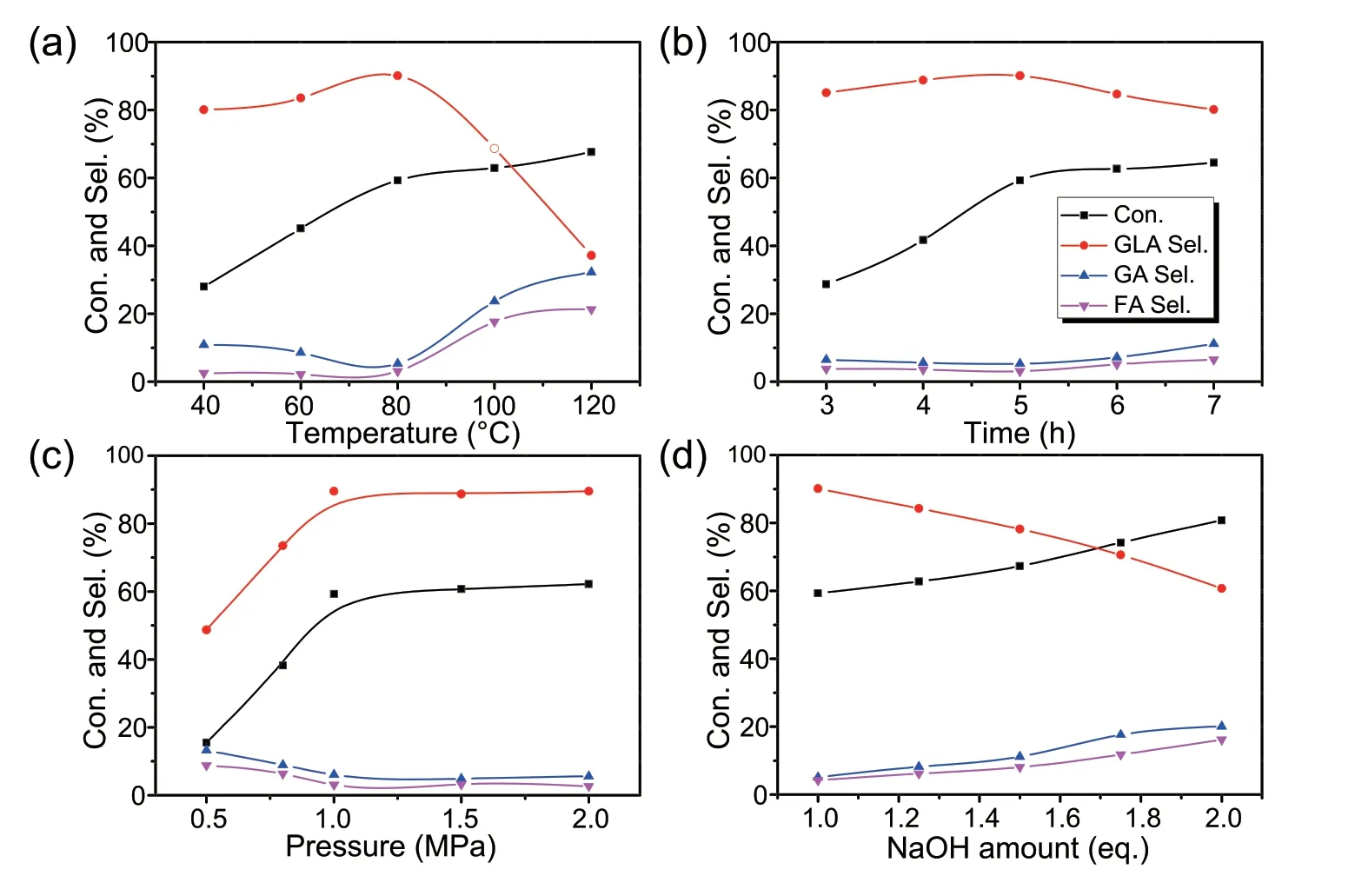
Fig.7.Influence of(a)reaction temperature,(b)reaction time,(c)pressure and(d)NaOH amounts on the selective oxidation reaction of glycerol on Pd/HAP-NO catalyst.Reaction conditions: 5.5 mmol glycerol,4.5 mL H2O,1 eq.NaOH,0.03 g catalyst,80°C,5 h,1 MPa O2.For each figure,there is a specific parameter changed.
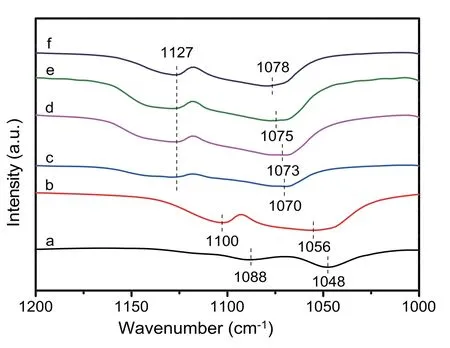
Fig.8.FT-IR spectra of(a)pure glycerol and the adsorption states of glycerol over(b)4%OA-Pd/HAP-NO,(c)HAP,(d)Pd/HAP-Ac,(e)Pd/HAP-Cl and(f)Pd/HAP-NO.
The recycling of the Pd/HAP-NO catalyst has been performed for five runs to study the catalytic stability.As shown in Fig.9,it is clearly observed that no apparent deactivation of Pd/HAP-NO in the GLA yield,indicating the good recyclability in the consecutive catalytic recycles.The XRD pattern of the spent Pd/HAP-NO (Pd/HAP-NO-R) after the 5th run is shown in Fig.S7,which suggests that the apatite structure of HAP is well maintained.Moreover,from the HRTEM image of the Pd/HAP-NO-R catalyst(Fig.S8),it is found that there is no noticeable change in morphology,although a slight aggregation of Pd species occurred.Actually,the BET surface area of Pd/HAP-NO-R shows almost the same as that of the fresh catalyst,revealing that the porous structure of the catalyst is highly stable and do not collapse even under the hydrothermal reaction conditions in spite of a minor decrease of the pore size (Table 1).The Pd content of Pd/HAP-NO-R sample keeps almost the same as that of the fresh catalyst(1.03 wt%).This suggests that Pd leaching from Pd/HAP-NOR is negligible after the catalytic recycles.Therefore,the asobtained Pd/HAP-NO catalyst has been proved as an efficiently heterogeneous catalyst in the selective oxidation of the glycerol into GLA.Finally,the chemical state of Pd species on the Pd/HAP-NO-R is also investigated by XPS (Fig.S9).It indicates that the Pd(II) species seem to suffer from decrease from 71.3% to 56.2% during its recycling process,indicating that the partial Pd(II) species might be reduced by surface active hydrogen species,but the catalyst can still maintain stable GLA yield.The excellent recyclability of the Pd/HAPNO catalyst clearly arises from the preservation of apatite framework sufficiently.On one hand,the carboxylic acid products are consumed by the added base to form cor111responding salts,thus preventing the collapse of the skeleton of HAP in the alkaline media,and the framework related Pd (0)species enable to activate molecular oxygen.On the other hand,the Pd(II) species are still present in spite of the partial reduction in the catalytic run.Overall,the HAP support not only retains the oxidation activity of Pd species but also enhances the stability due to the strong alkali resistance.
Finally,the catalytic performance of the present catalyst has been compared with that of other earlier report in the oxidation of glycerol to glyceric acid.As shown in Table S1,the efficient conversion usually can be achieved with a molar ratio of NaOH to glycerol above 4.Only a few reports were related to conversion at a lower molar ratio of NaOH to glycerol,and these reactions were normally carried out at low glycerol concentration.Although the present Pd/HAP-NO catalyst could afford lower conversion of glycerol than some of the reported systems,the catalyst exhibits great advantages of excellent selectivity to GLA,high concentration of glycerol in aqueous phase and low molar ratio of NaOH to glycerol in the reaction system.In particular,the present catalytic system can also be extended to the selective oxidation of the other vicinal diols into cor111responding α-hydroxycarboxylic acids.
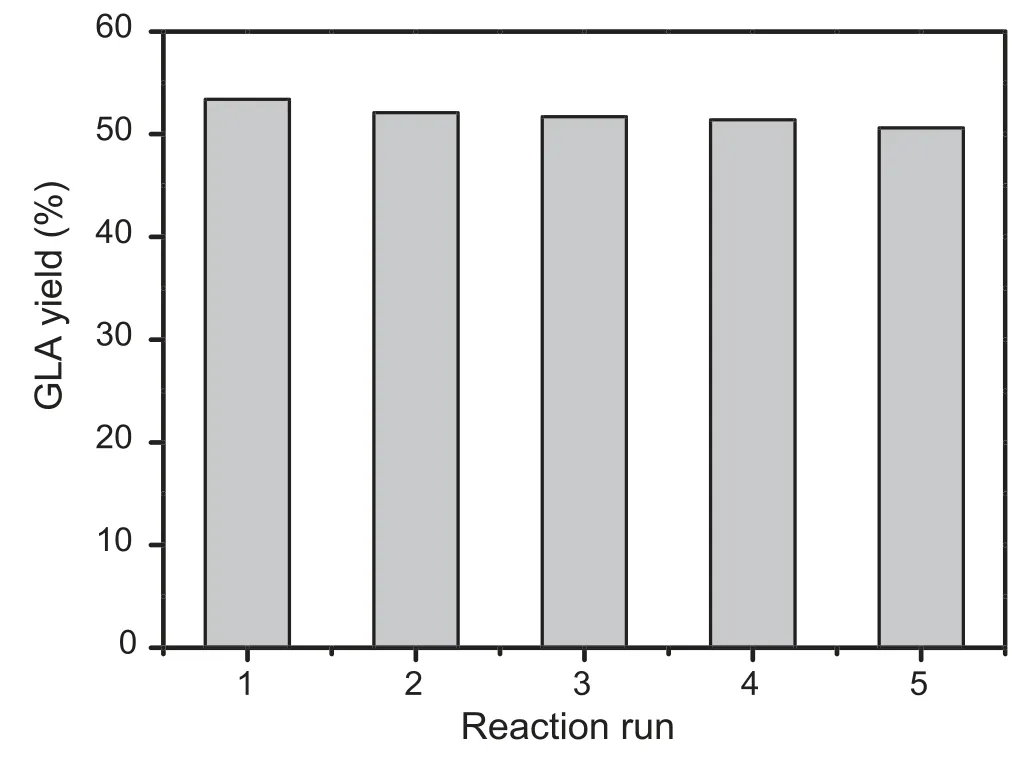
Fig.9.Catalytic recyclability over Pd/HAP-NO for selective oxidation of glycerol.Reaction conditions: 5.5 mmol glycerol,4.5 mL H2O,1 eq.NaOH,0.03 g Pd/HAP-NO,80°C,5 h,1 MPa O2.
4.Conclusions
Glycerol oxidation promoted by HAP-supported Pd catalysts combined with O2under alkaline aqueous solution condition was developed.This methodology provides an efficient heterogeneous catalyst for the selective oxidation of glycerol to GLA under very mild reaction conditions,affording a high GLA selectivity and promising reusability.As revealed by characterization of the catalyst,the excellent performance results from the strong surface basicity of catalysts and highly dispersed Pd(II) and Pd (0) species on HAP,which resists the over-oxidation or C-C cleavage of glycerol and thus enhances the selectivity to GLA.The stabilization of Pd catalysts caused by the strong interaction between active Pd species and surface basic sites of HAP.Notably,a coordination between the vicinal hydroxyl group and Ca2+sites in the HAP framework plays a crucial role in glycerol activation,where the secondary hydroxyl group of glycerol kept untouched while the primary hydroxyl of glycerol is converted into carboxyl group,offering an excellent selectivity to αhydroxycarboxylic acid from the vicinal diols.H2O not only played a crucial role in acting as a solvent,but also was involved in oxidation reaction.This study would afford a versatile route toward selective oxidation of bio-derived polyols,which can be further extended to a wide range of applications that entail catalytic biomass upgrading.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are grateful for support from the National Natural Science Foundation of China (21773061,21978095),Innovation Program of Shanghai Municipal Education Commission (15ZZ031),and the Fundamental Research Funds for the Central Universities.
Appendix ASupplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gee.2020.11.018.
 Green Energy & Environment2022年4期
Green Energy & Environment2022年4期
- Green Energy & Environment的其它文章
- Multivariate MOF for optimizing atmospheric water harvesting
- Lignin-based carbon fibers: Formation,modification and potential applications
- Charactering and optimizing cathode electrolytes interface for advanced rechargeable batteries: Promises and challenges
- Metal-organic frameworks-derived metal phosphides forelectrochemistry application
- Surface-mediated iron on porous cobalt oxide with high energy state for efficient water oxidation electrocatalysis
- Oxygen-deficient SnO2 nanoparticles with ultrathin carbon shell for efficient electrocatalytic N2 reduction
