Design and in-situ construct BiOI/Bi/TiO2 photocatalysts with metal-mediated heterostructures employing oxygen vacancies in TiO2 nanosheets
Chenchen Zhng,Wenbin Chen,Dirong Hu,Hnjie Xie,Yibing Song,,Binbin Luo,Yiwen Fng,Wenhu Go,Ziyi Zhong,
a Department of Chemistry,Shantou University,Guangdong,515063,China
b Department of Chemical Engineering,Guangdong Technion-Israel Institute of Technology (GTIIT),Guangdong,515063,China
c Technion-Israel Institute of Technology (IIT),Haifa,32000,Israel
Abstract The conventional p-n heterojunction photocatalysts suffer from the incompatibility between the interfacial charge transfer efficiency and the redox ability of charge carriers.To optimize the interfacial charge transfer of the conventional BiOI/TiO2 p-n photocatalyst,we synthesized the BiOI/Bi/TiO2 ternary photocatalyst with sandwiched metallic bismuth (Bi0) by the oxygen-vacancy assisted method.The DFT calculation and structural characterizations confirmed the reaction of the electron-rich oxygen vacancies in the 2D-TiO2 nanosheets(TiO2-NS)with the adsorbed BiO+ species.This reaction broke the Bi-O bonds to form Bi0 nanoparticles in-situ at the interface but still maintained the p-n heterojunction well.The NO-TPD and XRD analyses for samples correlated the Bi0 formation with the oxygen vacancy concentrations well.The sandwiched Bi0 functioned as an electronic transfer mediator like that in the Z-scheme heterostructure.Comparing with 0.20 BiOI/TiO2-NP (NP,Nanoparticles),0.20 BiOI/Bi/TiO2-NS-a (NS,Nanosheet) showed a much improved catalytic performance,i.e.,duplicated apparent quantum yield(AQY) and triplicated reaction rate constant (k).Also,the formation mechanism and the reaction mechanism were investigated in detail.This work provides a new strategy for the improving of the conventional p-n photocatalysts and new insights into the nature of the photocatalysis.© 2020 Institute of Process Engineering,Chinese Academy of Sciences.Publishing services by Elsevier B.V.on behalf of KeAi Communications Co.,Ltd.This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Keywords: TiO2 nanosheets;Oxygen vacancy;Metallic bismuth;Sandwiched heterostructure;Photocatalysis
1.Introduction
The construction of p-n heterojunction can effectively extend the light absorption range and spatially separate of the photogenerated charge carriers in photocatalysts because the p-n heterojunction possesses a built-in electric field and the energy-band regulation effect caused by the Fermi level alignment [1-5].However,while this hybrid structure gains the above advantages,it unavoidably sacrifices the redox ability of the photogenerated charge carriers,which leads to a weaker driving force that may fail to drive a specific photocatalytic reaction.To overcome this problem,in 1979,Bard et al.proposed the Z-scheme photocatalytic concept to maximize the redox ability of the heterojunction systems.The Z-scheme photocatalyst consists of two different semiconductors (PS I and PS II) and an acceptor/donor (A/D) pair[6].Since then,Z-scheme photocatalysts have attracted considerable attention and have been developed continuously.In 2006,Tada et al.proposed the concept of an all-solid-state Z-scheme photocatalyst,which replaced the A/D pair with a solid electron-mediator (such as Pt,Ag,and Au) [7].In 2013,Yu et al.reported a direct Z-scheme heterojunction photocatalyst,which consisted of two semiconductors with matching band structures but without the use of an electron mediator between them [8].The excellent photocatalytic performance of Z-scheme photocatalysts is attributed to the four main features: (1) preservation of useful photogenerated electrons and holes with high redox ability,(2) separation of the reductive and oxidative active sites,(3)advantage of using two semiconductors and (4) enhancement of the light response.However,there are considerable challenges for the facile,efficient,economic and large-scale preparation of high-quality Z-scheme heterojunction photocatalysts for practical applications.For the traditional Z-scheme photocatalysts,A/D pairs can be operated only in the solution and are pH-sensitive,limiting their use in the gas-solid photocatalytic reactions[9].The introduction of interfacial noble metals increases the process difficulty and the fabrication cost for the all-solid-state Z-scheme photocatalysts [10].Two semiconductors with appropriate band structures are a prerequisite for successfully applying the direct Z-scheme heterojunction photocatalysts,which greatly limits its diversity and availability [11].
Many research groups have tried to design the interface structure of conventional p-n heterojunctions to make the separation efficiency and redox ability of charge carriers compatible.For example,Pang et al.reported an illuminationassisted process to induce uniform p-type semiconductor Bi2O3nanocrystals on carbon bridged n-type semiconductor TiO2nanotube arrays,in which the carbon could trap the electrons.The carbon-bridged Bi2O3/TiO2nanotube arrays changed the electron transfer mechanism from p-n heterojunction photocatalyst to Z-scheme photocatalyst and exhibited a highly selective photoelectrochemical determination performance [12].Yin et al.demonstrated that the embedding of metal Pd into the interface between n-type C3N4and p-type Cu2O could further enhance the interfacial charge transfer and increase the redox ability of charge carriers through the design of C3N4-Pd-Cu2O stack nanostructure.Enabled by this unique design,the achieved hydrogen evolution is dramatically higher than that of its counterpart C3N4-Cu2O structure without Pd embedding [13].These studies highlight the importance of introducing a new conductive component into the p-n heterojunction interface to improve the interfacial charge transfer.In most studied cases,the new conductive element is not derived from the two semiconductors that make up the p-n heterojunction,but noble metals and carbon-containing materials,which will weaken the interfacial coupling between the components,and increase the process difficulty and the fabrication cost [14-16].To enhance the interfacial coupling between the p-n semiconductors,forming a conductive component layer or particles at the heterojunction interface directly from the two contacted semiconductors probably should be a practical solution or preferred option.So far,there are no literature on how to in-situ develop a conductive component from the original p-n components at the heterojunction interface.
Due to the unique photoelectric properties and abundant surface defects on the high-exposed {001} facets,twodimensional (2D) TiO2nanosheets are an ideal candidate supports [17-23].For instance,Liu et al.used the ultra-thin TiO2nanosheets with ample oxygen vacancies as the substrate to support and stabilize well-dispersed Pt0nanoparticles (ca.1.1 nm) [24].Wan et al.reported a method for stabilizing single Au atoms by generating defects on the surface of supports [25].Tian et al.used the Ti3C2MXene as a titanium source to construct a variety of photocatalysts for enhanced photocatalytic H2production over the TiO2nanosheets [26-29].On the other hand,BiOI is a visible-light-driven p-type semiconductor with a unique anisotropic layered structure,in which the [Bi2O2] slabs have weak Bi-O bonds and the two iodine atoms are connected by Van der Waals force [30,31].These characteristics make it easily reducible and promising for constructing photocatalysts with the defected TiO2nanosheets to generate specific and advanced heterostructure at the interface.For example,the generation of Bi0islands at the interface may act as an effective conductive mediator to promote interfacial electron transfer [32,33].
In this work,we first conducted a theoretical calculation to confirm the feasibility of the metallic Bi0formation by employing the reducing function of the oxygen vacancies in TiO2nanosheets,then in-situ constructed the BiOI/Bi/TiO2-NS ternary photocatalyst with a sandwiched heterostructure.This BiOI/Bi/TiO2ternary photocatalyst possesses the functions of the p-n junction for energy-band manipulation and of the Z-scheme photocatalyst for photogenerated charge carrier separation and high redox capability.When evaluated in photodegradation of RhB in water,it showed much enhanced catalytic activity and AQY as compared with the BiOI/TiO2binary catalyst with the same Bi/Ti molar ratio.To our best knowledge,this simple and feasible approach for the in-situ construction of the BiOI/Bi/TiO2ternary heterostructure by employing the abundant oxygen vacancies on TiO2nanosheets has not yet reported in the literature.This method changes the traditional BiOI/TiO2p-n heterostructure into the high-performance BiOI/Bi/TiO2photocatalyst with optimized interfacial charge dynamics.
2.Experimental
2.1.Materials
Titania P25 was purchased from Degussa,Hulls Corporation,Germany.Titanium dioxide (anatase TiO2,100 nm,>99%),titanium (IV) butoxide (TBT,>99%),bismuth nitrate pentahydrate (Bi(NO3)3·5H2O,99%),potassium iodide (KI,99%),sodium hydroxide (NaOH,96%),p-benzoquinone(C6H4O2,99%),Rhodamin B (RhB,99%),disodium ethylenediaminetetraacetate (EDTA-2Na,99%) were supplied by Adamas Reagent Co.,Ltd.(Shanghai,China).Hydrofluoric acid (HF,40 wt%),sulfuric acid (H2SO4,98%),hydrochloric acid (HCl,36-38%),anhydrous ethanol (C2H6O,99%),isopropanol (IPA,99%),ethylene glycol (EG,99%) were obtained from XiLong Chemical Co.,Ltd.(Shantou,China).All the materials were used without further purification.
2.2.Synthesis of TiO2 samples
The TiO2nanosheets (TiO2-NS) were prepared by a wet chemical method modified from the literature[34].In a typical preparation,25 mL of titanium (IV) butoxide was added dropwise into an aqueous solution of HF (15 mL,24 wt%)with magnetic stirring.Subsequently,the mixture was transferred into a 100 mL Teflon-lined autoclave and maintained at 240°C for 24 h.The resulting bluish paste was collected by filtration,washed thoroughly with deionized water,and dried at 80°C for 12 h.The obtained solid samples were further calcined in air or helium atmosphere at 500°C for 2 h to defluorinate,and then the final products were denoted as TiO2-NS-a or TiO2-NS-h,respectively.The commercially available anatase TiO2(100 nm) was denoted as TiO2-NP.TiO2nanobelts (TiO2-NB) and TiO2microspheres (TiO2-MS) were also synthesized by the hydrothermal method,and their preparations were summarized in the Supporting Information.
2.3.Preparation of heterostructure catalysts
BiOI/Bi/TiO2-NS with different Bi/Ti molar ratios (0.05:1,0.10:1,0.20:1 and 0.30:1,respectively)were synthesized by a solvothermal method.For example,the preparation procedures of 0.20:1 BiOI/Bi/TiO2-NS-a are as follows:2 mmol of TiO2-NS-a and 0.4 mmol of Bi(NO3)3·5H2O were dispersed in 20 mL of ethylene glycol,followed with the addition of 0.4 mmol of KI in ethylene glycol (20 mL) dropwise,and the slurry was continuously stirred for 60 min.The mixture was then transferred into a 100 mL Teflon-lined autoclave,sealed,and maintained at 160°C for 24 h.The precipitate was collected by centrifugation (8000 r min-1,15 min),washed thoroughly,and dried at 80°C for 12 h.For comparison,0.20 BiOI/TiO2-NP,0.20 BiOI/TiO2-NB,and 0.20 BiOI/TiO2-MS were prepared by the same method.
2.4.Evaluation of photocatalytic activity
The photocatalytic activities of the synthesized catalysts were evaluated in the photodegradation of RhB in an aqueous solution.A 300 W Xe lamp was used as the light source.All experimental procedures were kept the same except for using different catalysts.10 mg of catalyst was suspended in 50 mL of RhB (20 mg L-1) aqueous solution treated with ultrasonic irradiation for 15 min to form a homogeneous mixture.Then the suspension was stirred in the dark for 90 min to reach the adsorption/desorption equilibrium.After that,the reaction mixture was exposed to the light irradiation using 300 W Xe lamp.During the reaction,a small portion of the suspensions was taken from the reactor at the same time interval,and the catalyst was removed from the suspensions by centrifugation.Finally,the change in the concentration of RhB with irradiation time was monitored by UV-vis spectrophotometer.
The calculation of the apparent quantum yields(AQY)was determined using the equation: AQY (%)=[the number of degraded RhB (mol)][the number of photons entered into the reaction cell (mol)]-1× 100%.The number of photons entered into the reaction cell was determined by a chemical actinometry employing potassium ferrioxalate under the monochromatic light (436 nm) irradiation (please see details in Supporting Information).
2.5.Radical scavenging experiment
To know the primary reactive species participating in the photodegradation of RhB,we employed isopropanol (IPA,5 mL),benzoquinone (BQ,0.5 g) and ethylene diamine tetraacetic acid (EDTA-2Na,1 g) for scavenging hydroxyl radicals (·OH),superoxide radicals (·) and photogenerated holes (h+),respectively.The functions of different radical species in the photocatalytic process were confirmed based on their impacts on photocatalytic efficiency.
2.6.Photoelectrochemical measurements
3.0 mg of as-prepared samples were uniformly mixed with 1 mL of deionized water,and drop 10 μL onto a 3 cm×1 cm indium tin oxide(ITO)glass.Subsequently,the ITO glass was dried at 60°C for 6 h in a vacuum.The photoelectrochemical measurements were performed on a Zahner pp211 electrochemical station (Zahner Germany) at room temperature.435±7 nm monochromatic light was used as the illumination source.A power density of 180 W m-2was measured for the monochromatic light.A standard three-electrode system was used,consisting of the ITO glass as photoelectrode,an Ag/AgCl electrode as reference electrode,and a Pt foil as counter electrode in a quartz cell containing 0.1 mol L-1PBS electrolyte.The photocurrent density vs.time (I-t) curves of the prepared photoelectrodes were measured at an applied potential of 0 V vs.Ag/AgCl under chopped light irradiation(light on/off cycles: 20 s) for 80 s.
2.7.The DFT calculations
The first-principle calculations were performed with the projector augmented wave (PAW) method based on the DFT[35].The exchange-functional was treated using the generalized gradient approximation (GGA) of Perdew-Burke-Ernzerhof(PBE)functional.The cut-off energy of the plane-wave basis was set at 450 eV for optimizing the calculations of the atoms and cells.The vacuum spacing in a direction perpendicular to the plane of the catalyst was at least 10 Å.The Brillouin zone integration was performed using 3 × 3 × 2 Monkhorst and Pack k-point sampling for a surface and interface structure.The self-consistent calculations apply a convergence energy threshold of 10-5eV.The maximum Hellmann-Feynman force for each ionic optimization step is 0.05 eV Å-1.In addition,spin polarization is considered in all calculations.The reaction energy is calculated by the ΔE=Eproduct-Ereaction,in which the Eproductis the energy of the product,and the Ereactionis the reaction energy.
2.8.Characterization
X-ray diffraction (XRD) experiments were carried out using a Bruker D8 Advance X-ray diffractometer (Cu Kα).The optical properties of the samples were characterized by a Lambda 950 UV-vis Spectrometer in which BaSO4was employed as the internal reflectance standard.The morphology and microscopic structure of samples were measured using a field emission scanning electron microscopy(FE-SEM,Gemini 300) and a transmission electron microscope (TEM,JEOL JEM-2100f),respectively.The oxidation states of Ti,O,Bi,and I atoms were determined by X-ray photoelectron spectroscopy (XPS) using a Thermo ESCA Lab250Xi spectrometer (Al Kα).All peak positions were calibrated with the C 1s peak (284.8 eV).The photoluminescence (PL) spectra of the samples were recorded on a Hitachi F-7000 spectrophotometer,and the excitation wavelength was set at 320 nm.NO temperature-programmed desorption (NO-TPD) was performed on AMI-300 equipped with a TCD detector,using Helium(>99.999%)as carrier gas with a flow rate of 30 cc min-1.In a typical experiment,100 mg of TiO2powder was pretreated in the Helium flow at 300°C for 120 min.After cooling to 50°C,the NO/He gas(5%) was introduced in the system to reach adsorption saturation.Next,to remove the weakly absorbed NO,the NOTiO2sample was purged with the Helium flow at 80°C for 30 min,and then heated up to 900°C with a nominal heating rate of 10°C min-1for desorption of NO.
3.Results and discussion
3.1.Theoretical calculation analysis and construction of the heterostructure
It is reported that prototypical TiO2nanosheets have a large number of oxygen vacancies on the high-exposed{001}facets[36,37],and the bismuth (III) ions are readily hydrolyzed to form BiO+species.We can probably prepare a BiOI/Bi/TiO2ternary heterostructure through the interaction of the electronrich oxygen vacancies with the easily reducible BiO+to form the Bi0nanoparticles in-situ at the interface of the p-n heterojunction.To verify the rationality and feasibility of this preparation approach,at first,we theoretically modeled the adsorption and reaction of BiO+on the oxygen vacancies in the TiO2{001}facets(Fig.1a).In the DOS plot(Fig.1d),the oxygen vacancies in TiO2nanosheets interact with BiO+to form the defect resonance peaks and have a broad range of common peaks in the valence band,indicating that there is an electron interaction between them.Furthermore,the discrete occupied spin gap states and the conduction band states of BiO+-defected TiO2{001} slab model are significantly less than that of the defected TiO2{001} slab model (Fig.1c-d),and the charge density difference calculation suggests the bonding BiO+can accumulate electrons from the localized electrons of two unsaturated Ti atoms near the oxygen vacancies of the defected TiO2{001} slab model (Fig.1b).The minimum reaction energy of the breaking of Bi-O bond on defected TiO2{001} slab model is -0.37 eV (Fig.1e),which means that the interface between the defected TiO2{001}facets and the BiO+is unstable,and a spontaneous reaction of them can take place to form Bi0.
Recognizing the crucial role of oxygen vacancies in the formation of Bi0,we used three different TiO2supports(commercial TiO2-NP,synthesized TiO2-NS-a,and TiO2-NSh) and employed the NO-TPD technique to determine their oxygen vacancy concentrations.Then,we prepared the composite catalysts with these supports according to the scheme displayed in Fig.1h.It is reported that two NO molecules(Osurf-N) captured by oxygen vacancies could be decomposed into N2via surface vacancy diffusion mechanism at high temperatures;thus,the area of desorption peak can be used to evaluate the oxygen vacancies concentration [38-40].As shown in Fig.1f,the area of desorption peak follows the order of TiO2-NP<TiO2-NS-a<TiO2-NS-h,suggesting that the concentration of oxygen vacancies in the TiO2nanosheets with larger specific surface area is higher than that in the TiO2nanoparticles,and more oxygen vacancies can be generated under harsh treatment conditions,i.e.,in a Helium flow at 500°C.The XRD patterns (Fig.1g) reveal that all the TiO2supports are anatase phase (JCPDS file no.21-1272),indicating the formation of the oxygen vacancies in supports has no substantial effect on their crystal structures.For the composites,the two diffraction peaks at 29.3°and 31.6°can be ascribed to BiOI(JCPDS file no.06-0249).As evidenced,the additional characteristic peaks of Bi0(2θ=27.1°and 39.6°)along with BiOI are observed in the XRD patterns of 0.20 BiOI/Bi/TiO2-NS-a and 0.20 BiOI/Bi/TiO2-NS-h.The observed XRD characteristic peaks of Bi0in 0.20 BiOI/Bi/TiO2-NS-h are much stronger than those in 0.20 BiOI/Bi/TiO2-NS-a,suggesting the formation of more Bi0in the former.To determine whether the formation of Bi0is a general phenomenon in the coupling process of BiOI and TiO2,we further used the other TiO2supports with various morphologies to deposit BiOI (Fig.S1).The XRD results show that Bi0is not observed in the composites using TiO2-MS and TiO2-NB as the supports(Fig.S2).These results indicate that,at the interface,the formation of the Bi0is indeed dependent on the concentration of oxygen vacancies in TiO2supports.It should be emphasized that the synthesis conditions of 0.20 BiOI/TiO2-NP,0.20 BiOI/Bi/TiO2-NS-h and 0.20 BiOI/Bi/TiO2-NS-a,i.e.,solvothermal synthesis in ethylene glycol at 160°C,are identical,which excludes the possibility of the reduction of BiOI by the alcohol solvent at this reaction temperature.
3.2.Structural characterization of the catalysts,particularly the heterostructure
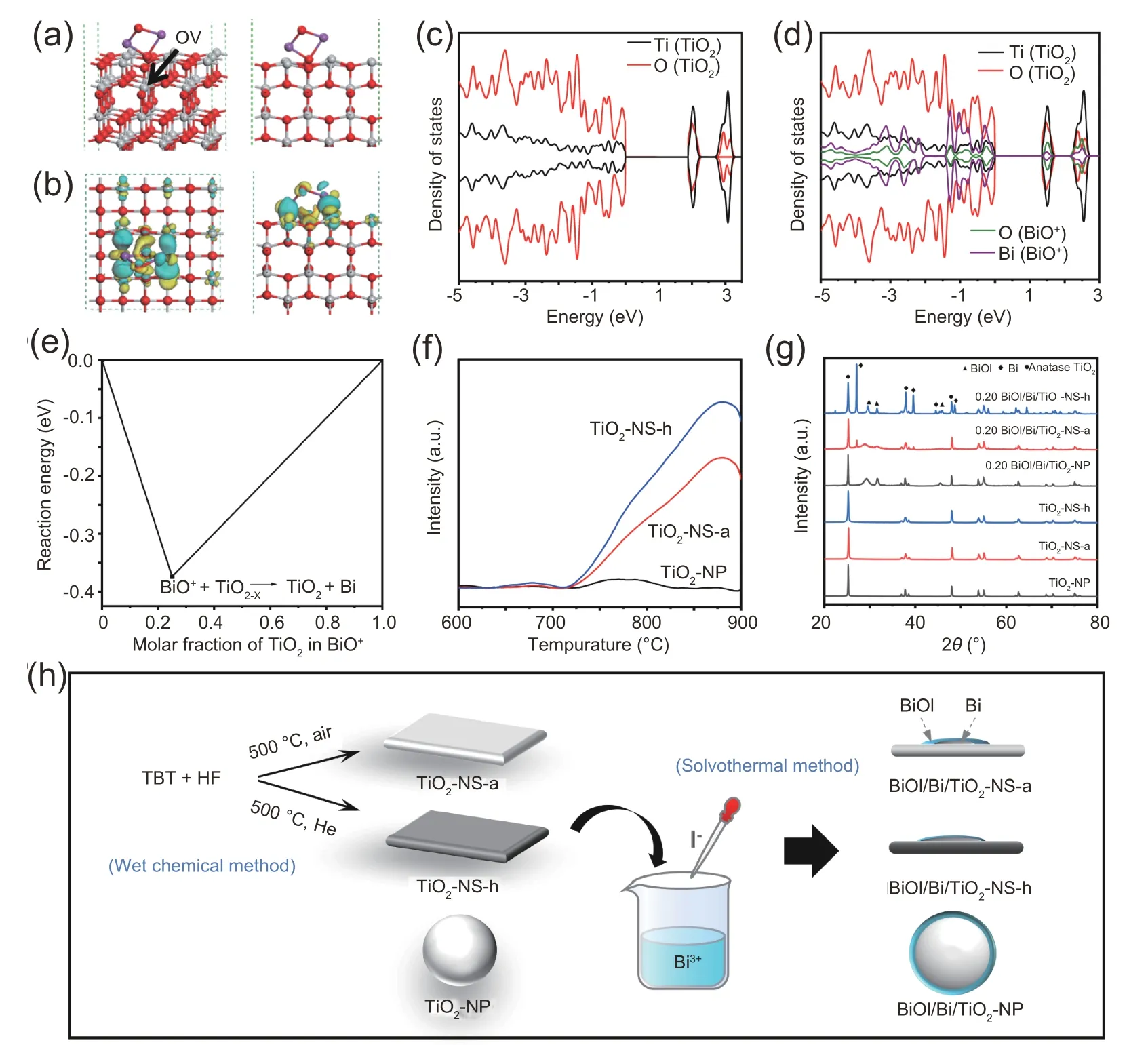
Fig.1.The structural model(a)and the charge density difference for the most stable adsorption configuration(b)of BiO+ion on defected TiO2{001}facets.(Red,white and purple spheres represent oxygen,titanium and bismuth atoms,respectively.Light blue and yellow represent charge loss and charge accumulation,respectively.)(c)The density of states(DOS)plot of the defected TiO2{001}slab model and(d)the BiO+ion adsorbed on the oxygen vacancy in the TiO2{001}slab model.(e)The interface reaction of defected TiO2{001}facets with BiO+.The reaction energy is calculated by the equation of ΔE=Eproduct-Ereaction.The Eproduct is the energy of product,and the Ereaction is the energy of the reaction.(f)XRD patterns of various TiO2 supports and their cor111responding composites with BiOI.(g) NO-TPD spectra of TiO2-NP,TiO2-NS-a and TiO2-NS-h.(g) The preparation scheme of BiOI/Bi/TiO2 ternary composites.
To further understand the structure of 0.20 BiOI/Bi/TiO2-NS-a ternary composite,a series of characterizations were conducted.FE-SEM images reveal the nanosheet structure of TiO2-NS-a with a width of about 170 nm and thickness of about 30 nm (Fig.2a).After coupling with BiOI,some isolated particles(red circled)are observed on the{001}facets of TiO2-NS-a (Fig.2b).The high-resolution TEM image of 0.20 BiOI/Bi/TiO2-NS-a (Fig.2c) shows an apparent low contrast caused by the stacked TiO2-NS-a and the introduced Bi-containing species.Furthermore,lots of small and isolated Bi0nanoparticles are observed (Fig.2d),and the complicated lattice fringes suggest that the BiOI layer may cover the Bi0nanoparticles.Additionally,the elemental mapping images of the Bi and Ti elements show that the enrichment region of the Bi element matches well with that of the Ti element (Fig.2e and f),indicating the good dispersion of Bi-containing species on the surface of TiO2-NS-a.
XPS analyses were further performed to investigate the near-surface chemical composition and element valence states of TiO2-NS-a and 0.20 BiOI/Bi/TiO2-NS-a.The general survey spectrum of 0.20 BiOI/Bi/TiO2-NS-a(Fig.S3a)shows the existence of Ti,Bi,I,O elements.The Ti 2p core-level spectra exhibit spin-orbital doublets(Fig.3a),Ti 2p1/2and Ti 2p3/2.It is obvious that the Ti 2p1/2peak of 0.20 BiOI/Bi/TiO2-NS-a became broader,which is attributed to the shoulder peak of Bi 4d3/2appeared at around 465.5 eV[41].In addition,a small Ti 2p3/2peak can be discerned at 457.9 eV,which is deemed as Ti3+sites[42].Correspondingly,the O 1s core-level spectra of 0.20 BiOI/Bi/TiO2-NS-a in Fig.3b can be fitted with three deconvolution peaks at 529.9 eV,530.8 eV,531.6 eV,cor111responding to Bi-O bonds,Ti-O bonds and the O-atoms near oxygen vacancy,respectively [25,43-48].After forming the composite,the peak area of the oxygen vacancy decreases significantly.It is noteworthy that the Ti and O binding energies of 0.20 BiOI/Bi/TiO2-NS-a exhibit an overall shift to higher values.The variation of binding energy relates to the change of surface electron density,which indicates the electron on TiO2-NS-a may be transferred for the construction of p-n heterojunction and for reducing Bi3+to Bi0.Unexpectedly,only Bi3+is observed in the Bi 4f core-level spectra(Fig.3c),even the Bi0phase has been validated by XRD and TEM analysis.It is still not revealed in the XPS spectrum of 0.20 BiOI/Bi/TiO2-NS-a,indicating that Bi0should be sandwiched in the heterojunction interface and completely covered by the BiOI.These characterization results and analyses not only provide direct evidence for the location of Bi0,but also imply that the formation of Bi0is closely related to the oxygen vacancies on the surface of TiO2-NS-a.
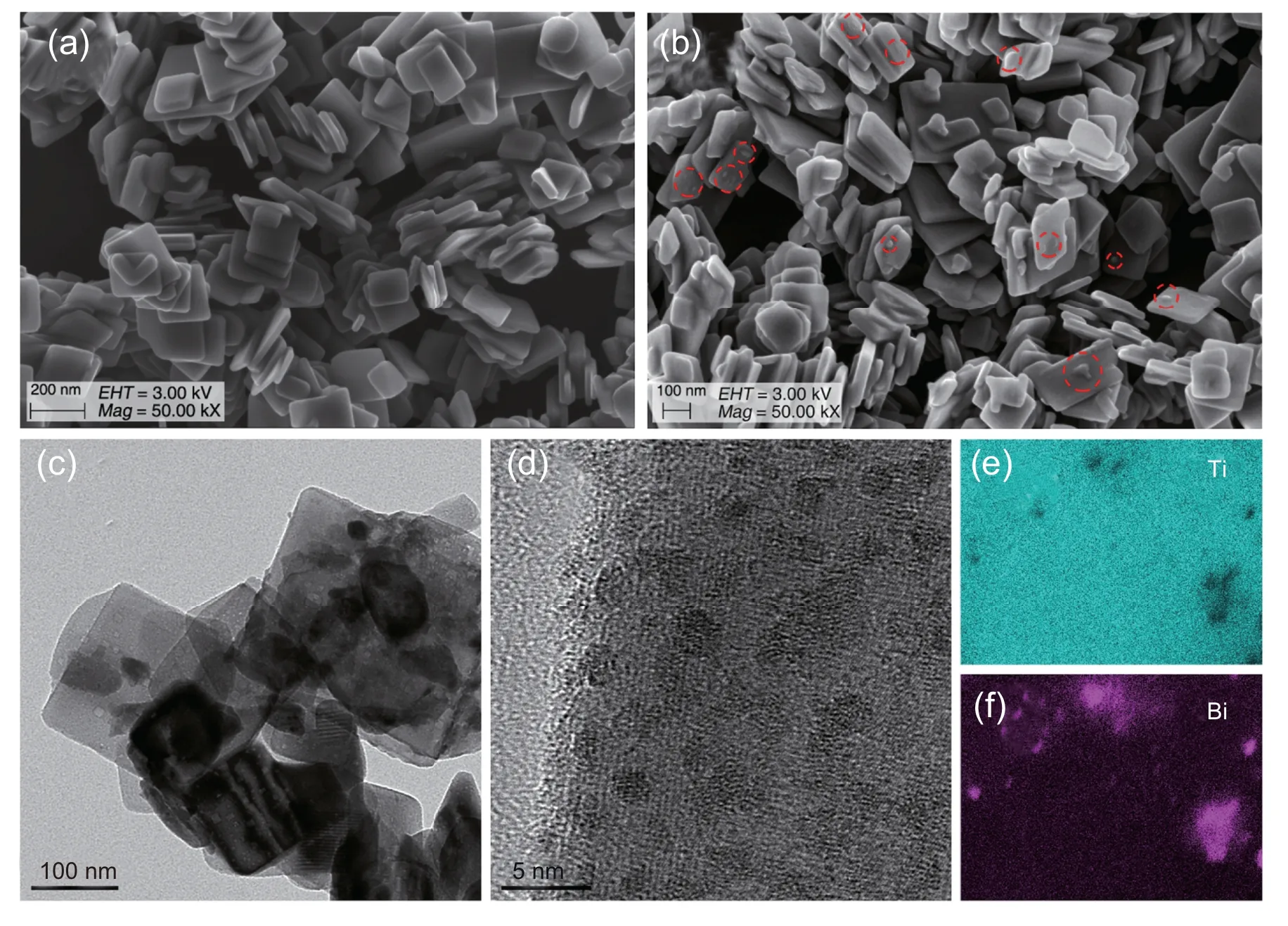
Fig.2.FE-SEM images of(a)TiO2-NS-a and(b)0.20 BiOI/Bi/TiO2-NS-a(c-d)TEM images of 0.20 BiOI/Bi/TiO2-NS-a and(e-f)the elemental mapping images of Ti and Bi.

Fig.3.XPS spectra of BiOI,TiO2-NS-a and 0.20 BiOI/Bi/TiO2-NS-a.(a),(b)and(c)are the high-resolution spectra of Ti 2p,O 1s and Bi 4f,respectively.(d)XRD patterns of BiOI/Bi/TiO2-NS-a with different Bi:Ti molar ratios and(e)the schematic illustration of the formation of the BiOI/Bi/TiO2-NS ternary heterostructure.
A series of BiOI/Bi/TiO2-NS-a samples with various BiOI loadings were prepared to investigate the formation mechanism of this ternary composite.The XRD patterns of the assynthesized samples with different Bi/Ti molar ratios are shown in Fig.3d.When the Bi/Ti molar ratio is 0.10:1,only are the characteristic peaks of Bi0observed.Increasing the Bi/Ti molar ratio to 0.20:1,the characteristic peaks of BiOI are observed along with those of Bi0.With the further increase of the Bi/Ti molar ratio to 0.30:1,the characteristic peak intensity of Bi0is almost the same,but the peaks of BiOI become stronger.Base on the above results,the formation mechanism of BiOI/Bi/TiO2-NS-a ternary heterostructure is thus proposed(Fig.3e).When the loading of BiOI is low,the ultra-thin BiOI layer attaches tightly to the surface of TiO2-NS-a,so that the oxygen vacancies can break the weak Bi-O bond and result in an almost complete conversion of BiOI to Bi0.However,with the further increase of the BiOI loading,a stable BiOI/Bi/TiO2-NS ternary heterostructure is formed with the continuous coverage of the in situ formed Bi0nanoparticles by the BiOI layer.Thus,the characteristic signal of Bi0is not observable in the Bi 4f core-level XPS spectra at high Bi:Ti ratios.
3.3.Optical absorption properties and photocatalytic activity
The optical absorption behaviors of different TiO2supports and their cor111responding composites were studied by UV-Vis diffuse reflectance spectra (DRS) measurement.As shown in Fig.4a,all the TiO2supports show a conspicuous absorption edge at around 380 nm.Intriguingly,TiO2-NS-a and TiO2-NSh exhibit a broad absorption tail starting from 400 nm to 800 nm,due to the existence of Ti3+defects that induce a continuous vacancy band of electronic states just below the conduction band edge of TiO2[49],which is consistent with the theoretical calculation.The discrete occupied spin gap state in the bandgap is pinned at about 2.02 eV,and the minimum electron transition energies from the valence band to the occupied gap states are about 2.12 eV (Fig.1c).In agreement with the literature reports[50-52],the formation of the occupied gap state of defect sites could lead to visible-light absorption.Upon coupling with BiOI,the absorption edges of the composite red shift to higher wavelengths,indicating they can be excited by visible light.Additionally,0.20 BiOI/TiO2-NP shows a similar absorption pattern to BiOI in the visible light region.Still,a significantly different absorption behavior of the ternary composite is observed.More specifically,the optical absorption of 0.20 BiOI/Bi/TiO2-NS-h with higher loading of Bi0becomes weaker in the range from 400 to 600 nm comparing with that of 0.20 BiOI/Bi/TiO2-NS-a,suggesting that the formation of Bi0is at the expense of BiOI,which leads to the decrease of the optical absorption capacity of the systems.An optimum amount of Bi0at the interface is a prerequisite for the better optical absorption performance of the whole composite system.On the other hand,the BiOI layer entirely covers the Bi0particle's surface,and their sizes are quite small.Thus,the impact of the SPR effect should be ignored.
We further studied the effect of the heterostructure on the separation and migration efficiency of photogenerated charge carriers by employing the photoluminescence (PL) technique.As shown in Fig.4b,the PL spectra surveyed at the strongest excitation wavelength of 380 nm show that the emission peak intensity of 0.20 BiOI/TiO2-NP is much stronger than that of 0.20 BiOI/Bi/TiO2-NS-a and 0.20 BiOI/TiO2-NS-h,suggesting that the separation and migration efficiency of the photogenerated carriers in the ternary composite is significantly enhanced.The effective transfer of the photogenerated charge carriers is attributed to Bi0at the heterojunction interface,which acts as the electron transfer mediator.To gain deeper insight into the charge transfer behaviors of the heterostructure,we measured the photocurrents of the samples under 435 ± 7 nm monochromatic light irradiation (Fig.4c).These photocurrents show an order of TiO2-NS-a <BiOI <0.20 BiOI/Bi/TiO2-NP <0.20 BiOI/Bi/TiO2-NS-h <0.20 BiOI/Bi/TiO2-NS-a,suggesting that the Bi0nanoparticles indeed promoted the interfacial charge transfer between BiOI and TiO2and enhance the photocurrent response.
The photocatalytic activities of the catalysts were evaluated by the photodegradation of RhB under 300 W Xe lamp irradiation,and the results are shown in Fig.4d and S5.Prior to the catalyst test,the blank experiment was carried out without any photocatalyst,and it was found that the removal rate of RhB was minimal.The combination of BiOI and TiO2can achieve remarkable property improvement.Furthermore,the photocatalytic activity of the catalyst with the sandwiched ternary heterostructure is superior to that of the binary ones,and 0.20 BiOI/Bi/TiO2-NS-a shows a much better catalytic performance than 0.20 BiOI/TiO2-NP and 0.20 BiOI/Bi/TiO2-NS-h.The photodegradation efficiency was replotted by ln(C0/C) versus reaction time,and the apparent reaction rate constant could be evaluated by the slope of the linear fitting of the results.As shown in Fig.S5a,linear fitting results show a good linear relationship,indicating the photocatalytic reaction is in good agreement with the pseudo-first-order reaction.Table 1 summarizes the apparent reaction rate constants(k)of different samples,where the k value of 0.20 BiOI/Bi/TiO2-NSa (13.02 × 10-3min-1) is much higher than those of all the other samples,around 4.55,4.34,2.98 and 2.0 times of the k value of TiO2-NS-a (2.73 × 10-3min-1),BiOI (3.00 × 10-3min-1),0.20 BiOI/TiO2-NP (4.37 × 10-3min-1) and 0.20 BiOI/Bi/TiO2-NS-h (6.49 × 10-3min-1),respectively.

Fig.4.(a)UV-Vis diffuse reflectance spectra,(b)PL spectra,(c)photocurrent vs.time(I-t)curves,(d)photodegradation of RhB experiments,(e)apparent quantum yield of TiO2-NS-a,BiOI and binary/ternary composites within monochromatic light(436 nm)irradiation for 5 min and(f)recyclability of ternary composites in photodegradation of RhB.
To evaluate the energy conversion efficiency of the catalyst in the degradation of RhB,apparent quantum yield (AQY)measurement was performed with monochromatic light(436 nm),and the results are shown in Fig.4e and Table 1.The AQY roughly reflects the relative quantity of effective photocarriers diffused to the catalyst surface to participate in the RhB degradation reaction.As seen,the AQY for the RhB degradation of 0.20 BiOI/Bi/TiO2-NS-a reaches 0.464% at 436 nm,which is much higher than that of TiO2-NS-A and BiOI,and twice that of 0.20 BiOI/TiO2-NP.Upon a closer look,we can get more details and conclusions.(i) the single component of TiO2-NS-a and BiOI.For TiO2-NS-a,due to the presence of a small number of oxygen vacancies,it can generate additional energy levels near the conduction band of pristine TiO2(only responsive to ultraviolet light of<380 nm),so it has a certain absorption at 436 nm of visible light (this can be seen from UV-Vis absorbance spectrum).However,due to the small amount of absorption,the AQY is still low.Although BiOI has good visible light absorption (as can be seen in the UV-vis absorbance spectrum),it has a relatively fast recombination rate of the photogenerated carrier[53].So,the quantum yield of 436 nm laser excitation is not high.(ii)BiOI and TiO2composites.To the binary BiOI/TiO2-NP,the AQY is significantly improved compared to the single component,confirming the formation of p-n heterojunction and the built-in electric field promote the separation of photogenerated carriers.With the in-situ formation of the Bi0at the p-n heterojunction interface,the AQY of the ternary component systems is further improved based on the binary component system,indicating the Bi0in the ternary component systems acts as an electron transfer mediator alter the electron transfer paths and further regulate the redox ability.The difference of AQY between the 0.20 BiOI/Bi/TiO2-NS-a and 0.20 BiOI/Bi/TiO2-NS-h indicates that the formation of Bi0will consume part of BiOI;thus,there should be an optimal composition ratio in the ternary component systems that should be followed to ensure an excellent photocatalytic activity.

Table 1 Photocatalytic performance of different samples for photodegradation of RhB.
The stability of photocatalyst is an essential indicator of practical applications.Therefore,the durability of BiOI/Bi/TiO2-NS ternary photocatalysts was evaluated by the cyclic degradation of RhB.As shown in Fig.4f,the tested photocatalysts exhibit a stable and efficient photocatalytic activity up to the five successive cycling experiments.In particular,the photocatalytic activity of 0.20 BiOI/Bi/TiO2-NS-a remained 94.0% after five cycling tests,showing only 3.29% of attenuation compared with the first run.The composition and morphology of the 0.20 BiOI/Bi/TiO2-NS-a sample after the five successive cycling experiments were also measured,almost the same as before(Fig.S6).These results demonstrate that the 0.20 BiOI/Bi/TiO2-NS-a ternary composite has excellent catalytic activity and good durability.
3.4.The photocatalytic reaction mechanism over BiOI/Bi/TiO2
It is generally accepted that,for an excellent photocatalyst,both the high separation efficiency of photogenerated charge carriers and the strong light absorption capability are necessary.The above characterization results clearly show that the sandwiched BiOI/Bi/TiO2-NS-a ternary composite has a strong photoabsorption in the visible region,the high separation efficiency of charge carriers,and excellent photocatalytic performance.However,the photocatalytic reaction mechanism over this catalyst system is still unclear,or the enhanced catalytic activity should be further clarified.
To further probe into the photocatalytic mechanism of BiOI/Bi/TiO2-NS-a and understand the role of the Bi0in the ternary component systems,we also carried out the radical scavenging experiments in the photodegradation of RhB,and the results are shown in Fig.5a.Isopropanol (IPA),benzoquinone (BQ),and EDTA-2Na were employed as the scavengers for purging·OH,and h+,respectively.For the TiO2-NS-a,BiOI and 0.20 BiOI/TiO2-NP,the addition of EDTA-2Na significantly reduced the photodegradation rate of RhB.In contrast,the addition of IPA and BQ showed had less effect on the reaction,indicating that h+is the dominant active radical species,while ·has a little contribution to the photocatalytic reaction.This result can be rationally explained by the fact that the reduction of absorbed O2to·is hard to be realized because the CBs of TiO2-NS-a and BiOI(calculated by Eqs.S2 and S3)are lower than the standard reduction potential of O2/·(-0.33 eV)[54].On the contrary,in the case of 0.20 BiOI/Bi/TiO2-NS-a,the presence of BQ scavenger reduced the degradation rate of RhB,indicating that·is the main active radical species.The change of the dominant active species from h+to ·in the reaction suggests that the photogenerated electrons on 0.20 BiOI/Bi/TiO2-NS-a have a high reduction potential enough to reduce the adsorbed O2to·,which is hard to be realized in the conventional BiOI/TiO2binary composite that follows the double-transfer mechanism.
Based on our experimental results and our understanding,we,therefore,propose the electron transfer mechanism of the BiOI/Bi/TiO2-NS-a photocatalyst and the role of the Bi0nanoparticles (Fig.5b).(1) When the p-type semiconductor BiOI combines with the n-type semiconductor TiO2-NS-a,the whole energy band in p-region and n-region will move upwards and downwards due to the transfer of electrons from TiO2-NS-a to BiOI until the Fermi energy levels (Ef) of the two semiconductors are aligned,thus forming a p-n heterojunction [55,56].(2) In the BiOI/Bi/TiO2-NS-a ternary composite,the existence of Bi0nanoparticles at the heterojunction interface can weaken the built-in electric field of the p-n heterojunction,which effectively inhibits the transfer of photogenerated charge carriers driven by the built-in electric field.Therefore,to a certain extent,it alleviates the significant decrease of redox potential caused by the double transfer mechanism of the photogenerated charge carriers commonly occurring on the p-n junction semiconductor photocatalysts.(3) On the other hand,the existence of the Bi0nanoparticles also provides a new electron transfer mediator similar to the Zscheme mechanism[57,58].It ensures the effective separation and high redox ability of photogenerated charge carriers.
Our experimental results support the above-proposed mechanism well.(1) In the BiOI/Bi/TiO2-NS-a ternary composite,the formation of Bi0nanoparticles at the interface implies the occurrence of the electron transfer between TiO2and BiOI,which gives strong evidence for the creation of the p-n heterojunctions.(2) The radical scavenging experiments show that the redox ability of photogenerated electrons in the ternary composite is significantly improved.Still,neither type-II nor p-n heterojunctions can explain the experimental phenomena.(3) The catalytic activity of the 0.20 BiOI/Bi/TiO2-NS-a is superior,and the cor111responding AQY measurement and PL spectra results also confirm that this ternary composite possesses higher photoquantum efficiency.Hence,we can reasonably deduce that,after excluding the SPR effect,the sandwiched Bi0nanoparticles act as a metal-media that enables the BiOI/Bi/TiO2-NS-a to combine both the advantages of the p-n heterojunction for energy band manipulating and those of the Z-scheme heterojunction for photogenerated charge carriers separation and high redox capability.

Fig.5.(a)The photocatalytic performance of the samples under a series of radical scavenging experiments.(b)Band energy diagrams of BiOI and TiO2,BiOI/TiO2 binary composite and BiOI/Bi/TiO2 ternary composite.(Ef1,Ef2,and Ef represent the Fermi levels of pure TiO2,BiOI,and composites,respectively).
Therefore,the whole reaction process can be elaborated by the following equations.Under the light irradiation,the electrons on the VB of TiO2-NS-a and BiOI are excited to their CB(Eqs.(1)and(2)).And then,the electrons on the CB of TiO2-NS-a migrate to the VB of BiOI via the Bi0and further recombine with the photogenerated holes in the VB of BiOI(Eq.(3)).Due to the weakened built-in electric field,the migration of electrons from the CB of BiOI to the CB of TiO2-NS-a is significantly suppressed.Finally,the photoexcited electrons on the CB of BiOI with higher reduction potential can reduce the absorbed O2to ·,which is strong oxidation radical for the degradation of the reactant species(Eqs.(4)and(5)).The holes accumulated in the VB of TiO2-NS-a can directly oxidize the reactant species (Eq.(6)) and oxidize the OH-to form a small amount of ·OH (Eqs.(7) and (8)).This photocatalytic mechanism provides a reasonable explanation for the superior photocatalytic activity of the BiOI/Bi/TiO2-NS-a ternary composite to that of BiOI/TiO2binary ones.

4.Conclusions
In summary,we have reported a novel method for the preparation of highly efficient BiOI/Bi/TiO2ternary photocatalyst with sandwiched heterostructure instead of the formation of the conventional BiOI/TiO2p-n photocatalyst.In this preparation,part of the adsorbed BiO+species interacts with the electron-rich oxygen vacancies in the TiO2nanosheet surface and is reduced into Bi0.The DFT calculations confirm the feasibility of this reaction,and the formation of the Bi0nanoparticles and the ratio of BiOI/Bi0are correlated well with the oxygen vacancy concentration and the loading of BiOI.In this BiOI/Bi/TiO2-NS-a catalyst,the Bi0nanoparticles can weaken the built-in electric field of the p-n heterojunction and reduce the transfer of photogenerated charge carriers driven by the built-in electric field,thus being able to alleviate the significant decrease of redox potential commonly occurring in the p-n junction semiconductor photocatalysts.On the other hand,the existence of the Bi0nanoparticles allows electron transfer like that in the Z-scheme mechanism,enabling the effective separation and higher redox ability of photogenerated charge carriers.As a result,this synthesized BiOI/Bi/TiO2-NS-a photocatalyst possesses both the advantages of the p-n junction for energy band manipulating and of the Z-scheme heterojunction for photogenerated charge carriers separation and high redox capability,which is evidenced by its significantly improved photocatalytic performance as compared with the BiOI/TiO2-NP binary photocatalyst.Such a design concept via utilizing oxygen vacancies in conventional metal oxides is instructive and applicable to the preparation of some other active photocatalysts for various applications.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We acknowledge that this work is financially supported by the National Natural Science Foundation of China (No.21043006 and 51702205),the Education Department of Guangdong Province (No. 2018KTSCX063 and 2013KJCX0081),the Science and Technology Planning Project of Guangdong Province (No.2014A020216045),the 2020 Li Ka Shing Foundation Cross-Disciplinary Research Grant (2020LKSFG09A) and the Guangdong Key Discipline Fund at GTIIT,and are grateful to Prof.Mingde Li at Shantou University for his kind help in AQY measurement.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gee.2020.11.013.
 Green Energy & Environment2022年4期
Green Energy & Environment2022年4期
- Green Energy & Environment的其它文章
- Multivariate MOF for optimizing atmospheric water harvesting
- Lignin-based carbon fibers: Formation,modification and potential applications
- Charactering and optimizing cathode electrolytes interface for advanced rechargeable batteries: Promises and challenges
- Metal-organic frameworks-derived metal phosphides forelectrochemistry application
- Surface-mediated iron on porous cobalt oxide with high energy state for efficient water oxidation electrocatalysis
- Oxygen-deficient SnO2 nanoparticles with ultrathin carbon shell for efficient electrocatalytic N2 reduction
