Neutrophil-to-lymphocyte ratio in sporadic amyotrophic lateral sclerosis
Qian-Qian Wei,Yan-Bing Hou,Ling-Yu Zhang,Ru-Wei Ou,Bei Cao,Yong-Ping Chen,Hui-Fang Shang
Abstract The neutrophil-to-lymphocyte ratio (NLR) is considered a robust prognostic biomarker for predicting patient survival outcomes in many diseases.However,it remains unclear whether it can be used as a biomarker for amyotrophic lateral sclerosis (ALS).To correlate NLR with disease progression and survival in sporadic ALS,1030 patients with ALS between January 2012 and December 2018 were included in this study.These patients were assigned into three groups according to their NLR values:Group 1 (NLR <2,n=544 [52.8%]),Group 2 (NLR=2–3,n=314 [30.5%]),and Group 3 (NLR >3,n=172 [16.7%]).All patients were followed up until April 2020.Patients in Group 3 had a significantly older onset age,a lower score on the Revised ALS Functional Rating Scale,and rapidly progressing disease conditions.Furthermore,faster disease progression rates were associated with higher NLR values (odds ratio=1.211,95% confidence interval [CI]:1.090–1.346,P <0.001)after adjusting for other risk factors.Compared with Groups 1 and 2,the survival time in Group 3 was significantly shorter (log-rank P=0.002).The NLR value was considered an independent parameter for the prediction of survival in ALS patients after normalizing for all other potential parameters (hazard ratio [HR]=1.079,95% CI:1.016–1.146,P=0.014).The effects on ALS survival remained significant when adjusted for treatment (HR=1.074,95% CI:1.012–1.141,Ptrend=0.019) or when considering the stratified NLR value (HR=1.115,95% CI:1.009–1.232,Ptrend=0.033).Thus,the NLR may help to predict the rate of disease progression and survival in patients with sporadic ALS.The study was approved by the Institutional Ethics Committee of West China Hospital of Sichuan University,China (approval No.2015 (236)) on December 23,2015.
Key Words:amyotrophic lateral sclerosis;Cox analysis;inflammation;lymphocytes;monocytes;neutrophils;prognosis;survival rate
Introduction
Amyotrophic lateral sclerosis (ALS) is one of the most lethal and irreversible progressive motor neurodegenerative diseases.It is pathologically characterized by degenerating motor neurons that are mainly located in the brainstem and spinal cord regions (both upper and lower) (Brown and Al-Chalabi,2017).Its etiology remains unknown,but multiple factors may contribute to its development,including genetics,exposure to environmental toxins,and trauma (Su et al.,2016).The survival window for ALS is relatively wide,ranging from months to over a decade.However,the median survival time is relatively narrow,typically between 3 and 5 years after diagnosis (Westeneng et al.,2018).The complex role of neuroinflammation involving multiple cell types plays an important role in the disease process of ALS.Both cellular autonomous motor neurodegeneration and noncellular autonomous degeneration of non-neuronal cells,such as astrocytes and microglia,occur in ALS (Philips and Robberecht,2011).Furthermore,ALS-mediated modulation of the activation of peripheral immune cells and central nervous system-specific microglial and glial cells has also been reported (Malaspina et al.,2015).
Beers et al.(2011) reported that endogenous regulatory T-lymphocytes ameliorate ALS in mice,but this immune cell population is unable to precisely predict disease onset.However,it does correlate with disease progression in ALS patients.Depending on the aggressiveness of disease progression,ALS patients exhibit varying degrees of immune activation profiles (Zhao et al.,2013).Notably,activation of the early immune response involves cytokines and polarized immune cells as part of the protective mechanisms,while the later response is primarily associated with cytokine storms and persistent immune activation,which leads to rapid progression of the disease (Zhao et al.,2013).Thus,there is a critical need to identify early prognostic biomarkers for disease progression.
Altered leukocyte profiles in peripheral blood are a potential biomarker of ALS because the rate of disease progression may be associated with the complex coordination between different immune response pathways.A previous study assessing peripheral immune cells in ALS demonstrated that a higher neutrophil-to-CD16+monocyte ratio correlates with more rapid disease progression (Murdock et al.,2016).Another study reported that an increase in neutrophil count and a reduced number of CD4+T cells might be significantly correlated with disease advancement (Murdock et al.,2017).These results suggest a crucial role of the peripheral immune system in ALS pathogenesis.The neutrophil-to-lymphocyte ratio (NLR),a numerical value obtained by dividing the number of neutrophils by the number of lymphocytes in the peripheral blood,is considered a robust prognostic biomarker for predicting patient survival outcomes in many diseases,including cancer and coronary artery disease (Arbel et al.,2012;Scilla et al.,2017).Usually,higher NLR values are associated with poorer survival outcomes (Arbel et al.,2012;Scilla et al.,2017).Additionally,NLR is reportedly correlated with patient mortality in acute ischemic stroke (Celikbilek et al.,2014;Pektezel et al.,2019).However,the effects of NLR on ALS remain uncertain.One recent study of 322 ALS patients reported that NLR reflects the degree of neuroinflammation and might be useful as a prognostic biomarker for ALS(Choi et al.,2020).However,the association between NLR and important clinical features,such as clinical phenotype,disease staging,and disease progression,remains unclear in ALS.Studies with larger sample sizes are required to further address the predictive value of NLR in ALS after adjusting for potential confounding factors.Therefore,the present study was designed to analyze the relationships between NLR and both clinical features and prognostic outcomes in a large cohort of ALS subjects,in combination with other known prognostic factors.
Participants and Methods
Design
All participating subjects in this retrospective,cross-sectional,observational study had been referred to the Tertiary Motor Neuron Disease Treatment Center (Department of Neurology,West China Hospital of Sichuan University,Chengdu,China),located in the Sichuan province of southwest China.Only patients diagnosed with either definite,probable,or possible ALS between January 2012 and December 2018 were recruited into the current study,strictly following the revised El Escorial eligibility criteria for ALS (Brooks et al.,2000).Patients classified as possible ALS at the time of registration were confirmed to have probable or definite ALS during follow-up.Most patients were diagnosed with ALS for the firsttime in our hospital.
Clinical data collection
Information about demographic features was collected through questionnaires,as previously described (Weisskopf et al.,2004).Disease-related variables,including the age of onset,onset region (bulbar or non-bulbar),disease duration,diagnostic delay,phenotypes,and disease stages according to the King’s College staging system (Valdmanis and Rouleau,2008),were recorded.Patients in stage 1 or 2 were divided into the early-stage subgroup,and patients in stage 3 or 4 were divided into the late-stage subgroup.The Revised ALS Functional Rating Scale (ALSFRS-R) was used to score disease severity (Kollewe et al.,2008).Total ALSFRS-R scores range from 0 to 48,and higher scores indicate better functioning.The disease progression rate was presented as the change in ALSFRS-R scores per month.Patients were divided into the faster disease progression subgroup and the slower disease progression subgroup based on the median rate of disease progression (0.62).Patients with a putative hereditary ALS background (obtained from interviews or genetic analysis),juvenile ALS,or with incomplete data were excluded.The body mass index (BMI) was calculated as weight (kg) divided by height (m)2.
Laboratory data collection
Blood samples from the cubital vein were collected from subjects in the morning by venipuncture following overnight fasting (>8 hours) and were analyzed after admission.Laboratory parameters including white blood cell (monocytes,basophils,neutrophils,eosinophils,and lymphocytes) counts,red blood cell count,platelet count,hematocrit,mean corpuscular volume,mean corpuscular hemoglobin,red blood cell distribution,hemoglobin A1c (HbA1c),and albumin levels were evaluated in the Biochemistry Laboratory of West China Hospital of Sichuan University.Complete blood cell analysis was performed using an automated XE-5000 hematology counter (Sysmex,Kobe,Japan).The NLR was calculated as the ratio of the neutrophil count to the lymphocyte count.The neutrophil-to-monocyte ratio (NMR) was calculated as the ratio of the neutrophil count to the monocyte count.Patients were excluded from the study if blood tests were unable to be performed within 1 month before or after the initial registration assessment.Patients were followed up at an interval of 3 or 6 months from the date of registration by our expert neurologists (QQW,LYZ,and YBH),either by telephone or one-to-one on-site interviews.Death information was collected from family reports and provincial public security bureau records.Results were collected for the following treatment procedures:riluzole and edaravone medications,percutaneous endoscopic gastrostomy,and non-invasive positive pressure ventilation.All participants were aware of the study protocols and gave signed written consent(Additional file 1) for their participation.The Institutional Ethics Committee of West China Hospital of Sichuan University,China approved the study (approval No.2015 (236))on December 23,2015 (Additional file 2).This study was conducted in accordance with the ethical principles of theDeclaration of Helsinkiand followed the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE)statement (Additional file 3).
Statistical analysis
This study analyzed approximately 15 associated factors for progression and survival.According to the empirical formula of multi-factor analysis sample estimation,sample size (N)=number of research factors (n) × 5–10.The sample size calculated by the empirical formula was then increased by 10% to control the impact of loss to follow-up on the results.The results of this formula indicated that this research needed at least 165 cases.We thus included a large sample size in this study.
All analyses were conducted using SPSS 26.0 (IBM,Armonk,NY,USA).According to a previous study (Arbel et al.,2012),ALS patients were subdivided into three groups:Group 1 (NLR values <2),Group 2 (NLR values 2–3),and Group 3 (NLR values>3).Comparisons of continuous variables were performed using the Student’st-test or one-way analysis of variance.The chi-squared test was used to compare categorical variables.To assess the disease-related effects of NLR in the different subgroups,we also related NLR to the following clinical features:age,sex,onset region,clinical phenotype,diagnostic criteria,disease staging,and disease progression.We performed multivariate logistic regression analysis using normalized values for the age of onset,sex,onset region,albumin level,HbA1c level,and BMI to validate whether a higher NLR value was able to predict a faster disease progression rate in ALS patients.The disease progression rate was set as the dependent variable.The survival rate was calculated using Kaplan-Meier curves and log-rank tests,and considered the survival time between the date of disease onset (diagnostically verified) and death or the censoring date (April 2020).We also performed a multivariable Cox proportional hazards regression model to evaluate the effect of NLR on the risk of death after symptom onset.The hazard ratios (HRs) and their 95% confidence intervals (CIs) were then calculated.We also performed a sensitivity analysis using ‘time since registration’ instead of ‘time since symptom onset’ as the underlying time scale,to explore the potential influence of different time scales on the study results.In addition,we conducted a sensitivity analysis using NMR instead of NLR to explore its predictive effect on survival.Values ofP<0.05 were considered statistically significant.
Results
NLR levels in ALS patients and controls
A total of 2002 patients were registered in our hospital at the end of 2018.Of these,972 patients who had incomplete data were excluded (Additional Figure 1).The age,sex,BMI,diagnostic delay,and disease progression rates did not differ significantly between included and excluded patients (data not shown).Comparative analyses of the demographic and NLR values of the ALS patients vs.age-matched control subjects are shown inAdditional Table 1.The age,sex,and BMI values were not significantly different between ALS patients and controls (allP>0.05).ALS and multiple system atrophy patients had higher NLR values than healthy subjects (allP<0.05).
The demographic and clinical features of ALS patients are shown inTable 1.The patients were subdivided into three groups according to their NLR values:544 patients (52.8%)were in Group 1 (NLR values <2),314 patients (30.5%) were in Group 2 (NLR values 2–3),and 172 patients (16.7%) were in Group 3 (NLR values >3).There were significant differences between the three groups in sex,onset age,ALSFRS-R score,ALS progression rate,and percentage of early disease stage.Figure 1shows the relationship between NLR and various clinical aspects of ALS.The onset age,sex,probability of an ALS diagnosis,King’s College staging,disease progression,and ALSFRS-R score were significantly related to NLR (allP<0.05).Laboratory data from the 1030 ALS patients included in the three groups are shown inTable 2.Group 3 patients exhibited significantly higher levels of neutrophils,monocytes,white blood cells,NLR,and NMR compared with the other groups(allP<0.05).The average lymphocyte count of Group 3 patients was the lowest among all three groups (P<0.001).Furthermore,the percentage of neutrophils and lymphocytes in white blood cells were significantly different between the groups.Group 1 patients had significantly lower hemoglobin counts compared with the other groups (P=0.047).
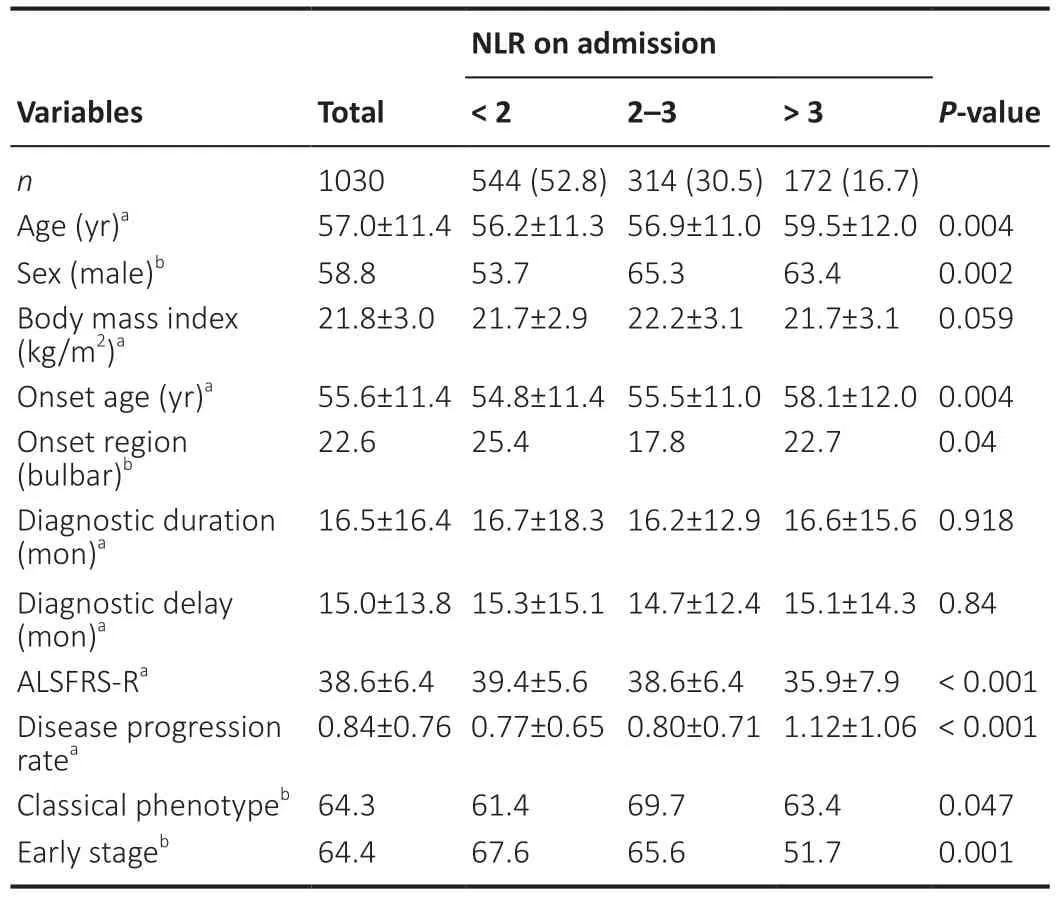
Table 1|Comparisons of demographic and clinical features of ALS patients among the three subgroups according to NLR
The logistic regression model indicated that faster disease progression was associated with higher NLR values (odds ratio=1.211,95% CI:1.090–1.346,P<0.001) after adjusting for other potential risk factors,such as onset age,sex,onset region,BMI,HbA1c,and albumin.
Survival analyses based on NLR
Upon the completion of follow-up studies,677 patients(65.7%) had died,353 patients (34.3%) were alive,and no patients were lost to follow-up.The mean survival time was 40.2 months.Considering all 1030 ALS patients,Kaplan-Meier analysis revealed that the survival time was significantly different among the three subgroups,stratified according to their NLRs (log-rankP=0.002,Figure 2).Patients in Group 3 had worse outcomes.The survival time was significantly shorter in Group 3 compared with Group 1 (estimated median survival time:32.0 monthsvs.42.1 months,log-rankP<0.001).The estimated median survival time was 42.0 months in Group 2,which was significantly longer than that of Group 3 (log-rankP=0.008).The survival time between Groups 1 and 2 did not differ significantly (log-rankP=0.551).

Figure 1|Relative NLR stratified by clinical characteristics in patients.
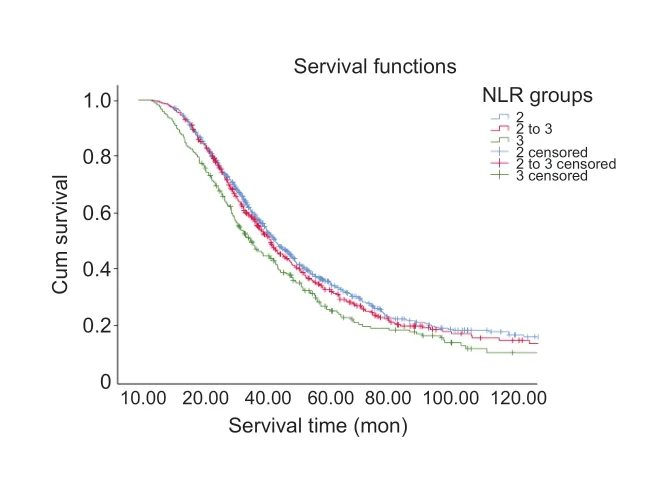
Figure 2|Kaplan-Meier survival curves of ALS patients according to NLR stratifications.
Univariate and multivariate models were used to explore potentially prognostic factors of survival.Higher neutrophil,NLR,and NMR values were associated with an increased risk of mortality.In contrast,higher albumin levels were correlated with a lower risk of mortality in the univariable Cox regression analysis.After adjusting for age,sex,disease stage,phenotype,onset region,BMI,ALSFRS-R,disease duration,albumin,and HbA1c,the NLR (continuous variable) exhibited potential as an independent parameter for survival prediction in the multivariate Cox regression analysis (HR=1.079,95%CI:1.016–1.146,P=0.014) (Table 3).Similarly,following the normalization of treatment strategies,the NLR (continuous variable) still showed an independent effect on survival in ALS patients (HR=1.074,95% CI:1.012–1.141,P=0.019).We explored the Cox model by stratifying the NLR into three groups to determine the predictive value of a higher NLR.As expected,a higher NLR (categorized variable) was correlated with an increased risk of mortality (HR=1.115,95 CI%:1.009–1.232,Ptrend=0.033).The HR for Group 3 patients (NLR>3) relative to Group 2 patients (NLR <2) was 1.249 (95% CI:1.015–1.537,P=0.035) after adjusting for other covariates.Furthermore,after adjusting for treatment strategies,the NLR(categorized variables) still exhibited an independent effect on survival in ALS (HR=1.121,95% CI:1.014–1.240,Ptrend=0.026).Using ‘time since registration’ instead of ‘time since symptom onset’ generated similar results for NLR (HR=1.082.95% CI:1.021–1.146,P=0.007).Moreover,sensitivity analyses using NMR rendered similar results,indicating that a higher NMR was associated with a greater risk of mortality (HR=1.015,95% CI:1.000–1.030,P=0.045).

Table 2|Comparisons of laboratory data of ALS patients among the three subgroups according to NLR

Table 3|Exploration of a putative prognostic effect of the NLR on survival in ALS using multivariable Cox regression analyses
Discussion
In the present study,we investigated the associations between clinical characteristics or laboratory data and NLR subgroups.We also explored the prognostic effects of the NLR(tested at registration) on survival.Our results indicated that a higher NLR in peripheral blood at the time of registration was associated with a faster disease progression rate and had a negative effect on survival.
It has been well documented that ALS pathology involves key immunological components (Zhao et al.,2013).Immune changes—increased percentages of total leukocyte populations,especially—have been detected in humans with ALS.Increased numbers of neutrophils in peripheral blood have also been reported in ALS patients,and were correlated with ALSFRS-R scores (Chiò et al.,2014).Moreover,a significant correlation between increased neutrophil number and the rate of disease progression has been observed in ALS patients,suggesting that there is a fine balance between the protective and pathological immune responses.Additionally,modulation of the functional activation of ALS pathologyspecific immune cells influences the aggressiveness and speed of disease progression in ALS rodent models (Murdock et al.,2016,2017).Results from our binary logistic regression analysis are in agreement with these previous findings,and also extrapolate from them regarding the crucial roles of immune systems in the pathomechanisms of ALS.Indeed,a rapid advancement of the disease condition was associated with higher NLR values in the current study.Moreover,neutrophils may act as a proinflammatory factor that influences the onset and progression of neurodegeneration,and might also contribute to disrupting the brain-spinal cord barrier,which reportedly aggravates ALS pathobiology(Garbuzova-Davis and Sanberg,2014).In contrast,neutrophil infiltration might be associated with enhanced tissue atrophy.Other studies have also suggested the possibility that neutrophils are involved in neuronal repair because increased numbers of neutrophils,rather than other cell entities,accompany the repair process in the central nervous system (Butterfield et al.,2006;Kurimoto et al.,2013).Indeed,neutrophils have been identified as one of the fast-response cell types that promptly initiate repair processes in the brain and spinal cord.Neuroinflammation is also regulated by the increased activation of microglia,which are the resident immune cells of the brain (Kwon and Koh,2020),thus suggesting a complex association between multiple immune cells and the ALS disease process.
A reduced T-lymphocyte population in peripheral blood has been reported to associate with rapidly progressing ALS symptoms (Murdock et al.,2017).A previous study documented significant decreases in circulating CD4+T cells in peripheral blood.However,the levels of CD4+T cells do not correlate with ALSFRS-R scores (Murdock et al.,2016).Another study found no significant changes in CD4+T cell populations between ALS and healthy subjects,but reported that lower numbers of CD4+T cells may correlate with disease advancement in ALS patients (Murdock et al.,2017).This correlation has been confirmed in mouse models,where regulatory T cells prime the immune system to protect affected tissues and simultaneously slow the progression rate of ALS (Beers et al.,2011).A decreased population of circulating lymphocytes may reflect the relocalization of T-lymphocytes—especially specific populations of T cells—to specific brain regions (Zhao et al.,2013).Thus,a higher value of NLR may result from increased neutrophil production and/or T-lymphocyte relocalization to the central nervous system,leading to clinical heterogeneity in ALS.However,we did not examine regulatory function in the present study.Future investigations should evaluate the clinical potential of these biomarkers,their precise roles as destructive or protective factors,and their response to pathological immune activation in ALS.
Several studies have indicated that NLR modulation is correlated with mortality rate in cardiovascular and neurological diseases,such as acute ischemic stroke (Celikbilek et al.,2014;Pektezel et al.,2019).Recently,an increase in NLR values has been suggested as an independent predictive biomarker for poor prognostic outcomes in ALS patients(Choi et al.,2020).The present prognostic role of NLR in ALS outcomes should be considered strictly based on its precise distribution pattern among subjects of a particular cohort.In the current study,Kaplan-Meier analysis revealed that patients with the highest level of NLR had significantly shorter survivaltimes.Cox multivariate regression analysis demonstrated that the NLR value was an independent prediction parameter for ALS survival,even when all other survival parameters were normalized.After adjusting for treatments,an independent effect of NLR on survival remained.Furthermore,the stratified NLR was associated with a higher risk of mortality during follow-up.Taken together,these results suggest the critical involvement of inflammatory pathways in ALS prognosis.However,their involvement in ALS pathophysiology remains incompletely understood.Similarly,another study found that survival was positively correlated with peripheral lymphocyte count,although this correlation lost significance after adjusting for other variables (Chiò et al.,2014).A review study highlighted the fine balance between the activation of proinflammatory and anti-inflammatory mechanisms for modulating the rates of ALS disease progression and survival(Thonhoff et al.,2018).In the current study,we revealed immune alterations in ALS and demonstrated that the NLR may be a potential biomarker for ALS survival.Additional studies using longitudinal samples are required to characterize the role for this new metric combined with other peripheral inflammatory biomarkers.
To date,several determining factors for the survival of ALS patients have been extensively investigated,such as clinical,cognitive,and genetic characteristics.The age at onset,onset region,and progression rate all had significant effects on the survival rate in our Cox analysis.We revealed that latelife onset,bulbar onset,and lower ALSFRS-R scores were correlated with shorter survival;these observations were consistent with the findings of previous studies (Chiò et al.,2009;van Rheenen et al.,2012).Other parameters included in routine laboratory tests,such as albumin,HbA1c,and serum C-reactive protein (CRP),have also been examined in previous studies for their predictive role in the survival of ALS patients.These previous studies reported that ALS prognosis can be influenced by albumin,which is associated with an inflammatory state (Chiò et al.,2014;Sun et al.,2020).Our previous study explored the relationship between HbA1c levels and survival in ALS patients,and revealed that higher HbA1c levels at registration correspond to a greater risk of mortality (Wei et al.,2017).Activation of CRP is acutely regulated by proinflammatory cytokines following its secretion from hepatocytes in response to inflammatory signaling.It has been reported that higher initial CRP levels might correlate with faster disease progression and poorer survival in ALS (Lunetta et al.,2017;Beers et al.,2020).Another study also indicated that CRP may not be directly associated with survival rates,but might be useful as a potential marker to evaluate the efficacy of anti-inflammatory therapies in ALS(De Schaepdryver et al.,2020).Further analyses are required to reveal how changes in CRP levels are associated with progression rates and disease conditions.
It is important to mention that our study suffers from certain limitations that need to be addressed in future investigations.First,we only estimated the associations between baseline NLRs and survival.Future long-term longitudinal studies that test the NLR at different time points and disease stages will allow us to explore the precise functions of the immune response in the pathomechanisms of ALS.Second,although an association between CRP and ALS survival has been explored in several previous studies,data about CRP was unavailable in the present study.Third,many disease factors that might influence the NLR were unable to be taken into consideration,such as metabolic,infectious,and drug-related parameters.In addition,a subgroup analysis is necessary for patients with putative hereditary ALS in the future.Last,potential bias in patient selection might have affected our interpretation of the results because all participating patients were recruited through a single center only.
Our study explored the potential of the baseline NLR as a prognostic biomarker for ALS patient outcomes.We revealed that the NLR might help to predict the rate of disease progression and survival duration in patients with sporadic ALS,thus suggesting potentially novel roles for inflammatory signaling in ALS diagnosis and therapies.To better understand the clinical potential of this biomarker,further investigation,including real-time monitoring of NLR modulation in longitudinal studies,is warranted.
Acknowledgments:The authors thank the ALS patients for their participation in this study.
Author contributions:Study conception and organization:QQW‚BC‚HFS;study execution and data collection:QQW‚RWO‚YBH‚LYZ‚BC‚YPC;statistical analysis:QQW‚RWO;manuscript draft:QQW;manuscript review:HFS.All authors approved the final version of the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:This study was supported by the Science and Technology Bureau Fund of Sichuan Province of China‚No.2020YFS0220;the China Postdoctoral Science Foundation‚No.2019M 653427;Postdoctoral Research Project‚West China Hospital‚Sichuan University of China‚No.2019HXBH029;and Health Commission of Sichuan Province of China‚No.20PJ038 (all to QQW).The funders had no roles in the study design‚conduction of experiment‚data collection and analysis‚decision to publish‚or preparation of the manuscript.
Institutional review board statement:This study was approved by the Institutional Ethics Committee of West China Hospital of Sichuan University‚China (approval No.2015 (236)) on December 23‚2015.
Declaration of patient consent:The authors certify that they had obtained all appropriate patient consent forms from the conscious patients.In the forms‚the patients had given their consent for their images and other clinical information to be reported in the journal.The patients and their families had understand that their names and initials will not be published and due efforts will be made to conceal their identity.
Reporting statement:This study followed the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement.
Biostatistics statement:The statistical methods of this study were reviewed by the biostatistician of the West China Hospital‚Sichuan University‚China.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal‚and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License‚which allows others to remix‚tweak‚and build upon the work non-commercially‚as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Bartłomiej Baumert‚Pomeranian Medical University‚Poland.
Additional files:
Additional file 1:Informed consent form (Chinese).
Additional file 2:Hospital ethics approval (Chinese).
Additional file 3:STROBE checklist.
Additional file 4:Open peer review report 1.
Additional Table 1:Comparisons of demographic and neutrophil-tolymphocyte ratio of ALS patients and controls.
Additional Figure 1:Flow chart of the study design.
- 中国神经再生研究(英文版)的其它文章
- Towards a comprehensive understanding of p75 neurotrophin receptor functions and interactions in the brain
- Microglia regulation of synaptic plasticity and learning and memory
- Stroke recovery enhancing therapies:lessons from recent clinical trials
- Functional and immunological peculiarities of peripheral nerve allografts
- MicroRNA expression in animal models of amyotrophic lateral sclerosis and potential therapeutic approaches
- Significance of mitochondrial activity in neurogenesis and neurodegenerative diseases

