Striatal oxidative damages and neuroinflammation correlate with progression and survival of Lewy body and Alzheimer diseases
Huifangjie Li,William C.Knight,Jinbin Xu
Abstract Neurodegenerative diseases are a class of chronic and complex disorders featuring progressive loss of neurons in distinct brain areas.The mechanisms responsible for the disease progression in neurodegeneration are not fully illustrated.In this observational study,we have examined diverse biochemical parameters in the caudate and putamen of patients with Lewy body diseases (LBDs) and Alzheimer disease(AD),shedding some light on the involvement of oxidative damage and neuroinflammation in advanced neurodegeneration.We performed Spearman and Mantel-Cox analyses to investigate how oxidative stress and neuroinflammation exert comprehensive effects on disease progression and survival.Disease progression in LBDs correlated positively with poly (ADP-Ribose) and triggering receptors expressed on myeloid cell 2 levels in the striatum of LBD cohorts,indicating that potential parthanatos was a dominant feature of worsening disease progression and might contribute to switching microglial inflammatory phenotypes.Disease progression in AD corresponds negatively with 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxo-dG) and myeloperoxidase concentrations in the striatum,suggesting that possible mitochondria dysfunction may be involved in the progression of AD via a mechanism of β-amyloid entering the mitochondria and subsequent free radicals generation.Patients with lower striatal 8-oxo-dG and myeloperoxidase levels had a survival advantage in AD.The age of onset also affected disease progression.Tissue requests for the postmortem biochemistry,genetics,and autoradiography studies were approved by the Washington University Alzheimer’s Disease Research Center (ADRC) Biospecimens Committee (ethics approval reference number:T1705,approval date:August 6,2019).Recombinant DNA and Hazardous Research Materials were approved by the Washington University Environmental Health &Safety Biological Safety Committee (approval code:3739,approval date:February 25,2020).Radioactive Material Authorization was approved by the Washington University Environmental Health &Safety Radiation Safety Committee (approval code:1056,approval date: September 18,2019).
Key Words:Alzheimer disease;disease progression;Lewy body diseases;microglia;neurodegeneration;oxidative damage;striatum;survival
Introduction
Neurodegenerative diseases are disorders defined by progressive impairment of motor and/or cognitive function.Neurodegenerative diseases lead to severe and restlessly functional impairments for millions of people annually,affecting individuals’ life quality and happiness,burdening their families and caregivers,as well as raising many healthrelated public concerns.There are no powerful therapies to prevent or reduce disease progression.However,available treatments can alleviate some symptoms,accompanied by ineffective consequences over time and final disruptive symptoms.One of the most significant challenges in neurodegenerative disease therapeutics is the clarification of clinically meaningful endpoints to measure progression.The pathogenesis of different neurodegenerative diseases shares specific characteristics,as featured with Alzheimer disease(AD),Parkinson’s disease (PD),amyotrophic lateral sclerosis(ALS),and Huntington’s disease (Cho,2010;Cummings,2017;Katsuno et al.,2018).
Increased levels of oxidative damages have been reported in all three stages of AD–mild cognitive impairment (MCI),early AD,and late-stage AD (Nunomura et al.,2001;Reed et al.,2009a,b),supporting the hypothesis that oxidative damages may be one of the common pathological mechanisms at different AD stages.The decreases in the antioxidant enzyme activity may promote enlarged productions of free radicals,leading to increased oxidative damages in the nuclear and mitochondrial DNA during the progression from mild cognitive impairment to early AD/AD (Migliore et al.,2005;Guidi et al.,2006;Wang et al.,2006).Studies are consistent with the assumption that oxidative damage originates in the mild cognitive impairment brain is an early AD event and contributes to the disease progression (Stephan et al.,2012).β-amyloid (Aβ) has been shown to enter mitochondria,generate free radicals,and cause oxidative damage in postmortem brain neurons from patients with AD and transgenic animal AD models (Cardoso et al.,2001;McLellan et al.,2003;Reddy,2006).PD is the second most universal neurodegenerative disease featuring the aggregation of α-synuclein (α-syn) and the loss of dopamine neurons in the substantia nigra.As the resident macrophages and the chief immune defenders of the central nervous system (CNS),microglia protect against the subsequent dangers following the accumulation of α-syn via different activated phenotypes(Sanchez-Guajardo et al.,2010).Microglia-mediated neuroinflammation and α-syn related disruption of cellular homeostasis promote each other;this positive feedback loop drives a worsening progression of neurodegeneration (Fellner et al.,2011;Schapansky et al.,2015).Pathologically,dying or dead dopaminergic neurons in PD release neuromelanin pigment,which could be one of the factors activating microglia (Wilms et al.,2003).Neuromelanin particles are phagocytized by microglia,inducing microglial activation,superoxide production,nitric oxide (NO),hydrogen peroxide(H2O2),and pro-inflammatory cytokines and chemokines(Zhang et al.,2011).Neuroinflammation and oxidative stress affect and potentiate each other,amplifying persistent and progressive neurodegeneration in PD.PD dementia (PDD),dementia with Lewy bodies (DLB),and PD have common clinical and neuropathological features and have been aggregated conceptually across the spectrum of Lewy body diseases (LBDs) (Lippa et al.,2007).
Recently,considerable advances in genome-wide association studies (GWASs) have classified genetic loci for Mendelian(or familial) neurodegenerative diseases (Gandhi and Wood,2010;Pihlstrøm et al.,2017).The dominant disorders are mostly sporadic and late-onset diseases caused by a complex of proteins misfolding and accumulating due to genetic mutations and environmental factors.Distinct biochemical entities in various brain areas have contributed to the understanding of neurodegenerative disease pathogenesis.The striatum contains two important sub-regions that receive input from different cortical regions–the caudate and putamen (Draganski et al.,2008).The caudate nucleus is implicated in diverse performances,including procedural learning and working memory (Seger and Cincotta,2005;Hannan et al.,2010).The dorsal posterior putamen receives its principal input from the motor and sensorimotor cortices and contributes extensively to the regulation of motor circuits(Del Campo et al.,2016).
Our laboratory has devoted considerable effort to the research of the striatum in patients with neurodegenerative diseases.We have found that the interactions between dopamine and oxidative damage play critical roles in advanced neurodegeneration (Li et al.,2020a).Chronic oxidative stress most likely leads to microglial phenotype changes between dystrophy and senescence (Han et al.,2019;Xu et al.,2019).Interestingly,we have observed that oxidative damages,generated by misfolded tau proteins and dopamine metabolism,give rise to microglial dysfunction,which reversely reduces tau propagation (Li et al.,2020b).The physiological crosstalk between disease progression in neurodegenerative diseases and oxidative damage and neuroinflammation is still a lingering question.In this study,we analyzed diverse biochemical parameters,determined by our previous research and the clinical presentations of study patients,to provide a comprehensive attempt at addressing the possible correlations between striatal oxidative damages,inflammation,and neurodegenerative progression and survival.
Materials and Methods
Ethics statement
Following local ethical committee protocols,patients gave written consent (Additional file 1) before cognitive impairment or responsible caregivers handed over approval antemortem or postmortem consent (Washington University Institutional Review Board,Washington University School of Medicine).Tissue requests for the postmortem biochemistry,genetics,and autoradiography studies were approved by the Washington University Alzheimer’s Disease Research Center(ADRC) Biospecimens Committee (ethics approval reference number:T1705,approval date:August 6,2019).Recombinant DNA and Hazardous Research Materials were approved by the Washington University Environmental Health &Safety Biological Safety Committee (approval code:3739,approval date:February 25,2020).Radioactive Material Authorization was approved by the Washington University Environmental Health &Safety Radiation Safety Committee (approval code:1056,approval date:September 18,2019) (Additional file 2).The study involving human tissue samples was conducted in accordance with theDeclaration of Helsinki.
Subjects
Clinically and neuropathologically well-analyzed human braintissues were gained from the Knight ADRC and the Movement Disorders Center brain bank at Washington University School of Medicine.Initially,we collected tissues from a population of 10 PD patients (7 men,3 women) aged 69–87 years at death (mean:78 ± 2),8 PDD patients (7 men,1 woman) aged 66–87 years at death (mean:77 ± 3),10 DLB patients (5 men,5 women) aged 69–89 years at death (mean:81 ± 2),27 AD patients (13 men,14 women) aged 62–94 years at death(mean:82 ± 2),and 10 age-matched congnitively control cases(6 men,4 women) aged 72–93 years at death (mean:83 ± 2)who passed away from non-neuronal causes.The arbitrary clinical distinction between DLB and PDD was achieved using the McKeith et al.criteria (Hughes et al.,1992;McKeith et al.,2005).Dementia level was classified by the Clinical Dementia Rating (CDR) scale (Emre et al.,2007).Participants with a CDR≥ 1 were enrolled following their clinical evaluation using the CDR criteria for diagnosing dementia in PD.AD pathological changes were evaluated using Braak staging (Thal and Braak,2005).Braak stages of amyloid-beta deposition are as follows:(A) the initial accumulates in the basal neocortex,(B) accumulations that extend into the adjacent areas of the neocortex,and (C) heavy accumulations throughout the entire cortex.Stages of neurofibrillary pathology are classified by their presentation in the transentorhinal (I–II),limbic (III–IV),and neocortical (V and VI) regions.There were no significant discrepancies in the average age and postmortem interval time across groups.All AD cases featured neurofibrillary tangles (NFTs) (V:13 patients;VI:14 patients) and significantly differed from those of the age-matched control cases (Average amyloid-beta:A;NFTs:II).Also,all LBDs showed NFTs.PD stages were defined by the Hoehn and Yahr scale (Hoehn and Yahr,2001).Sustained response to levodopa (L-dopa)is the supportive and positive criteria for LBDs,which were evaluated through L-dopa test (Abramsky et al.,1971).The clinical information and pathological characteristics are recapitulated inTable 1.
Tissue collection
Brain tissues were harvested at the time of autopsy;subsequently,the right hemisphere was coronally sectioned and snap-frozen using liquid nitrogen vapor.Then,tissue blocks were preserved at–80°C.Frozen coronal sections (20µm) were prepared for autoradiography study.The caudate and putamen were carefully cut using a scalpel from adjacent cryosections and tested for biochemistry.
Laboratory determinations of eight biochemical parameters
All tissue samples or tissue slices were prepared immediately before the assays.Concentrations of 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxo-dG),8-oxo-7,8-dihydroguanosine(8-oxo-G),myeloperoxidase (MPO),and poly (ADP-Ribose)(PAR) in the samples were determined to evaluate oxidative damages developed in the striatum of patients and agematched controls.Levels of triggering receptors expressed on myeloid cell 2 (TREM2) in the striatum were analyzed to understand the possible microglial phenotype changes promoted by oxidative damages.Densities of translocator protein (TSPO) and tau fibrils were quantified to estimate the correlations between microglia dystrophy and tauopathy.Additionally,dopamine levels in the caudate and putamen were quantitatively measured.The detailed experimental procedures for biochemistry and quantitative autoradiography assays can be found in our previous reports (Li et al.,2020a,b).Methods are briefly described below.All data are summarized inTable 2.

Table 1|Demographic characteristics of the study subjects
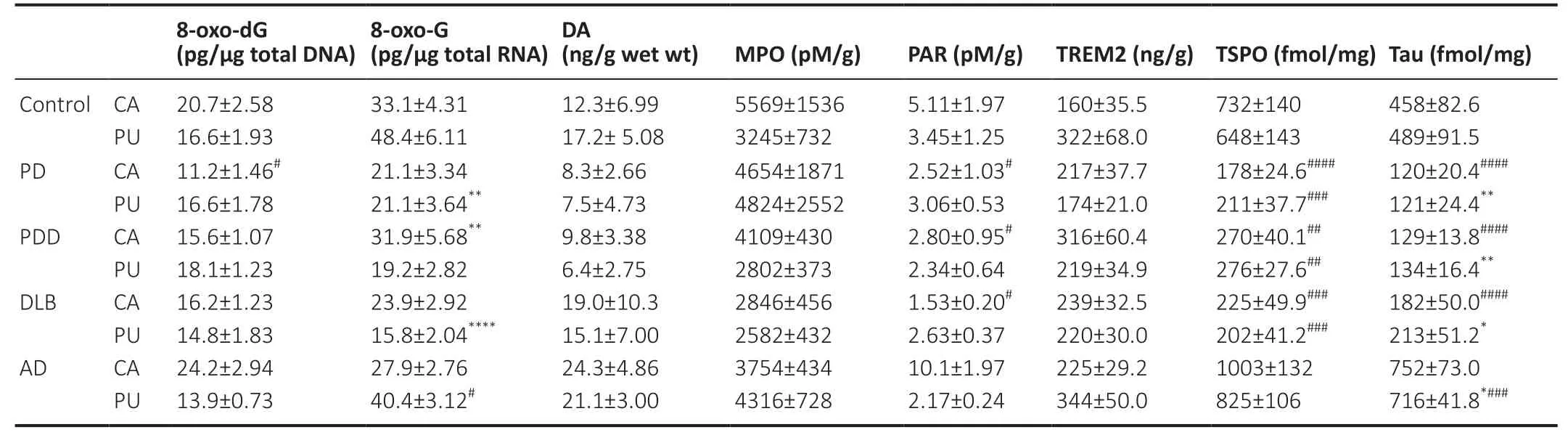
Table 2|Biochemical parameters of study participants
8-oxo-dG and 8-oxo-G quantification
Total DNA and RNA in the caudate and putamen of study brains were extracted separately using the Qiagen QIAamp DNA Mini Kit (Qiagen,Valencia,CA,USA;Cat# 51304) and the Qiagen RNeasy Plus Micro Kit (Qiagen;Cat# 74034).The concentrations of 8-oxo-dG and 8-oxo-G were determined separately using the OxiSelect oxidative DNA damage ELISA(Cell Biolabs,Inc.,San Diego,CA,USA;Cat# STA-320) and the OxiSelect oxidative RNA damage ELISA kits (Cell Biolabs,Inc.,Cat# STA-325-5).
MPO,PAR,and TREM2 quantification
Brain samples were homogenized in commercially available lysis buffer (for MPO and TREM2,Fisher Scientific,895347,Pittsburgh,PA,USA) or RIPA buffer (for PAR),the supernatant was collected to determine the expression of MPO,PAR,and TREM2 in the caudate and putamen using the Myeloperoxidase Human ELISA kit (Abcam,ab119605,Cambridge,MA,USA),PAR ELISA kit (Cell Biolabs,Inc.,XDN–5114,San Diego,CA,USA),and theTREM2 Human ELISA kit(Biomatik,EKU07882,Wilmington,DL,USA),respectively.
Dopamine quantification
Brain samples were homogenized with PBS (Fisher Scientific;Cat# 50-983-207),and the supernatant was collected for quantification of dopamine levels in the caudate and putamen using the dopamine ELISA kit (BioVision,Inc.,Milpitas,CA,USA,Cat# K4219).
TSPO and tau fibril quantification
The densities of TSPO and tau fibril in the caudate and putamen from study brains were quantified using autoradiography,[3H]PBR28 (80 Ci/mmol,CAS Number:253307-72-1) and [3H]MK6240 (21.5 Ci/mmol,CAS Number:1841078-87-2) were chosen as radioligands,respectively.The TSPO gene was also genotyped to screen and exclude the lowaffinity binders for [3H]PBR28 autoradiography binding data analysis.
Statistical analysis
Spearman’s correlation coefficient (rs) was calculated to assess the strength of the correlation between continuous variables.In this study,we estimated the correlations between patients’clinical presentations:age at the onset of disease,disease progression,Braak NFT stage,Braak Aβ stage,PD stage,and 8 biochemical parameters expressed in the caudate and putamen samples from patients with neurodegenerative diseases.Disease progression was calculated from the ageof the onset to the age of death and expressed in years.We used a log-rank (Mantel-Cox) test to estimate the relationships between patient survival and the 8 biochemical parameters.Mantel-Cox curves for the patient’s survival were constructed according to high concentrations of the biochemical parameter (above the mean value) and low concentrations of the biochemical parameter (below the mean value).Statistical analyses were achieved using GraphPad Prism 6.0 (GraphPad Software Inc.,San Diego,CA,USA;RRID:SCR_002798) for Windows and IBM SPSS Statistics version 23 (IBM,Armonk,NY,USA;RRID:SCR_002865).P<0.05 was considered statistically significant.No sample size calculations,blinding,or randomizations were performed during the statistical analyses.
Results
Demographic characteristics and biochemical parameters of study subjects
In this study,65 participants,aged between 62 and 94,met the clinical diagnostic criteria:10 PD subjects,8 PDD subjects,10 DLB subjects,27 AD subjects,and 10 healthy control subjects.Demographic characteristics of the disease and age-matched control groups are presented inTable 1.There were no significant differences in postmortem interval,age at death,brain weight,age at onset,and disease progression,suggesting that our results were not affected by these factors.Braak neurofibrillary tangle stage (NFT) stages observed in the AD group significantly differed from controls.
Moreover,levels of nucleic acid oxidative species and dopamine,expressions of MPO,PAR,and TREM2,as well as densities of TSPO and tau fibrils in the caudate and putamen of the patients with neurodegenerative diseases and controls were identical (Table 2).
Correlations of biochemical parameters and disease progression in patients with neurodegenerative diseases
In the Spearman correlation analyses,PDD progression positively depended on the concentration of PAR in the caudate (rs=0.786,P=0.036;Figure 1A).We observed a positive correlation between the TREM2 levels in the putamen of PDD patients and disease progression (rs=0.786,P=0.036;Figure 1B).Then we examined whether the microglial expression could be related to PDD disease progression.It appears that there was a negative correlation between TSPO levels in the putamen of the PDD group and disease progression (rs=–0.857,P=0.014;Figure 1C).We further examined the LBD cohorts,and Spearman analyses revealed positive correlations between PAR concentration in the caudate of LBD brains and disease progression (rs=0.539,P=0.010;Figure 1D),as well as TREM2 expression in the putamen of LBD brains and disease progression (rs=0.501,P=0.013;Figure 1E).Interestingly,we found significant negative correlations between 8-oxo-dG concentration in the putamen of AD brains and disease progression (rs=–0.453,P=0.020;Figure 1F),as well as MPO levels in the caudate of AD brains and disease progression (rs=–0.594,P=0.003;Figure 1G).
Correlations between the age of the onset and disease progression in patients with neurodegenerative diseases
Thought-provokingly,we found that the age of onset associated with disease progression in all the neurodegenerative diseases studied,as shown inFigure 2.Spearman analyses uncovered negative correlations between the age of onset and disease progression in PD,PDD,DLB,and AD cohorts (Figure 2A–D).These results supported the hypothesis that the older the patients were,the more severe their clinical and neurophysiologic features,and that onset age mainly altered the rate of progression (Kempster et al.,2010;Pagano et al.,2016).
Correlations between PD stage and biochemical parameters in patients with Lewy body diseases
LBD patients were stratified into different PD stages according to the Hoehn and Yahr scale (Hoehn and Yahr,2001).We investigated the relationships between biochemical parameters in the striatum and PD stages in LBD groups.A significant negative correlation was observed between PD stage and PAR concentration in the putamen of LBD brains(rs=–0.462,P=0.023;Figure 3A).Additionally,a significant positive relationship was found between PD stage and TREM2 concentration in the putamen of LBD cohorts (rs=0.421,P=0.036;Figure 3B),which was in agreement with the positive correlation between disease progression and TREM2 levels in the putamen of LBD cases.
Impact of biochemical parameters in the striatum on the survival of patients with AD
In the AD cohort,Mantel-Cox analyses revealed that patients with a lower 8-oxo-dG concentration in the putamen had a significant survival advantage over those with higher 8-oxo-dG levels (P=0.0195;Figure 4A),indicating that DNA oxidative damage in the putamen played a critical role in patient survival.Besides,there was a significant difference in survival between AD cases with a higher MPO concentration in the caudate and those with lower MPO concentration in the same brain area (P=0.0003;Figure 4B),suggesting MPO level in the caudate is a potential positive risk factor for survival in AD patients.
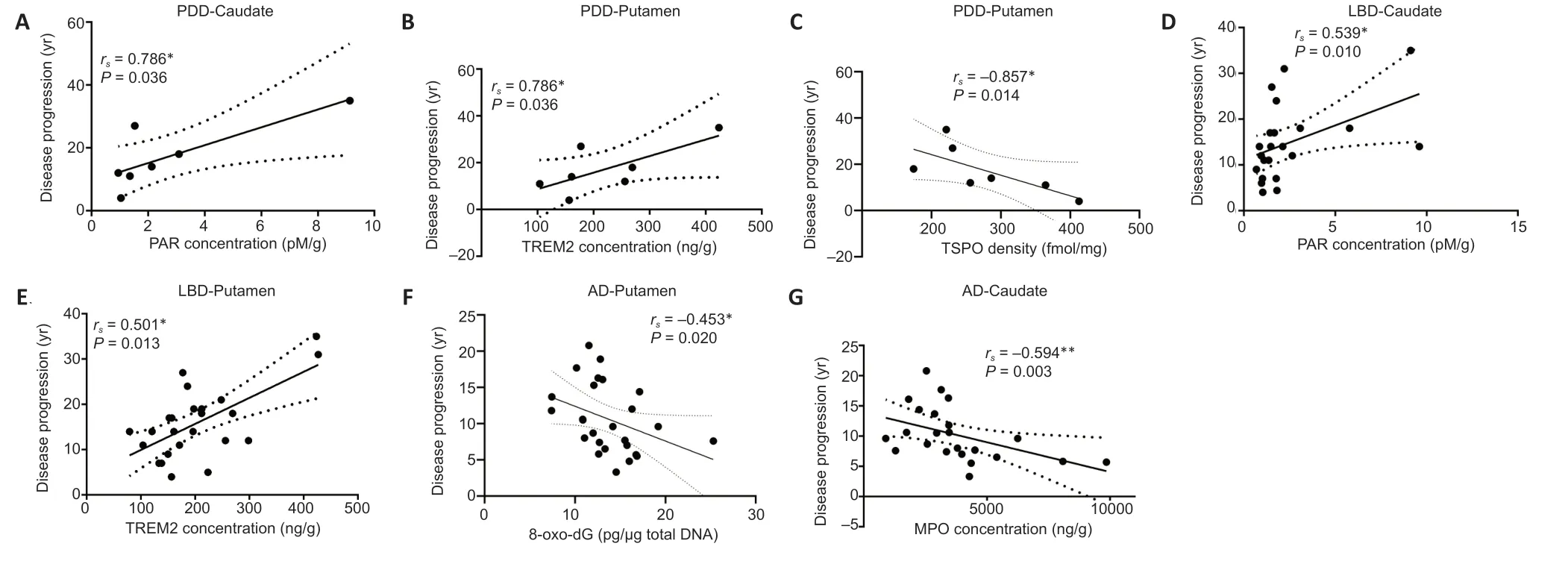
Figure 1|The correlations of biochemical parameters in the caudate and putamen of patients with diseases and disease progression.

Figure 2|The correlations of the age of onset and disease progression.
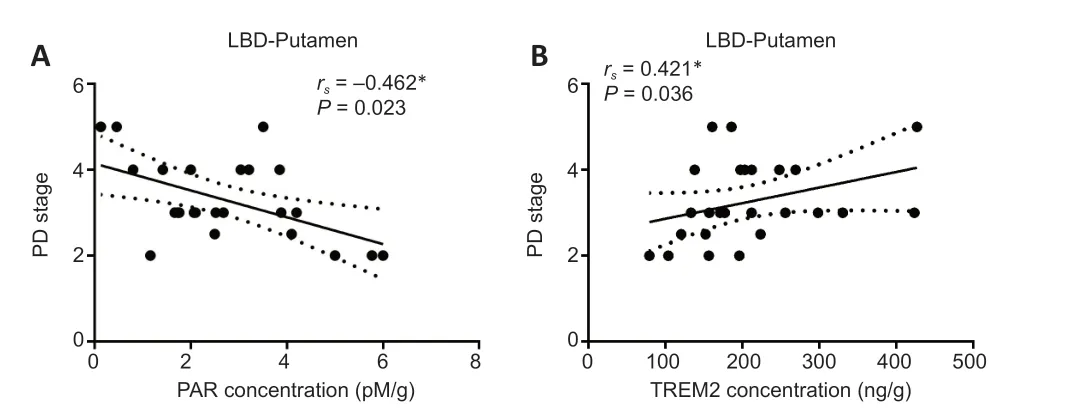
Figure 3|The correlations between PD stage and biochemical parameters in the putamen of patients with LBD.

Figure 4|Impact of biochemical parameters in the striatum on the AD patient survival.
Discussion
Neurodegenerative diseases are disorders featured by progressive dysfunction of the motor and/or cognition.Pathogenically,these diseases share common characteristics–a vicious pathogenic cycle,including the accumulation of insoluble proteins,disturbance of the redox homeostasis,activation of glial cells,and death/loss of neurons.
Disease progression in Lewy body diseases
LBDs are a family of neurodegenerative disorders identified by the presence of Lewy bodies,α-synuclein-positive inclusions in the brain.LBDs mainly consist of PD,DLB,and PDD.DLB is diagnosed as dementia that develops before or within 1 year of PD,and PDD is dementia manifesting in the context of confirmed PD (McKeith et al.,2005;Emre et al.,2007).However,numerous metabolic and functional deficiencies developed in the early stage of PD brains are promoted by oxidative stress and mitochondrial abnormalities rather than Lewy pathology (Ferrer,2009).
We found significant positive correlations between disease progression and PAR concentrations in the caudate of PDD cases and LBD cohorts.Coincidently,PAR-dependent cell death has been described to be prevalent in slowly progressing dopaminergic neurodegeneration in PD mouse models (Lee et al.,2013).Parthanatos–one form of cell death–was named from the integration of“PAR”and“Thanatos”,Thanatos being the god of death in Greek mythology (Fatokun et al.,2014).Parthanatos can be generated by the accumulation of PAR accompanied by the overactivation of PAR polymerase-1.Excesses of PAR stimulates the mitochondrial release of apoptosis-inducing factor (AIF),which,together with macrophage migration inhibitory factor,enters the nucleus and cleaves genomic DNA into large fragments (Park et al.,2020).Aminoacyl-tRNA synthetase complex interacting multifunctional protein-2 has been reported to be critically involved in the dopaminergic cell death mediated by parthanatos,indicating that PAR polymerase-1 inhibition may practically slow the progression of Parkinson disease(Lee et al.,2013).On the other hand,overactivation of PAR polymerase-1 plays a crucial role in causing deterioration of the blood-brain barrier,whose defense slows down PD progression (Wu et al.,2014).
Impressively,in contrast to the caudate results,we found a significant negative correlation between the PD stage and PAR concentration in the putamen of LBD brains.PD stages are defined by the Hoehn and Yahr Scale (Hoehn and Yahr,2001).Patients’ motor symptoms and secondary physical features get worse as the disease progresses,as well as the advanced PD stages,which can be proved further by the significant positive correlation between PD stage and disease progression in the LBD patients (Additional Figure 1;rs=0.479,P=0.015).These results might indicate asymmetric biochemical and pathological changes in the caudate and putamen of patients with LBDs.The putamen of PD patients has been defined with more deficiency in dopamine storage capacity,decreased dopaminergic projections from the substantia nigra,increased dopamine D2 receptor,and loss/or lower benzodiazepine receptor and muscarinic receptor when compared with the caudate of PD and AD cases (Griffiths et al.,1994;Rinne et al.,1995;Otsuka et al.,1996;Kumakura et al.,2006).We firstly reported the PAR concentrations in the caudate and putamen of patients with neurodegenerative diseases.However,by virtue of the small sample size for these groups,asymmetric alterations in the PAR levels of the caudate and putamen still need to be more robustly elucidated in further studies.
It was remarkable to find significant positive correlations between disease progression/PD stage and TREM2 expressions in the putamen of PDD cases and the LBD cohorts.TREM2 is expressed entirely by microglia within the central nervous system,acting as a primary immune checkpoint that modulates the microglial homeostatic pathway (Jay et al.,2017).TREM2 upregulation in the late-stage of AD progression was reported in postmortem brains (Perez et al.,2017).Emerging evidence shows that microglia can polarize into classic inflammatory M1 and immunosuppressive M2 phenotypes.TREM2 is beneficial for switching between the M1 and M2 microglial phenotypes and is especially essential for M2 microglia polarization (Belloli et al.,2017).In a previous report,diverse stages of diseaseassociated-microglia were modulated independently and/or dependently by TREM2.This finding,along with our results,suggests high microglial heterogeneity in individual patients during the disease progression or stages (Masuda et al.,2020).Upregulation of TREM2 levels in the late disease progression seems to be a compensatory attempt of TREM2 to alleviate the microglial over-activation or ameliorate dystrophic,or senescent,microglial types (Zhang et al.,2018).This hypothesis was supported further by the significant negative correlation between disease progression and TSPO density in the putamen of PDD patients–a marker of brain microglia activation.
Disease progression and survival in AD
Information obtained from studies of familial AD models might also be applicable to sporadic AD,where increased age-dependent oxidative damages have been clarified as a potential key factor leading to a more rapid disease progression (Melov,2004;Manczak et al.,2005).Mechanistically,Aβ enters mitochondria and generates free radicals,leading to oxidative damages in postmortem AD brain cells and,in turn,disrupts mitochondrial function (McLellan et al.,2003;Crouch et al.,2005).Therefore,mitochondrial dysfunction is tightly involved in the onset and progression of AD,which might be an alternative therapeutic target (Yang et al.,2020).The significant negative correlations between disease progression and 8-oxo-dG concentration in the putamen,as well as MPO levels in the caudate of patients with AD,lends evidence for this hypothesis.
8-oxo-dG,the guanine oxidation product of DNA,can cause GC to AT transversions.The increased DNA damage and decreased DNA repair found in AD patients have been closely correlated to the deficiency in the nonhomologous end joining repair pathway (De Zio et al.,2012).Studies conducted up to now are consistent with the assumption that oxidative stress initiated early in AD brains may contribute to AD progression.Survival analyses of AD cases point towards an inherently higher risk of mortality in AD patients with a higher 8-oxo-dG concentration in the putamen.
The MPO protein,released from azurophilic granules,can generate cytotoxic reactive oxygen species (ROS) and can also form neutrophil extracellular traps when combined with other neutrophil proteins (Aratani,2018).Clinical studies have revealed that alterations in the neutrophil activity and levels of ROS and neutrophil extracellular traps are associated with AD disease progression (Vitte et al.,2004;Dong et al.,2018).Pathologically,MPO has been found in the frontal cortex and hippocampus of AD brains,co-localized with amyloid plaques and neurofibrillary tangles (NFTs).Moreover,higher MPO concentration in the blood might be relevant to elevated levels of oxidative stress markers in AD patient blood samples(Gellhaar et al.,2017;Swardfager et al.,2017).The Mantel-Cox curve of patient’s survivals showed that AD patients with a lower MPO level in the caudate had a survival advantage over those with a higher level.
ROS reacts with cellular components and leads to disruption of redox homeostasis and cell death.ROS also accelerates the disease progression and ultimately influences life span.Moreover,survival time was linked with age of diagnosis,living status,medication treatment,and epilepsy history.
The correlation of age of onset and disease progression
Depending on the age of onset,PD patients have been classified into four phenotypes in a new investigation:younger than 50,50–59,60–69,and older than or equal to 70 years (Pagano et al.,2016).The older the patients were,the more severe their clinical presentations,including motor dysfunction and cognitive impairment.This might be explained by their more significant dopaminergic deficiency and increased reduction of α-syn and total tau proteins in their cerebrospinal fluid,indicating that onset age closely affects the rate of disease progression.On the contrary,earlyonset AD–defined by a young age of onset (<65 years)–presents with worse neurodegeneration and a more rapid disease progression,probably due to chronic microglial overactivation and abnormal glucose hypometabolism (Tondo et al.,2020).Intriguingly,in this study,we found significant negative correlations between age of onset and disease progression in PD,PDD,DLB,and AD.These trends seem to indicate that the younger age of onset,the longer survivaltime patients experience.However,the common mechanism and therapy target for slowing disease progression needs to be explored.
The coupling found in our analysis between the patients’biochemical parameters and disease progression and survival is interesting,although limited by the subject numbers and the brain regions studied.Thus,the more systematic,extensive,and cross-sectional investigations in a larger population of patients with different brain regions would further validate bonafide biomarkers and facilitate understanding of the mechanisms involved in disease progression.Postmortem studies using the end-stage cases may not reflect the biomarker changes of the clinical phases.Therefore,our results need to be further confirmed by additional clinical trials and compared with antemortem cerebrospinal fluid and plasma oxidative damage measures.
Conclusions
Using 8 biochemical parameters examined from the caudate and putamen of patients with neurodegenerative diseases,we have performed Spearman and Mantel-Cox analyses to explore the correlations between these biochemical parameters,disease progression,and survival.These results supported the concept that the two characteristic features of neurodegenerative diseases,disruption of redox homeostasis and neuroinflammation,can potentiate each other and lead to the loss and dysfunction of neurons that affect disease progression.Furthermore,we found that older age of onset was associated with a faster disease progression in the neurodegenerative cohorts studied.The apparent lack of large cohort recruitments hardly supported some degree of generalizability of our findings.Essential future directions for research could include studies examining factors that may influence the progression and survival rates reported in the present study with larger cohorts of late-onset diseases and early-onset diseases induced by genetic mutations.Although the exact detailed mechanisms remain clarified,our observations might help understand these diseases’progression and survival rates.
Acknowledgments:We thank all participants and their families for their commitment and dedication to advancing research of diagnosis and treatment for PD and AD‚as well as the Knight-ADRC and MDC research staff for their contributions.We also thank Drs.John C.Morris‚Joel S.Perlmutter‚and Nigel Cairns‚Ms.Erin E.Franklin‚and Mr.Michael Baxter of the Knight Alzheimer’s Disease Research Center Neuropathology Core at Washington University School of Medicine‚for coordination of thetissue preparation and expert technical assistance.We thank Christopher Sawyer and other staff members at the Genome Technology Access Center (GTAC) of Washington University in St.Louis for helping with genotyping studies.
Author contributions:Study concept‚design‚and supervision:JX.Qualitative analysis and statistics of data:HL‚WCK‚and JX.Preparation oftissue:WCK‚HL‚and JX.Writing the draft with feedback from all authors:HL.All authors approved the final version of the paper.
Conflicts of interest:The authors declare no conflict of interest.
Financial support:This study was funded by NIH R01NS092865 and P30AG06644.
Institutional review board statement:Tissue requests for the postmortem biochemistry‚genetics‚and autoradiography studies were approved by the Washington University Alzheimer’s Disease Research Center (ADRC) Biospecimens Committee (ethics approval reference number:T1705‚approval date:August 6‚2019).Recombinant DNA and Hazardous Research Materials were approved by the Washington University Environmental Health &Safety Biological Safety Committee(approval code:3739‚approval date:February 25‚2020).Radioactive Material Authorization was approved by the Washington University Environmental Health &Safety Radiation Safety Committee (approval code:1056‚approval date: September 18‚2019).
Declaration of participant consent:The authors certify that they have obtained all appropriate participant consent forms.In the forms‚the participants have given their consent for their images and other clinical information to be reported in the journal.The participants understand that their names and initials will not be published‚and due efforts will be made to conceal their identity.
Reporting statement:This study followed the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement.
Biostatistics statement:The statistical methods of this study were reviewed by the biostatistician of Washington University School of Medicine in USA.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:All of the deidentified data published in this communication will be available upon request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal‚and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License‚which allows others to remix‚tweak‚and build upon the work non-commercially‚as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files:
Additional Figure 1:Correlations between PD stage and disease progression in the patients with Lewy body diseases.
Additional file 1:Model consent form.
Additional file 2:Ethical Approval Documentation.
- 中国神经再生研究(英文版)的其它文章
- Towards a comprehensive understanding of p75 neurotrophin receptor functions and interactions in the brain
- Microglia regulation of synaptic plasticity and learning and memory
- Stroke recovery enhancing therapies:lessons from recent clinical trials
- Functional and immunological peculiarities of peripheral nerve allografts
- MicroRNA expression in animal models of amyotrophic lateral sclerosis and potential therapeutic approaches
- Significance of mitochondrial activity in neurogenesis and neurodegenerative diseases

