Cerebral dopamine neurotrophic factor transfection in dopamine neurons using neurotensin-polyplex nanoparticles reverses 6-hydroxydopamine-induced nigrostriatal neurodegeneration
Manuel A.Fernandez-Parrilla ,David Reyes-Corona ,Yazmin M.Flores-Martinez ,Rasajna Nadella ,Michael J.Bannon ,Lourdes Escobedo ,Minerva Maldonado-Berny,Jaime Santoyo-Salazar,Luis O.Soto-Rojas,Claudia Luna-Herrera,Jose Ayala-Davila,Juan A.Gonzalez-Barrios,Gonzalo Flores,Maria E.Gutierrez-Castillo0,Armando J.Espadas-Alvarez0,Irma A.Martínez-Dávila,Porfirio Nava,Daniel Martinez-Fong,,
Abstract Overexpression of neurotrophic factors in nigral dopamine neurons is a promising approach to reverse neurodegeneration of the nigrostriatal dopamine system,a hallmark in Parkinson’s disease.The human cerebral dopamine neurotrophic factor (hCDNF) has recently emerged as a strong candidate for Parkinson’s disease therapy.This study shows that hCDNF expression in dopamine neurons using the neurotensinpolyplex nanoparticle system reverses 6-hydroxydopamine-induced morphological,biochemical,and behavioral alterations.Three independent electron microscopy techniques showed that the neurotensin-polyplex nanoparticles containing the hCDNF gene,ranging in size from 20 to 150 nm,enabled the expression of a secretable hCDNF in vitro.Their injection in the substantia nigra compacta on day 21 after the 6-hydroxydopamine lesion resulted in detectable hCDNF in dopamine neurons,whose levels remained constant throughout the study in the substantia nigra compacta and striatum.Compared with the lesioned group,tyrosine hydroxylase-positive (TH+) nigral cell population and TH+fiber density rose in the substantia nigra compacta and striatum after hCDNF transfection.An increase in βIII-tubulin and growth-associated protein 43 phospho-S41 (GAP43p) followed TH+ cell recovery,as well as dopamine and its catabolite levels.Partial reversal (80%) of drugactivated circling behavior and full recovery of spontaneous motor and non-motor behavior were achieved.Brain-derived neurotrophic factor recovery in dopamine neurons that also occurred suggests its participation in the neurotrophic effects.These findings support the potential of nanoparticle-mediated hCDNF gene delivery to develop a disease-modifying treatment against Parkinson’s disease.The Institutional Animal Care and Use Committee of Centro de Investigación y de Estudios Avanzados approved our experimental procedures for animal use(authorization No.162-15) on June 9,2019.
Key Words:axonal growth;brain-derived neurotrophic factor;gene delivery;nanoparticles;neuritogenesis;neuronal cytoskeleton;neuroregeneration;neurorestoration;neurotrophic therapy;Parkinson’s disease;reinnervation;substantia nigra
Introduction
Neurodegeneration and progressive loss of nigrostriatal dopamine neurons in Parkinson’s disease (PD) lead to motor behavior deficits,which are the main symptom and the basis of early diagnosis (Reich and Savitt,2019).Since the current pharmacological therapy for PD is symptomatic,different neurotrophic factor approaches have been developed to halt neurodegeneration and restore the nigrostriatal dopamine system (Nasrolahi et al.,2018).Transfection of a neurotrophic gene in dopamine neurons is an attractive approach to provide a dual neurotrophic effect on the nigrostriatal dopamine pathway through the transgenic protein released from the cell body and terminals after its anterograde axonal transport(Martinez-Fong et al.,2012).
The nanoparticle system neurotensin (NTS)-polyplex enables the targeted gene delivery via NTS receptor 1 (NTSR1)internalization to dopamine neuronsin vitro(Hernandez-Baltazar et al.,2012) andin vivo(Alvarez-Maya et al.,2001).The nanoparticles result from a plasmid DNA (pDNA)compaction by the electrostatic binding of the synthetic karyophilic peptide KPRa and the conjugate of poly-L-lysine with NTS and HA2-fusogenic peptide (FP) known as NTScarrier (Lopez-Salas et al.,2020).The peptides act sequentially to transport the pDNA toward the cell nucleus (Martinez-Fong et al.,2012).NTS binds to NTSR1 to activate the internalization of the NTS-polyplex vector (Arango-Rodriguez et al.,2006;Hernandez et al.,2014;Espadas-Alvarez et al.,2017;Aranda-Barradas et al.,2018) into nigral dopaminergic neurons that highly express NSTR1 in the plasma membrane (Martinez-Fong et al.,2012).In the endosomes,acidity activates HA2-FP to rescue the pDNA from degradation (Navarro-Quiroga et al.,2002;Arango-Rodriguez et al.,2006),and KPRa,a nuclear localization signal (NLS),transports it from the cytoplasm to the cell nucleus (Lopez-Salas et al.,2020).
NTS-polyplex nanoparticle system efficiently delivers neurotrophic factor genes even to dopamine neurons with 6-hydroxydopamine (6-OHDA) lesion (Martinez-Fong et al.,2012).The transfection of the gene coding for human glial cell-derived neurotrophic factor (hGDNF) or brain-derived neurotrophic factor (BDNF) halts the advance of nigrostriatal dopamine system neurodegeneration (Gonzalez-Barrios et al.,2006;Hernandez-Chan et al.,2015).Furthermore,the transfection of the neurturin (NRTN) gene or BDNF gene reverses nigrostriatal system neurodegeneration (Razgado-Hernandez et al.,2015;Reyes-Corona et al.,2017).NTSpolyplex nanoparticle efficacy comes from producing the transgenic neurotrophic factor in the cell body,which enables its release from both the body and axonal projection terminals after its anterograde transport (Gonzalez-Barrios et al.,2006;Hernandez-Chan et al.,2015).
Cerebral dopamine neurotrophic factor (CDNF) has shown potential for PD therapy because of its antineuroinflammatory,neuroprotective and neurorestorative effects on chemically induced parkinsonism (Lindholm et al.,2007) that arise from two mechanisms.The first mechanism results from its location in the lumen of the endoplasmic reticulum (ER),whose primary function is the modulation of the unfolded protein response pathway to avoid ER stress and neuroinflammation (Liu et al.,2015),as shownin vitro(Cheng et al.,2013;Zhao et al.,2014;Arancibia et al.,2018) andin vivo(Nadella et al.,2014).The second mechanism implies the release of CDNF and its binding to unidentified receptors that activate intracellular signaling pathways that finally promote neuronal restoration (Lindholm and Saarma,2010),as shown by CDNF infusion in parkinsonian rodents and nonhuman primates (Garea-Rodriguez et al.,2016;Huttunen and Saarma,2019).
The preventive and neurorestorative effect of CDNF has been shown in PD models developed with 6-OHDA in rats and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in mice and nonhuman primates using several delivery approaches(Chmielarz and Saarma,2020).Its neurorestorative effect has been demonstrated using CDNF protein delivery to the striatum through single or repeated injections or continuous infusion in rats (Voutilainen et al.,2011),mice (Airavaara et al.,2012),and nonhuman primates (Garea-Rodriguez et al.,2016),and specifically into the subventricular zone (SVZ)of rats (Nasrolahi et al.,2019).CDNF gene delivery to the striatum has also been explored using the recombinant adenoassociated virus (AAV8) (Wang et al.,2017a).Importantly,intermittent monthly bilateral intraputamenal infusions of CDNF are currently being tested in a randomized placebocontrolled phase I–II clinical study in moderately advanced PD patients (Huttunen and Saarma,2019).Initial data suggest that CDNF is safe and shows encouraging therapeutic effects(Chmielarz and Saarma,2020).
The neurorestorative effect of hCDNF in gene therapy has been mainly shown by dopamine phenotype recovery and motor deficit reversal (Lindholm et al.,2007;Ren et al.,2013).However,the association with neurostructural markers and hCDNF presence in projection terminals remains unknown.Here,we explored whether the association between dopaminergic neural regeneration and hCDNF presence occurs using NTS-polyplex nanoparticle-mediated hCDNF gene delivery to dopamine neurons after 21 days of a 6-OHDA lesion.
Materials and Methods
Plasmid pNBRE-hCDNF
pNBRE3×-hCDNF plasmid (4485 bp) encodes hCDNF under the transcriptional control of the synthetic NBRE3× promoter that is responsive to Nurr1 in dopamine neurons (Sacchetti et al.,2001;Espadas-Alvarez et al.,2017).This plasmid was obtained from the plasmids pCR3.1-hCDNF and pGL2-Basic-NBRE3×-luc(Nadella et al.,2014).
Synthesis of the NTS-carrier and assembly of the NTS-polyplex
The detailed procedure of NTS-carrier synthesis,a conjugate of NTS (Sigma-Aldrich,St.Louis,MO,USA) and HA2 FP (>90% purity;Peptide 2.0;Chantilly,VA,USA) with poly-L-lysine(Sigma-Aldrich) was described previously (Arango-Rodriguez et al.,2006).
The NTS-polyplex nanoparticles were electrostatically assembled by binding the cationic NTS-carrier with the anionic KPRa-pNBRE3×-hCDNF complex (Figure 1A) in two steps using Dulbecco′s modified Eagle′s medium (DMEM;Thermo Scientific,Rockford,IL,USA) as a vehicle (Navarro-Quiroga et al.,2002;Hernandez-Baltazar et al.,2012).
First step.The KPRa-pNBRE3×-hCDNF complex is formed by incubating increasing concentrations (1–13 µM) of cationic KPRa (KMAPKKRK;>90% purity;Peptide 2.0;Chantilly,VA,USA) to an invariable 6 nM pNBRE3×-hCDNF (anionic).After 30-minute incubation,an electrophoretic retardation assay was performed by subjecting the samples at 80 V for 30 minutes in a 0.8% agarose gel using 40 mM Tris-Acetate,1 mM EDTA buffer,pH8 (TAE).The gel was stained with ethidium bromide (10 µg/mL) to select the KPRa concentration (6 µM)that delays pNBRE3×-hCDNF band migration up to half of the control band (Figure 1B),based on the criterion previously established (Navarro-Quiroga et al.,2002;Hernandez-Baltazar et al.,2012).A recent report shows that at that molar ratio (6µM KPRa:6 nM pDNA),the KPRa-pDNA complex leaves free negative charges to allow binding of the NTS-carrier in the following step (Lopez-Salas et al.,2020).
Second step.The NTS-polyplex nanoparticles are formed by incubating the KPRa-pNBRE3×-hCDNF complex (6 µM KPRa:6 nM pNBRE3×-hCDNF) with increasing concentrations (1–45 nM) of NTS-carrier.After 30 min incubation,the samples were subjected to electrophoretic retention gel assay using the conditions described above.According to the criterion previously established (Navarro-Quiroga et al.,2002;Arango-Rodriguez et al.,2006;Hernandez-Baltazar et al.,2012;Martinez-Fong et al.,2012),the optimum molar ratio was obtained at 30 nM NTS-carrier concentration,which follows the concentration producing the last visible retention of the KPRa-pNBRE3×-hCDNF band.The selected NTS-concentration produces the complete neutralization of the KPRa-pDNA complex charges (Lopez-Salas et al.,2020).The optimum molar ratio to assemble functional NTS-nanoparticles was 6 nM pNBRE3×-hCDNF:6 µM KPRa:30 nM NTS-carrier (Figure1C),the total amount of pNBRE3×-hCDNF injected into the substantia nigra pars compacta (SNc) was 260 ng (based on plasmid weight).
Electron microscopy analysis of NTS-polyplex nanoparticles
The Advanced Laboratory of Electron Nanoscopy of the Center for Research and Advanced Studies (CINVESTAV) characterized NTS-polyplex nanoparticles using field emission scanning electron microscopy (FE-SEM;Zeiss GmbH Auriga-39-16;Oberkochen,Germany) at 20 kV,transmission electron microscopy (TEM;JEM 2010,JEOL,Ltd.,Tokyo,Japan) at 200 kV,and scanning probe microscope (SPM;JSPM 5200;JEOL,Ltd.) in atomic force microscopy (AFM) mode.The detailed procedures for TEM (Arango-Rodriguez et al.,2006) and FESEM (Espadas-Alvarez et al.,2017) were previously reported.For AFM analysis,5 µL of NTS-polyplex nanoparticles containing the plasmid pNBRE3×-hCDNF in DMEM was added,and a thin smear was made over a glass slide and left for few minutes,then the sample was scanned by the SPM operated in AFM contact mode.Image processing and topography analysis were done with WinSPM Data Processing System Software (Version 2.15,R.B.Leane,JEOL Ltd.).
Biological functionality of NTS-polyplex nanoparticles in vitro
NTS-polyplex nanoparticle-mediated transfection of pNBRE3×-hCDNF plasmid was tested in N1E-115 cells (ATCC;Manassas,VA,USA) cotransfected with pCMV-Script-Nurr1 using lipofectamine 2000 (Life Technologies,San Diego,CA,USA)to express Nurr1 (N1E-115-Nurr1 cells) and thus activating NBRE3× promoter (Espadas-Alvarez et al.,2017).After 48 hours post-transfection,hCDNF was detected through immunofluorescence in cells counterstained with 1 µM Hoechst 33258 (Sigma-Aldrich) and quantified by enzymelinked immunosorbent assay (ELISA),as described below.
Animals
One hundred and twenty male Wistar rats of 2-month-old(body weight 210–230 g) were obtained from the animal facilities at CINVESTAV and housed in rooms with an inverted 12-hour light/dark cycle,constant temperature (23 ± 2°C),and humidity (55 ± 5%).Water and food were available ad libitum.Animals (n=120) were randomly grouped as follows:1) The healthy untransfected group (UT;n=20) evaluated on day 81 after the study initiation;2) The initial lesion group (L21;n=20) evaluated on day 21 after 6-OHDA (Sigma-Aldrich) lesion;3) The final lesion group evaluated on day 81 after 6-OHDA injection (L81;n=20);4) Three transfection groups (T15,T30,T60;n=20 per time point) that were all transfected on day 21 post-lesion (day 0) and evaluated on days 15,30 and 60 posttransfection (Additional Figure 1).
All procedures were performed under the current Mexican legislation,NOM-062-ZOO-1999 (SAGARPA),based on the Guide for the Care and Use of Laboratory Animals,National Research Council.Our experimental procedures for animal use were approved by the Institutional Animal Care and Use Committee of Centro de Investigación y de Estudios Avanzados(authorization No.162-15;approval date:June 9,2019).The total number of animals was 120,a minimum required by the experimental design in compliance with the Guide for the Care and Use of Laboratory Animals (The National Academies Collection:Reports funded by National Institutes of Health,2011).Throughout the study,all efforts were made to minimize animal suffering.
Intracranial injections of 6-OHDA
Rats were anesthetized with a mixture of ketamine (120 mg/kg) and xylazine (9 mg/kg) (both anesthetics were from PiSA Agropecuaria,Guadalajara,Jalisco,Mexico),injected intraperitoneally.6-OHDA was prepared at a concentration of 20 µg free base in 3 µL of phosphate-buffered saline solution(PBS) containing 0.2% ascorbic acid (Sigma-Aldrich) and injected into the left striatum at the following coordinates according to the stereotaxic atlas of the rat brain (Paxinos and Watson,1998):anteroposterior (AP),+7.7 mm from the interaural midpoint;mediolateral (ML),+4.0 mm from the intraparietal suture;dorsoventral (DV),–5.4 mm from the dura mater (Nadella et al.,2014).At 21 days post-lesion (L21),when apoptosis ended (Hernandez-Baltazar et al.,2013),3 µL of NTS-polyplex containing the plasmid pNBRE3×-hCDNF was injected into the lesioned SNc at a flow rate of 0.1 µL/min.The SNc coordinates (Paxinos and Watson,1998):AP,+2.5 mm from the interaural midpoint;ML,+2.0 mm from the intraparietal suture;DV,–6.7 mm from the dura mater (Nadella et al.,2014).
Dissection of cerebral nuclei for molecular and biochemical assays
The brain was obtained free of meninges and immediately submerged in cold PBS.Then 0.5-mm coronal slices were sectioned using a cold metallic rat brain matrix (Stoelting,Wood Dale,IL,USA) to dissect the SNc and striatum as previously reported (Flores-Martinez et al.,2018).Each sample was immediately stored in a respective Eppendorf tube at–70°C until used for ELISA,western blot,and highperformance liquid chromatography (HPLC).
ELISA
hCDNF levels were measured in pellets and culture medium of N1E-115-Nurr1 cells and SNc and striatum homogenates using ELISA type sandwich and SEG458Hu kit (Uscn Life Science Inc.,Wuhan,Hubei,China),as described previously (Nadella et al.,2014).The absorbance of samples was read at 450 nm using an iMarkTMMicroplate Absorbance Reader (Bio-Rad Laboratories,Hercules,CA,USA).The detection range was 15.6-1000 pg/mL.Total protein was determined using the PierceTM BCA protein assay kit (Thermo Scientific),and the values were expressed as pg/mg protein.
Western blot assay
βIII-Tubulin and GAP43p were detected by western blot in 50 µg of SNc homogenates following the procedure reported elsewhere (Hernandez-Baltazar et al.,2013).Total protein was determined using the PierceTM BCA protein assay kit(Thermo Scientific).The primary antibodies were incubated overnight at 4°C,whereas the secondary antibodies were incubated for 2 hours at room temperature.The primary antibodies were rabbit anti-βIII-tubulin (1:1000;T2200;Sigma-Aldrich) and rabbit anti-growth-associated protein 43 phospho-S41 (GAP43p;1;1:5000;Abcam;Cambridge,MA,USA).The secondary antibody was a donkey anti-rabbit IgG conjugated with horseradish peroxidase (HRP;1:5000;Zymed,Cambridge,MA,USA).After stripping,the membranes were incubated with a mouse monoclonal antibody against glyceraldehyde 3-phosphate dehydrogenase (GA3PDH;1:5000;AB8245;Abcam),followed by an HRP-conjugated goat antimouse (1:5000;Zymed).Proteins were detected using the ECL Western blotting system and Hyperfilm ECL (Amersham,Buckinghamshire,UK).ImageJ software v.1.52h (National Institutes of Health,Bethesda,MD,USA) was used for densitometry of protein bands whose values (arbitrary units)were doubly normalized first with the densitometric values of the GAPDH band and then with the values of control without treatment.
High-performance liquid chromatography
The contents of dopamine,DOPAC (3,4-dihydroxyphenylacetic acid),and HVA (homovanillic acid) were measured in 20µL of homogenates of the SNc and striatum by HPLC as described previously (Reyes-Corona et al.,2017).The pellets were resuspended in 120 µL of 0.1 M NaOH for protein determination using the PierceTM BCA protein assay kit(Thermo Scientific).The values were expressed as pg of catecholamine/µg protein.
Double immunofluorescence and tyrosine hydroxylaseimmunohistochemistry assays
Animals were transcardially perfused with 150 mL of cold PBS,followed by 150 mL of 4% paraformaldehyde in PBS.The brain was cut into successive coronal 30-µm slices for the double immunofluorescence procedure described elsewhere(Nadella et al.,2014;Reyes-Corona et al.,2017).The primary antibodies were incubated overnight at 4°C,whereas the secondary antibodies were incubated for 2 hours at room temperature.The primary antibodies were rabbit polyclonal anti-CDNF (1:1000;ProSci Inc.,San Diego,CA,USA),which detects both rat and human CDNF,mouse anti-tyrosine hydroxylase (TH;1:800;MAB7566;R&D Systems,Minneapolis,MN,USA),polyclonal rabbit anti-βIII-tubulin (1:300;T2200;Sigma-Aldrich),and rabbit anti-BDNF (1:500;ab 108319;Abcam).An irrelevant antibody of the same IgG subclass substituted the primary antibody in all the negative controls.The secondary antibodies were Texas Red goat anti-rabbit IgG(H+L) (1:400;Vector Laboratories;Burlingame,CA,USA),Alexa Fluor 488 chicken anti-mouse IgG (H+L) (1:200;Invitrogen Molecular Probes,Eugene,OR,USA),Texas Red horse antimouse IgG (H+L) (1:900;Vector Laboratories),and Alexa Fluor 488 chicken anti-rabbit IgG (H+L) (1:200;Invitrogen Molecular Probes;Eugene,Oregon,USA).Fluorescence labeling was analyzed with a multispectral confocal laserscanning microscope SP8 (TCS-SPE,Leica,Nussloch,Germany)at excitation-emission wavelengths of 405–465 nm (blue channel) for Hoechst nuclear staining,488–522 nm (green channel) for Alexa Fluor 488,and 568–635 nm (red channel)for Texas Red.Serial 1-µm optical sections were also obtained in the Z-series (scanning rate 600 Hz).The images were acquired using LAS AF software (Leica application suite;Leica Microsystems,Nussloch,Germany).
TH-immunohistochemistry was performed as described previously (Soto-Rojas et al.,2020c).The primary antibody was a mouse monoclonal anti-TH clone TH-2 (1:1000;Sigma-Aldrich) incubated overnight at 4°C,and the secondary antibody was a horse biotinylated anti-mouse IgG (H+L)(1:200;Vector Laboratories) incubated for 2 hours at room temperature.The immunohistochemical staining was observed with a Leica DMIRE2 microscope (Leica) through 1.6×,5×,and 20× objectives,and images were digitalized with a DC300F camera (Leica) for quantification of TH+bodies and terminals.
Neuron counting and ramification densitometry
ImageJ software v.1.49r (National Institutes of Health) was used to count TH+neurons in the SNc and measure the total density area of TH+fibers in the SNc and striatum (Soto-Rojas et al.,2020c).All background intensity was eliminated from the immunohistochemically stained area to quantify only TH+immunoreactivity.Counting of TH+cells and fibers was performed in five anatomical levels per nucleus of six independent rats per experimental condition.The mean value calculated from those quantifications was the final measurement in each time evaluated (n=6 for every experimental condition).The same procedure was followed to quantify immunofluorescence area density (IFAD).
Spontaneous behavior tests
Four rats randomly selected from each group were evaluated with tests that measure spontaneous behavior following the procedures described in detail elsewhere (Soto-Rojas et al.,2020a,b).Vibrissae-evoked forelimb placing test and limbuse asymmetry test assessed spontaneous motor asymmetry(Schallert and Woodlee,2004).The former test was evaluated through unsuccessful trials of the forepaw contralateral to the lesion expressed as a percentage.The beam walking test measured bradykinesia and dysbalance.The olfactory test was used to evaluate non-motor behavior (Van Kampen et al.,2015).
Circling behavior test
Ipsilateral turning behavior activated by DL-methamphetamine(8 mg/kg body weight,i.p.;Sigma-Aldrich),dose selected from our previous reports (Galarraga et al.,1987;Floran et al.,1990;Aceves et al.,1991),was evaluated a day after the spontaneous behavior testing and recorded at 1-minute intervals over 90 minutes (Reyes-Corona et al.,2017).We selected the rats that show ≥ 1000 total ipsilateral turns to avoid spontaneous recovery from the nigrostriatal lesion.
Statistical analysis
All values were expressed as the mean ± standard deviation(SD) obtained from a minimum of four independent rats.The comparison among the groups was made using a one-way analysis of variance followed by Bonferronipost hoctest using GraphPad Prism 5.0 (GraphPad Software Inc.,San Diego,CA,USA).The accepted significance wasP<0.05.
Results
Physical features of NTS-polyplex nanoparticles encompassing the plasmid pNBRE3×-hCDNF
NTS-polyplex nanoparticles comprise the NTS-carrier,a conjugate of poly-L-lysine with NTS and HA2 FP using the crosslinker SPDP,and the KPRa-pNBRE3×-hCDNF complex(Figure 1A).The assembly of those components involved two electrostatic reactions initiated with the formation of the KPRa-pNBRE3×-hCDNF complex monitored by the electrophoretic retardation,followed by its compaction by the NTS-carrier into functional nanoparticles monitored by the retention gel assay.The electrophoretic retardation assay(Figure 1B) showed that the band of pNBRE3×-hCDNF (6 nM)undergoes gradual retardation when KPRA concentration increases,indicating gradual neutralization of pNBRE3×-hCDNF negative charges (Lopez-Salas et al.,2020).Based on the criterion to select the KPRa concentration that leaves free negative charges of pDNA to allow NTS-carrier binding(Lopez-Salas et al.,2020),the chosen concentration was 6µM (Figure 1B).The electrophoretic retention assay showed that the band of KPRa (6 µM)-pNBRE3×-hCDNF (6 nM)complex is gradually retained in the gel well when NTS-carrier concentration increases (Figure 1C).We have demonstrated that the complete neutralization of pDNA negative charges by the NTS-carrier occurs at the concentration following that causing the band’s disappearance (Lopez-Salas et al.,2020).Based on this criterion,the chosen concentration was 30 nM NTS-carrier (Figure 1C).Therefore,the optimum molar ratio to assemble functional NTS-nanoparticles was 6 nM pNBRE3×-hCDNF:6 µM KPRa:30 nM NTS-carrier (Figure 1C).Electron microscopy analysis by FE-SEM displayed the spherical shape of NTS-polyplex nanoparticles at this optimum molar ratio,whereas TEM and AFM showed the classical toroids of polymer-based nanoparticles.The size measured by the three techniques was 20 to 150 nm (Figure 1D).These results support the accurate prediction of electrophoretic retardation and retention gel assays to form NTS-polyplex nanoparticles with a size that enables their internalization by the endosomal pathway (Arango-Rodriguez et al.,2006;Castillo-Rodriguez et al.,2014;Hernandez et al.,2014;Espadas-Alvarez et al.,2017;Aranda-Barradas et al.,2018).
hCDNF is released in N1E-115-Nurr1 cells
N1E115-Nurr1 cells were transfected with NTS-polyplex nanoparticles containing the plasmid pNBRE3×-hCDNF to test hCDNF expression driven by NBRE3× promoter.Transfection with lipofectamine was a control.ELISA measurements showed no hCDNF levels in untransfected cells (Figure 2A).In contrast,pNBRE3×-hCDNF transfection by NTS-polyplex nanoparticles or lipofectamine generated hCDNF in N1E115-Nurr1 cells (Figure 2A).hCDNF levels in the culture medium elicited by lipofectamine were higher than those by NTSpolyplex nanoparticle transfection (Figure 2A).However,the opposite trend was observed in cell extracts (Figure 2A).CDNF is a primarily ER lumen-located protein for bearing a KDEL-like ER retention sequence that can be secreted under cellular stress to protect neighboring cells in a paracrine fashion as MANF (Huttunen and Saarma,2019) by a possible mechanism depending on cell surface KDEL receptors(Henderson et al.,2013).Lipofectamine-induced cellular stress (Fiszer-Kierzkowska et al.,2011) could potentially explain the higher secretion of hCDNF compared with NTSpolyplex nanoparticles.While lipofectamine is highly efficient forin vitrotransfections,it exerts a degree of cytotoxicity on cultured cells (Rahimi et al.,2018;Wang et al.,2018),especially in cotransfections as was used here (Wang et al.,2018).In contrast,NTS-polyplex nanoparticle transfectionin vitrois limited by NTSR1 endocytosis (Espadas-Alvarez et al.,2017) but is safe in the concentration range used (Arango-Rodriguez et al.,2006).
The immunofluorescence assays also showed the absence of hCDNF immunoreactivity in untransfected cells and its presence in both cells transfected with lipofectamine or NTSpolyplex nanoparticles (Figure 2B).Altogether,these results show that the p3×NBRE-hCDNF plasmid is functional,enabling the expression of secreted hCDNF.
Dopamine neuron recovery from 6-OHDA lesion follows hCDNF gain
In agreement with previous reports (Nadella et al.,2014),double immunofluorescence assays against TH and CDNF revealed that CDNF was absent in TH+cells but present in TH–cells (Figure 3AandB) in both healthy and 6-OHDA lesioned SNc.However,15 days after hCDNF gene transfection,CDNF immunoreactivity appeared in TH+cells of the SNc with 21 days of 6-OHDA lesion,and its levels significantly increased from 30 to 60 days post-transfection,reaching the levels of healthy controls (Figure 3AandB).As expected,6-OHDA caused an 84.4 ± 1.1% significant decrease in TH immunoreactivity on day 21 that remained until day 60 post-lesion (Figure 3AandC).Interestingly,the progressive recovery of TH immunoreactivity in the transfected group(Figure 3AandC) followed the time course of hCDNF levels(Figure 3AandB).This association suggests that hCDNF may promote the recovery of dopaminergic neurons,which are the source of hCDNF,as confirmed by the confocal orthogonal view (Additional Figure 2).ELISA measurements using a specific antibody did not detect hCDNF in the SNc of healthy rats and untransfected rats at 21 or 81 days of the lesion.In contrast,hCDNF levels were present in the SNc since days 15 to 60 post-transfection without a statistically significant difference (Table 1).ELISA measurements in the striatum also yielded similar results (Table 1),suggesting that hCDNF traveled from the SNc to the striatum via anterograde axonal transport.
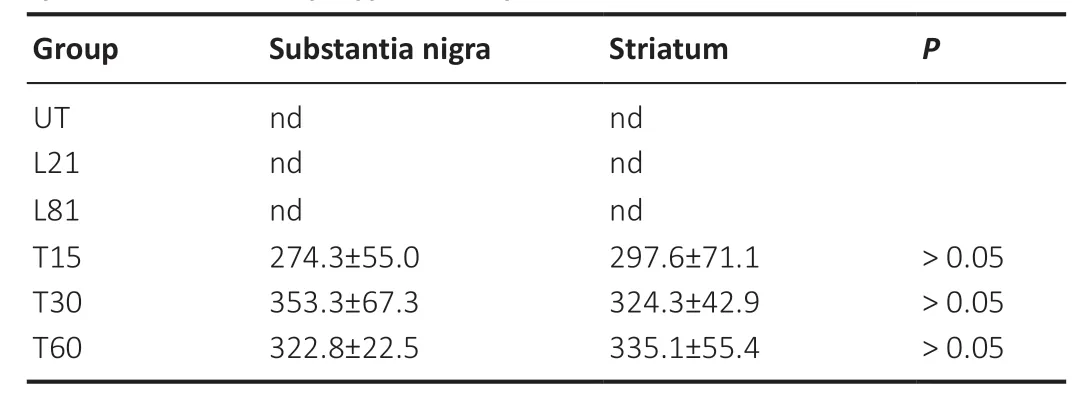
Table 1| hCDNF levels after CDNF gene transfection via nanoparticle system neurotensin-polyplex nanoparticles

Figure 1|Assembling of NTS-polyplex nanoparticles encompassing the plasmid pNBRE3×-hCDNF.

Figure 2|Production and release of hCDNF in N1E115-Nurr1 cells.
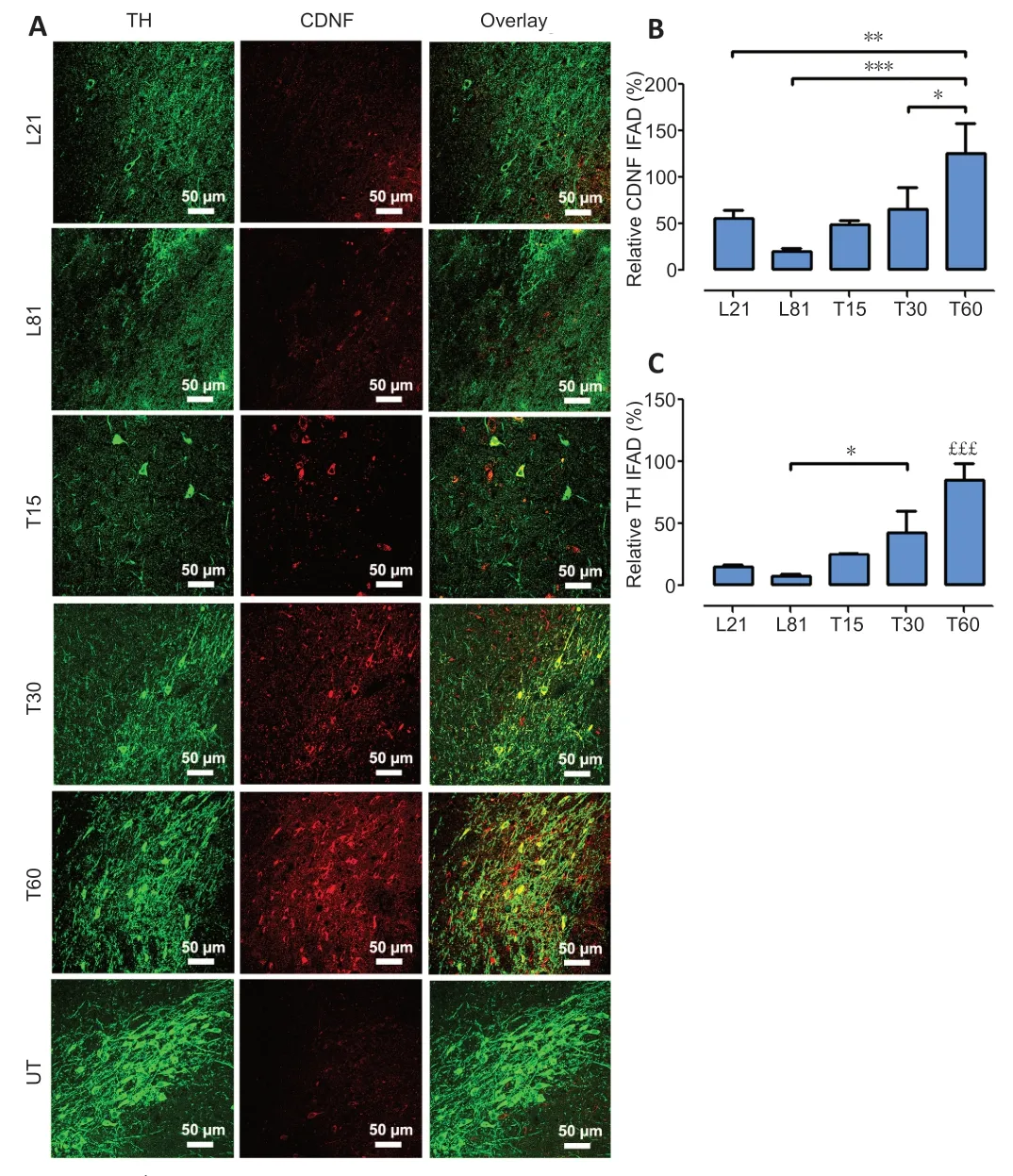
Figure 3|Dopamine neuron recovery from the 6-OHDA lesion follows the increase in CDNF levels in the SNc.
hCDNF gene transfection reverses the loss of dopamine neurons and striatal innervation
In the SNc,6-OHDA decreased TH+cell population by 88.7 ± 2.8%and branching density by 84.6 ± 9.9% (Figure 4A–C) compared with the UT group.Such a decrease remained unaltered until the end of the study,thus showing the absence of spontaneous recovery (Figure 4AandB).In contrast,hCDNF gene transfection caused a significant increase in both the number(reaching 63.1 ± 11.2%) and branching (75.9 ± 16.1%) of TH+cells as compared with untransfected lesioned rats (Figure 4).In the striatum,6-OHDA decreased TH+fiber density by 81.6± 4.7% compared with the UT group,and the recovery was 75.1 ± 7.6% after the intranigral hCDNF gene transfection compared with the untransfected lesioned rats (Figure 5).
In both nuclei,the recovery was progressive and significant since day 15 after transfection reaching 75.9 ± 16.1% (SNc)and 75.1 ± 7.6 % (striatum) of healthy condition levels at the end of the study (Figures 4and5).
hCDNF gene transfection recovers cytoskeleton in nigral dopamine neurons
The 6-OHDA lesion also significantly decreased the immunoreactivity of βIII-tubulin,a neuronal cytoskeleton protein (Katsetos et al.,2003),in the SNc on days 21 (30%)and 60 (70%) post-lesion compared with the healthy condition (Figure 6AandB).As compared with the lesion group,hCDNF gene transfection increased βIII-tubulin immunofluorescence,which was significant from day 30 posttransfection and reached 80% of the healthy condition (Figure6AandB).Western blot results of βIII-tubulin repeated the immunofluorescence pattern (Figure 6C) and also revealed a significant increase in GAP43p,a marker of axon growth and regeneration (Kawasaki et al.,2018),on days 15 and 30 post-transfection (Figure 6D).The TH immunofluorescence pattern in all the experimental groups (Figure 6AandE) was consistent with those shown inFigures 3Cand4B–C.These results suggest that hCDNF promoted dopamine neuron branching growth,consistent with the increased area density in the SNc (Figure 4AandC) and striatum (Figure 5).
Endogenous BDNF source is restored by hCDNF gene transfection
The double immunofluorescence analysis in the SNc showed that the primary source of BDNF is dopamine neurons (Figure 7A),confirming previous reports (Seroogy et al.,1994;Numan and Seroogy,1999).Therefore,the 6-OHDA lesion decreased the number of BDNF-expressing TH+cells but maintained high BDNF levels in TH–cells that decreased by 75% at the end of the study(L81) compared with the L21 condition (Figure 7A).Compared with the L81 condition,BDNF gain was 50% significantly higher on day 15 after hCDNF gene transfection and increased to 75% of healthy condition values on day 60 post-transfection (Figure 7AandB).At this time,most BDNF immunoreactivity was present in TH+cells,whose recovery pattern was similar to those shown above (Figures 3–6).These results suggest that BDNF recovery also contributes to the hCDNF-induced reversal of nigrostriatal dopamine neurodegeneration as shown by the gradual increase in relative TH IFAD (Figure 7C).
hCDNF gene transfection increases dopamine metabolism in the SNc and striatum
6-OHDA lesion decreased dopamine levels by 75% in the SNc(Figure 8A) and by 86.5% in the striatum (Figure 8B) compared with the healthy controls.In comparison with L21 and L81 groups,a significant increase in dopamine levels occurred on days 30 and 60 after hCDNF transfection.The recovery reached 45.2 ± 2.8% of the average healthy conditions (Figure 8AandB).However,the effect on DOPAC and HVA levels was different in each nucleus.In comparison with the healthy condition,those levels in the SNc of L21 and L81 groups were 650.32%higher,whereas the levels increased by 42.50% (DOPAC) and 68.54% (HVA) in the striatum of the same groups (Figure 8BandC).Nevertheless,DOPAC and HVA levels progressively increased in both nuclei after hCDNF transfection compared with lesion condition levels (Figure 8CandD).HVA levels in the SNc decreased at days 30 and 60 after transfection compared to the lesion groups;yet,the levels remained higher than healthy condition levels (Figure 8E).In the striatum,HVA levels decreased in the lesioned groups and recovered in the transfected groups to reach the highest values at 60 days compared with the UT values (Figure 8F).The index of the total DA catabolism rate after hCDNF transfection may indicate the scope of recovery,particularly in the striatum.
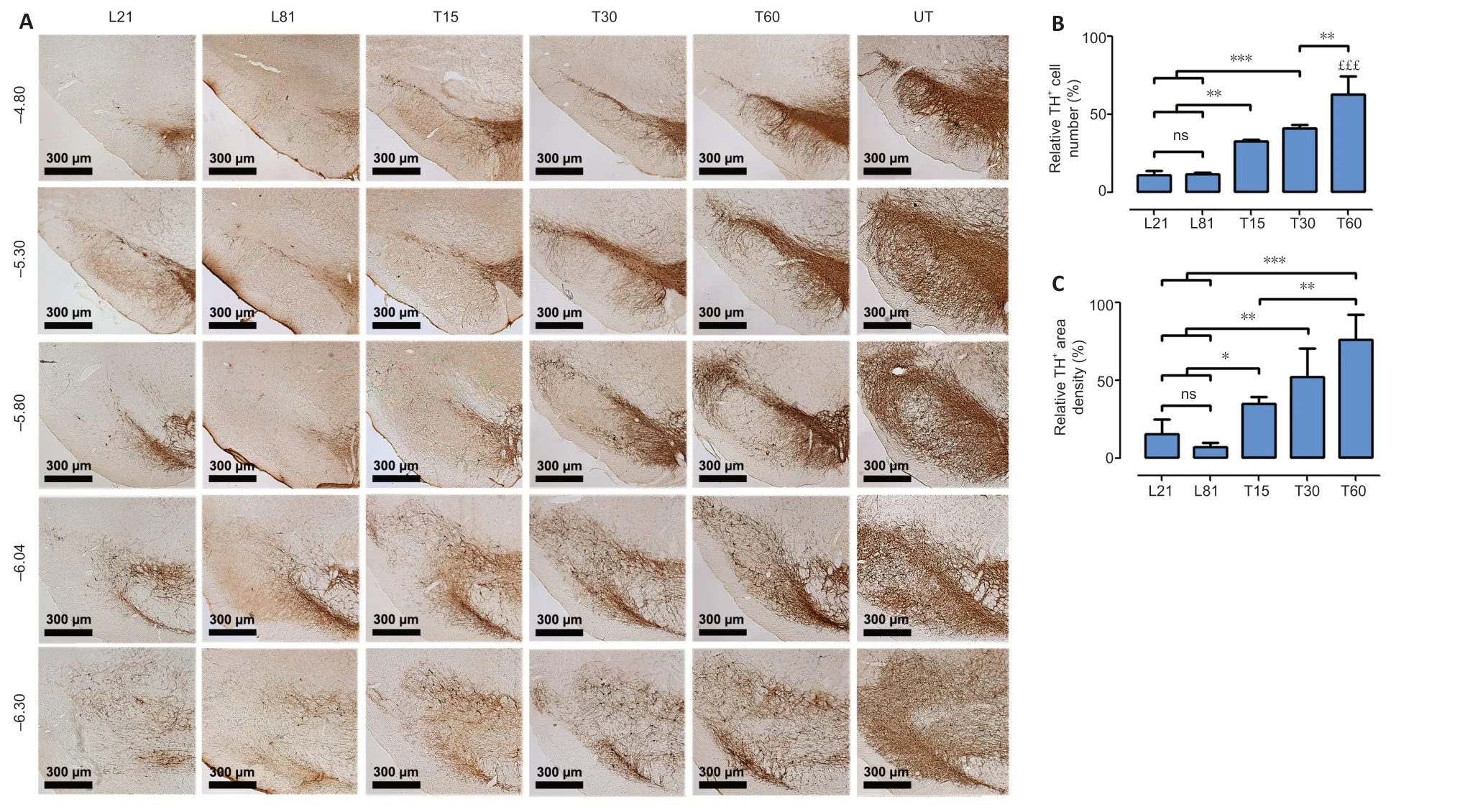
Figure 4|hCDNF gene transfection increases TH+ neuron population and branching density in the SNc of rats with 6-OHDA lesion.
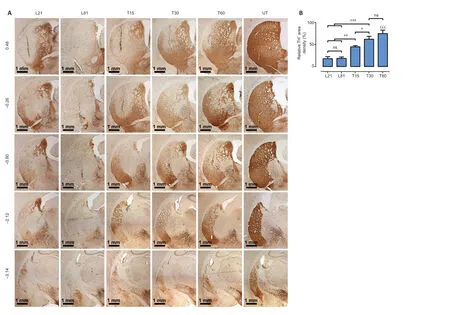
Figure 5|hCDNF gene transfection increases TH+ density area in the striatum of rats with 6-OHDA lesion.
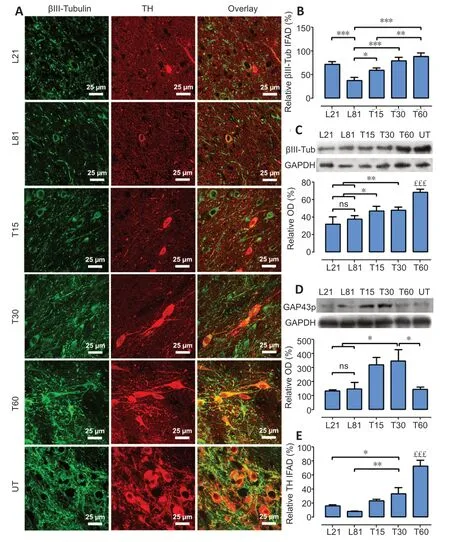
Figure 6|hCDNF gene transfection recovers neuron cytoskeleton in TH+cells of rats with 6-OHDA lesion.

Figure 7|hCDNF gene transfection restores BDNF levels in nigral dopamine neurons of rats with 6-OHDA lesion.

Figure 8|hCDNF gene transfection increases dopamine and catabolite levels in the SNc and striatum of rats with 6-OHDA lesion.
hCDNF transfection reliefs motor and non-motor behavior disability in 6-OHDA-lesioned rats
Functional recovery of the nigrostriatal dopamine system was shown through a drug-activated behavior test and four spontaneous behavior tests.The methamphetamineactivated circling behavior was 1227 ± 46.5 ipsilateral turning/90 min in the L21 group (Figure 9A) and remained unaltered in the L81 group (Figure 9A).This result shows that the methamphetamine at the dose used here did not cause dopaminergic degeneration in the contralateral side,as confirmed by TH immunohistochemistry results in the SNc and striatum (Additional Figure 3),thus normalizing rotational behavior.hCDNF transfection significantly reduced the methamphetamine-activated circling behavior overtime,although a minimum asymmetry (120 ± 41.2 ipsilateral turning/90 min) remained on day 60 after transfection (Figure9A).The vibrissae-evoked forelimb placing test and limbuse asymmetry test also revealed motor asymmetry in the same groups (Figure 9BandC).The disappearance of the asymmetry was complete from day 15 after hCDNF gene transfection in the vibrissae-evoked forelimb placing test(Figure 9B) and gradual in the limb-use asymmetry test (Figure 9C).The beam test revealed similar values of akinesia (Figure 9E) and motor discoordination (errors per step) in L21 and L81 groups (Figure 9F) that were gradually reduced by the hCDNF gene transfection until reaching healthy condition values(Figure 9F).The olfactory test showed that the rats with the lesion (L21 and L81) presented a 24.8 ± 6.4% reduction in thetime of permanence in the vanilla location (olfactory stimulus)when compared with the intact rats.In contrast,lesioned and transfected rats stayed longer in the vanilla location,reaching no statistical difference to healthy rats (Figure 9D).
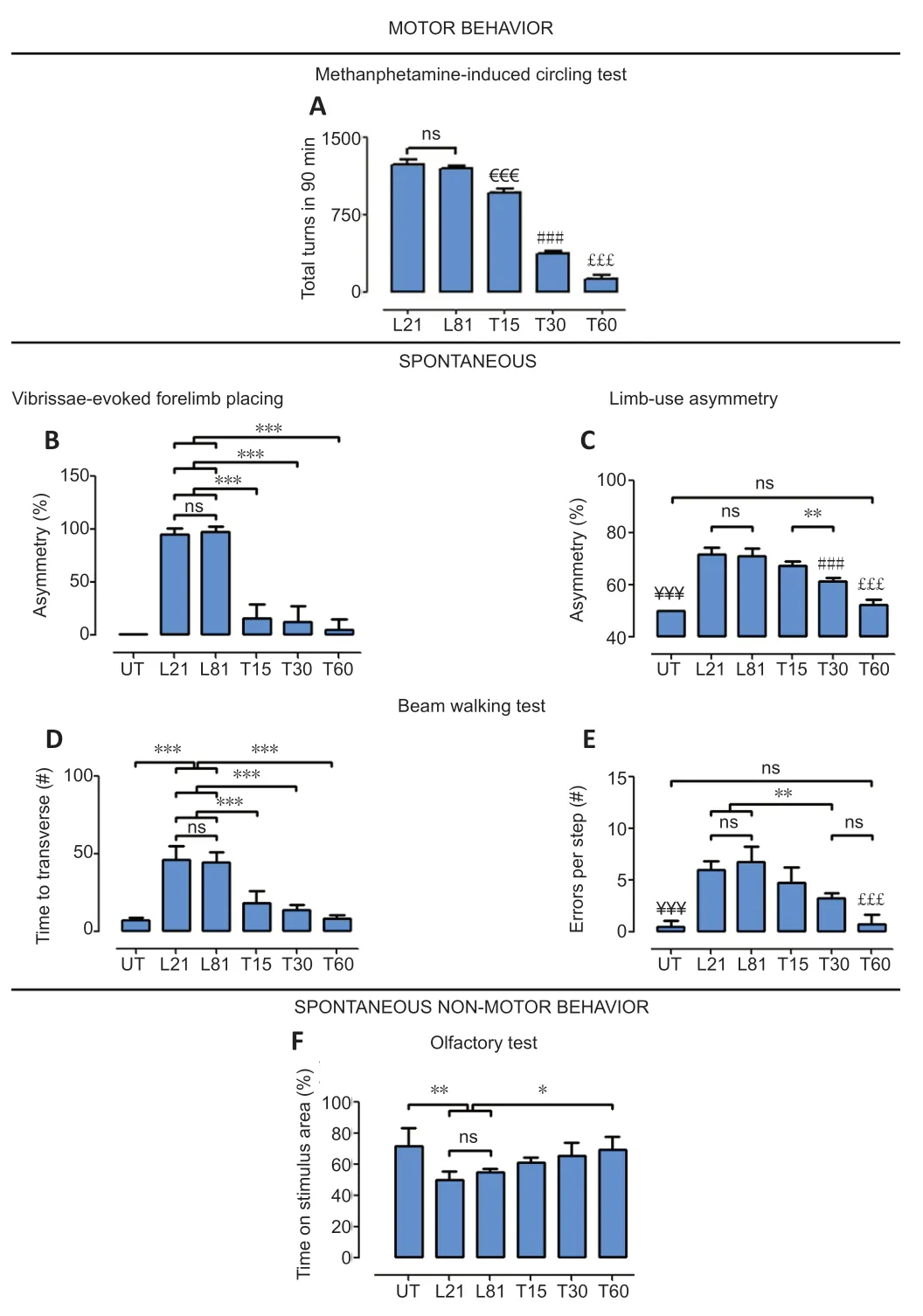
Figure 9|Effect of CDNF gene transfection on the motor and non-motor behavior of rats with 6-OHDA lesion.
Discussion
This work shows that hCDNF transfection in dopamine neurons surviving 6-OHDA lesion can provide a secretable hCDNF (shownin vitro) to both nigral cells and striatal terminals,thus promoting the restoration of the nigrostriatal system.The progressive rise of βIII-tubulin and GAP43p,two well-known markers of axon growth and regeneration(Katsetos et al.,2003;Kawasaki et al.,2018),suggests that hCDNF can also stimulate neuritogenesis.This suggestion is supported by the progressive sprouting of TH+nigral branches and striatal terminals following hCDNF transfection.In addition to the structural effect,hCDNF also stimulates the recovery of dopamine levels in the SNc and striatum that can account for the relief of motor and non-motor deficits.Remarkably,hCDNF transfection also increased endogenous BDNF levels in dopamine neurons with a similar time course to their restoration.This result suggests that BDNF could also mediate the hCDNF neurotrophic effect because BDNF is known to promote the survival and restoration of the nigrostriatal system in the 6-OHDA rat model (Hernandez-Chan et al.,2015;Razgado-Hernandez et al.,2015).
The progressive cell death and neuronal cytoskeleton loss caused by the intrastriatal injection of 6-OHDA in the rat reach a maximum by 21 days post-lesion,remaining 20% of surviving dopamine cells permanently (Hernandez-Baltazar et al.,2013).Because hCDNF transfection was performed at that time,then the recovery of the nigral TH+cells and striatal projections confirms the restorative hCDNF effect on phenotypic markers previously reported after AAV8-mediated intrastriatal gene delivery (Wang et al.,2017a) or hCDNF intracerebral infusion in 6-OHDA lesioned rodents (Lindholm et al.,2007;Voutilainen et al.,2011,2017;Airavaara et al.,2012) or marmoset monkeys (Garea-Rodriguez et al.,2016).Neurogenesis likely participates in dopamine cell population recovery because CDNF stimulates the proliferation and migration of neural progenitor cells of the subventricular zone in 6-OHDA-lesioned rats (Nasrolahi et al.,2019).This recovered neuronal fraction might take up the hCDNF released by the transfected neurons,thus explaining the increase in hCDNF between T15 and T30,given that the transfection was performed with a fixed amount of a plasmid.A similar phenomenon occurs with hGDNF,NRTN,and BDNF transfections (Gonzalez-Barrios et al.,2006;Razgado-Hernandez et al.,2015;Reyes-Corona et al.,2017).The CDNF uptake mechanism is still unknown;CDNF receptormediated endocytosis might participate,although a CDNF receptor has not yet been identified.
Moreover,the recovery of βIII-tubulin and TH+cells indicates that hCDNF transfection also restored the neuronal cytoskeleton of dopamine cells.The time course of the increased levels of GAP43p,a marker of axon growth and regeneration (Kawasaki et al.,2018),suggests that the more intense growth of neuronal cytoskeleton occurred in the first month after hCDNF transfection in this 6-OHDA lesion model.All these findings support that the hCDNF stimulates the regeneration of the nigrostriatal dopamine system in the 6-OHDA lesion model.Neuroprotective and neurorestorative effects of CDNF in other neurodegeneration models are also reported.Examples are contusion spinal cord injury (Zhao et al.,2016) and cerebral ischemia by middle cerebral artery occlusion (Matlik et al.,2014;Zhang et al.,2018;Anttila et al.,2019) in rats,as well as APP/PS1 model of Alzheimer’s disease(Kemppainen et al.,2015) and genetic models of amyotrophic lateral sclerosis in mice (Moreno-Igoa et al.,2012).
BDNF can also mediate the neurorestorative effect of hCDNF,as supported by the association of BDNF recovery with both hCDNF expression and restoration of dopamine neurons found here.BDNF is the endogenous neurotrophic source for dopamine neurons that influences them via autocrine and paracrine mechanisms through its high-affinity receptor TrkB (Seroogy et al.,1994;Numan and Seroogy,1999).The reduction of BDNF levels in the SNc may,therefore,contribute to the profound death of dopamine neurons induced by 6-OHDA in the rat.In contrast,the regeneration of the nigrostriatal dopamine system entails BDNF recovery,as shown here and by findings in 6-OHDA lesioned rats transfected with the BDNF gene using NTS-polyplex nanoparticles (Hernandez-Chan et al.,2015;Razgado-Hernandez et al.,2015).We found that BDNF levels remained high on day 21 post-lesion localized to non-dopamine cells and significantly decreased(80%) on day 81 post-lesion when dopamine cell loss was maximum (80%).Glial cells are thought to be a primary BDNF source (Hisaoka-Nakashima et al.,2016) on untransfected SNc with 21 days of 6-OHDA lesion.Ongoing experiments are conducted to clarify this issue and understand the lack of neurotrophic effect.These results suggest that BDNF should be recovered in dopamine neurons to exert neurotrophic effect by an autocrine and paracrine fashion on cell bodies and projection terminals after its antegrade transport (Venero et al.,2000;Gustafsson et al.,2003).
Preclinical gene therapy studies have shown that dopamine recovery in the nigrostriatal system is essential to reverse motor disabilities (Gonzalez-Barrios et al.,2006;Hernandez-Chan et al.,2015;Razgado-Hernandez et al.,2015;Reyes-Corona et al.,2017).HPLC measurements showed that hCDNF transfection increased dopamine and its main catabolites (DOPAC and HVA) levels in the SNc and striatum,suggesting dopamine utilization.Accordingly,the altered gait,motor discoordination,and motor asymmetry were utterly eliminated in rats transfected with hCDNF,but not entirely in methamphetamine-activated circling behavior.This result suggests that postsynaptic dopamine receptor hypersensitivity remains because a complete recovery of the nigrostriatal dopamine system was not achieved at the end of the study.The possibility that the methamphetamine dose used here(8 mg of racemic mixture=4 mg of D active enantiomer)might cause dopamine neurodegeneration in the contralateral side and normalize rotational behavior is ruled out because such a dose is in the previously reported safe range (1 to 5 mg active enantiomer) (Björklund and Dunnett,2019).This contention is supported by preserving TH+cells in nigral neurons and striatal terminals,the permanence of rotational behavior defects in the untransfected lesioned rats (L81),and the complete recovery of the spontaneous behaviors in the hCDNF transfected parkinsonian rats.Besides,the dose was acutely applied in a one-month intertrial in contrast to the neurotoxic dose that is acutely administered in three injections of 5 or 10 mg/kg at 3 or 4 hours of intervals (Wang et al.,2017b) or as a single 30 mg/kg injection (Ares-Santos et al.,2014;Yu et al.,2015).Our results are also consistent with the finding that hyposmia in 6-OHDA lesioned rats results from the loss of dopamine projections to the olfactory bulb from neurons in the SNc (Hoglinger et al.,2015).Therefore,olfaction improvement in hCDNF transfected rats indicates the reestablishment of nigro-olfactory projection following the recovery of the dopamine neuron population in the SNc.Similarly,the hCDNF-induced neural regeneration can also occur in other nuclei innervated by the nigrostriatal dopamine system.
Dopaminergic neural regeneration can be achieved with three CDNF delivery methods in parkinsonian rodents,namely direct protein injection or infusion or gene delivery.In shortterm studies on acute parkinsonism (1–2 weeks of the lesion),a single CDNF (10 µg) injection in the striatum or SVZ causes neural regeneration one week after administration (Airavaara et al.,2012) and proliferation and migration of progenitor cells three weeks after treatment (Nasrolahi et al.,2019).In long-term studies in acute or chronic parkinsonian rats,repeated injections or chronic infusion of CDNF protein in rats (Voutilainen et al.,2011) and marmoset monkeys (Garea-Rodriguez et al.,2016) is needed to induce a significant neurorestoration (Voutilainen et al.,2011).In chronic parkinsonism in the rat,a single CDNF administration requires combined deep brain stimulation (Huotarinen et al.,2018) or GDNF coadministration (Voutilainen et al.,2017) to decrease neurological deficits.These studies collectively point out that the continuous presence of CDNF is needed to produce sustained neuroregeneration of the dopaminergic nigrostriatal system.AAV8-mediated intrastriatal delivery of hCDNF gene produces sustained hCDNF production in chronic parkinsonian rats but requires the combined expression of aromatic amino acid decarboxylase gene to improve the recovery of motor function (Wang et al.,2017a).The decreased effectiveness of the intrastriatal CDNF delivery in chronic parkinsonism could be caused by its insufficient retrograde transportation by the scarce striatal terminals to cell bodies to elicit a maximal therapeutic response,as proposed for NRTN (Bartus et al.,2014).In contrast,CDNF overexpression in dopamine neurons can potentially affect the cell body and terminals after its anterograde axonal transport (Martinez-Fong et al.,2012).
The specificity of NTS-polyplex nanoparticles for dopamine neurons (Martinez-Fong et al.,1999;Alvarez-Maya et al.,2001;Navarro-Quiroga et al.,2002;Arango-Rodriguez et al.,2006;Hernandez-Baltazar et al.,2012) can account for hCDNF expression in the cell body and its distribution to the striatum,as shown previously with other transgenes in the 6-OHDA lesion model (Gonzalez-Barrios et al.,2006;Reyes-Corona et al.,2017).Their transfection efficiency yielded hCDNF levels in the range of those produced by AAV serotype 2 transduction in the 6-OHDA model (Back et al.,2013).These advantages result from proper compaction of NTS-polyplex components into functional nanoparticles shown by FESEM,TEM,and AFM.These results confirm the biophysical properties characterized by transmission and scanning electron microscopy,dynamic light scattering,and circular dichroism (Arango-Rodriguez et al.,2006;Castillo-Rodriguez et al.,2014;Hernandez et al.,2014;Espadas-Alvarez et al.,2017;Aranda-Barradas et al.,2018) used for a clinical formulation(Aranda-Barradas et al.,2018).The safety of NTS-polyplex nanoparticles (Hernandez et al.,2014) is another advantage that supports their clinical use.
Despite the success of NTS-polyplex nanoparticlesmediated hCDNF gene delivery in reversing 6-OHDA-induced parkinsonism,limitations regarding the animal model,administration route,and transgene regulation should be considered.The 6-OHDA model is the most frequently used but does not develop some pathological hallmarks in PD,such as α-synucleinopathy and neurotoxic A1 reactive astrocytes.NTS-polyplex nanoparticles-mediated hCDNF gene delivery should also be tested in the stereotaxic neurotoxin β-sitosterol β-d-glucoside (BSSG) model,known to develop spreading α-synucleinopathy and induction of neurotoxic A1 reactive astrocytes (Luna-Herrera et al.,2020;Soto-Rojas et al.,2020).Since NTS-polyplex nanoparticles do not cross the brainblood barrier (BBB),their administration is via an intracranial injection in the SNc.A less invasive procedure such as focused ultrasound could be implemented to open BBB transiently and allow the intravenous administration (Xhima et al.,2018).Last,the present study lacks a long-term assessment of hCDNF expression beyond 60 days post-transfection.Since the Nurr1 dependent NBRE3× promoter was used to drive hCDNF expression,the hCDNF production could be insufficient or absent in severe parkinsonism,known to reduce Nurr1 expression in dopaminergic neurons (Decressac et al.,2013) .
In conclusion,our results showed that hCDNF expression in dopamine neurons is a viable strategy to provide nigral neurons and striatal projection terminals with hCDNF,which reverses the nigrostriatal dopamine system neurodegeneration and motor and non-motor deficits.Additionally,the concomitant BDNF recovery can be expected to potentiate hCDNF neurotrophic effects.This approach can be used to develop a modifying-disease treatment for PD.The NTS-polyplex-mediated hCDNF gene transfection in dopamine neurons provides hCDNF to cell bodies and terminals and reverses the neurodegeneration of the nigrostriatal dopamine system.
Acknowledgments:The authors deeply thank Dr.Mart Saarma‚Institute of Biotechnology‚University of Helsinki‚for providing the plasmid pCR3.1-hCDNF.The authors also thank the Unit for Production and Experimentation of Laboratory Animals (UPEAL) of Cinvestav‚especially Rafael Leyva‚BSc‚Ricardo Gaxiola‚BSc‚and Mr.René Pánfilo Morales for animal handling.
Author contributions:MAFP performed immunostaining experiments‚stereotaxic surgeries‚HPLC assays‚analyzed the data‚made substantial contributions to the experimental design and manuscript writing.DRC‚LOSR‚and CLH participated in most immunofluorescence experiments and processed‚analyzed‚and interpreted the results.YMFM performed and analyzed ELISA and WB experiments.RN and MMB quantified dopamine and catabolites levels by HPLC‚RN also performed assays in vitro and participated in electron microscopy characterization directed by JSS.MJB provided NBRE3× sequence‚and LE designed and cloned pNBRE3×-hCDNF plasmid (4482 bp).JAD undertook the behavioral tests.JAGB supervised expression assays and statistical analysis‚and PN performed and analyzed GAP43p WB experiments.GF‚MEGC‚AJEA‚IAMD‚and DMF participated in the intellectual content‚conception‚and design of the study‚and edited the manuscript and figures.DMF also provided funding‚supervised experiments and data analysis‚and wrote the paper.All authors have contributed to writing and editing the manuscript and approved its final version.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:This study was supported by the Consejo Nacional de Ciencia Tecnología (Conacyt) de México (Grant # 254686‚to DMF).
Institutional review board statement:The experimental procedures for animal use were approved by the Institutional Animal Care and Use Committee of Centro de Investigación y de Estudios Avanzados(authorization No.162-15) on June 9‚2019.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal‚and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License‚which allows others to remix‚tweak‚and build upon the work non-commercially‚as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files:
Additional Figure 1:Experimental design.
Additional Figure 2:Location of CDNF (red immunofluorescence) in nigral dopamine neurons (green immunofluorescence).
Additional Figure 3:Methamphetamine did not cause dopaminergic degeneration in the contralateral side of the substantia nigra and striatum.
- 中国神经再生研究(英文版)的其它文章
- Towards a comprehensive understanding of p75 neurotrophin receptor functions and interactions in the brain
- Microglia regulation of synaptic plasticity and learning and memory
- Stroke recovery enhancing therapies:lessons from recent clinical trials
- Functional and immunological peculiarities of peripheral nerve allografts
- MicroRNA expression in animal models of amyotrophic lateral sclerosis and potential therapeutic approaches
- Significance of mitochondrial activity in neurogenesis and neurodegenerative diseases

