Baculoviral inhibitor of apoptosis protein repeatcontaining protein 3 delays early Wallerian degeneration after sciatic nerve injury
Min Cai ,Jian Shao ,Bryant Yung ,Yi Wang,Nan-Nan Gao,Xi Xu,Huan-Huan Zhang,Yu-Mei Feng,Deng-Bing Yao,
Abstract Wallerian degeneration is a complex biological process that occurs after nerve injury,and involves nerve degeneration and regeneration.Schwann cells play a crucial role in the cellular and molecular events of Wallerian degeneration of the peripheral nervous system.However,Wallerian degeneration regulating nerve injury and repair remains largely unknown,especially the early response.We have previously reported some key regulators of Wallerian degeneration after sciatic nerve injury.Baculoviral inhibitor of apoptosis protein repeat-containing protein 3 (BIRC3) is an important factor that regulates apoptosis-inhibiting protein.In this study,we established rat models of right sciatic nerve injury.In vitro Schwann cell models were also established and subjected to gene transfection to inhibit and overexpress BIRC3.The data indicated that BIRC3 expression was significantly up-regulated after sciatic nerve injury.Both BIRC3 upregulation and downregulation affected the migration,proliferation and apoptosis of Schwan cells and affected the expression of related factors through activating c-fos and ERK signal pathway.Inhibition of BIRC3 delayed early Wallerian degeneration through inhibiting the apoptosis of Schwann cells after sciatic nerve injury.These findings suggest that BIRC3 plays an important role in peripheral nerve injury repair and regeneration.The study was approved by the Institutional Animal Care and Use Committee of Nantong University,China (approval No.2019-nsfc004) on March 1,2019.
Key Words:apoptosis;baculoviral inhibitor of apoptosis protein repeat-containing protein 3;nerve degeneration;rat;Schwann cell;sciatic nerve injury;signal pathway;Wallerian degeneration
Introduction
Peripheral nerve injury may occur due to congenital,chemical,mechanical,or pathological causes,and has an intrinsic capacity for axon regeneration and functional recovery(Siemionow and Brzezicki,2009;Valls-Sole et al.,2011;Zochodne,2012;Caillaud et al.,2019;Liu and Wang,2020).Axonal injury induces responses to the proximal and distal axonal stumps after nerve fiber injury.Wallerian degeneration(WD) occurs on the distal side of an axon after it is cut or crushed.It is a complex biological process involving nerve degeneration and regeneration,followed by a series of postinjury morphological and molecular changes.Schwann cells(SCs) have an effect on nerve repair and regeneration in the peripheral nervous system (PNS) and studies of WD have revealed a level of coordination between axon degeneration and regeneration during peripheral nerve repair (Frostick et al.,1998;Scholz et al.,2009;Liu et al.,2019).SCs as major glia play a crucial role in the cellular and molecular events that contribute to WD.Thus,promoting SC activation might facilitate peripheral nerve repair.Upon nerve injury,PNS triggers rapid release of inflammatory cytokines.These cytokines recruit macrophages and regulate molecular and cellular processes in injured nerves.SCs might also be activated during anti-apoptotic processes to prevent damage from inflammatory cytokines and maintain nerve function integrity (Idriss and Naismith,2000;Zhao et al.,2010;Martini et al.,2013;Chen et al.,2015).The migration,proliferation,and apoptosis of SCs are very important for WD.Nerve remyelination is initiated after the contact of SCs with degenerating and regenerating axons.SCs guide axon growth by migration and proliferation to form the Büngner bands.The plasticity of SCs may have the ability to repair the injured nerves after injury (Dickens et al.,2012;Schleich et al.,2012;Tos et al.,2013;Peluffo et al.,2015;Wang et al.,2019).
With the development of microscopy,bioinformatics,and molecular biology,understanding the molecular control of nerve injury may provide insights into delaying nerve degeneration and promoting more rapid axon regeneration.Several studies have been carried out to increase the ability of nerves to repair and improve axonal regeneration after injury (Goethals et al.,2010;Peluffo et al.,2015;Wang et al.,2019).However,the molecular mechanisms regulating repair in peripheral nerves post-injury are incompletely understood,especially early activation.Although several technologies have been developed for the repair of nerve injury,recovery rate remains low.Nevertheless,understanding the molecular mechanisms of WD in peripheral nerve injury is crucial for developing new therapies for neuronal damage (Shamash et al.,2002;Jessen et al.,2015;Jessen and Mirsky,2016;Gamage et al.,2017;Gersey et al.,2017;Sango et al.,2017).
Previously,we have reported that baculoviral inhibitor of apoptosis protein (IAP) repeat-containing protein 3 (BIRC3) is a key regulator in nerve injury and repair (Wang et al.,2012;Li et al.,2014).The mechanism underlying the regulation of WD by BIRC3 is unknown.The effects of BIRC3 in tumor biology have been widely studied.But the roles of BIRC3 in the nervous system are seldom reported (Wang et al.,2012;Li et al.,2014;Piro et al.,2015;Gressot et al.,2017).Here,we explored the molecular pathways of BIRC3 that regulate both axon degeneration and regeneration during early WD in injured rat sciatic nerves.IAP,which regulates tumor necrosis factor-α (TNF-α) pro-apoptotic signaling pathway,contains at least one baculoviral IAP repeat.BIRC3 is the main regulator of IAP that belongs to the BIRC family.BIRC3 plays important roles in many malignancies,and it suppresses TNF-α stimulated cell apoptosis and prevents the formation of tumor necrosis factor receptor pro-apoptotic signaling pathway (Piro et al.,2015;Gressot et al.,2017).In addition,BIRC3 has been reported to be involved in the inflammation-mediated antiapoptotic gene network in injured sciatic nerves;however,the molecular mechanism is yet to be defined (Wang et al.,2012;Li et al.,2014;Piro et al.,2015;Gressot et al.,2017).Therefore,we explored the effects of BIRC3 and its antiapoptotic mechanisms on rat sciatic nerve repair post-injuryin vivoandin vitro.
Materials and Methods
Animal models of nerve injury and tissue preparation
Estrogen may affect nerve repair,so male Sprague-Dawley rats,weighing 180–200 g,were used to detect BIRC3 expression after sciatic nerve injury.The 1-day-old Sprague-Dawley rats were used for SCs culture.All rats were provided by the Experimental Animal Center of Nantong University (licence No.SYXK (Su) 2016-0031).All animal tests were conducted according to the Key Laboratory of Neuroregeneration Guidelines for the Care and Use of Laboratory Animals and the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.The methods used in this study were approved by the Institutional Animal Care and Use Committee of Nantong University (approval No.2019-nsfc004) on March 1,2019.All experiments were designed and reported according to the Animal Research:Reporting ofIn VivoExperiments (ARRIVE) guidelines.All rats were randomly divided into six groups (n=6) (Table 1).The rats in 0 hour group underwent a sham operation.Rats in the other groups underwent the sciatic nerve injury surgery as previously described (Weinstein and Wu,2001).All rats were anesthetized using an intraperitoneal injection of complex narcotics [85 mg/kg trichloroacetaldehyde monohydrate(RichJoint,Shanghai,China),42 mg/kg magnesium sulfate(Xilong Scientific,Guangzhou,China),and 17 mg/kg sodium pentobarbital (Sigma-Aldrich,St.Louis,MO,USA)].The sciatic nerve was identified and lifted through an incision on the lateral aspect of the mid-thigh of the right hind limb.Injury to a peripheral nerve following nerve transection normally results in the loss of connectivity of the injured neurons and target organs.WD involves axon damage and prevents axon regeneration (Wang et al.,2012;Li et al.,2014).The distal and proximal nerve stumps were separately inserted into nearby inter-muscular spaces to prevent nerve regeneration.Then the animals were housed in temperature-and humidity-controlled cages after the surgery,maintained under a 12-hour light/dark cycle,and were allowed free access to water and food (Table1).Rats in one group were euthanized by complex narcotics immediately assayed (0 hour) and rats in the other groups were euthanized at 3,6,12,18 and 24 hours post-surgery.Then a 1-cm segment of sciatic nerve was excised (Kumar et al.,2007;Jung et al.,2014;Rassu et al.,2017).

Table 1| Numbers of adult rats subjected to nerve injury
Primary SC culture and purification
In this study,SCs were cultured using the protocol of Weinstein and Wu (2001).After anesthesia by injection of a mixture of complex narcotics,the sciatic nerves of 1-day-old Sprague-Dawley rats were dissected and minced.Subsequently,the skin on the lateral side was incised and the nerve was exposed.Then,the neve sections were collected and then incubated at 37°C for 30–40 minutes in 3 mg/mL collagenase,and trypsinized for 10 minutes.Subsequently,primary cells were cultured at 37°C in humidified 5% CO2in poly-L-lysine coated plastic plates (RichJoint),maintained in Dulbecco’s modified Eagle’s medium (Invitrogen,Carlsbad,CA,USA) containing 10% fetal bovine serum (complete medium) (Invitrogen,Carlsbad,CA,USA),and treated with 10 µM cytosine arabinoside.Rabbit complement (Invitrogen,Carlsbad,CA,USA;Cat# M310203) mediated lysis and polyclonal anti-Thy1.1 antibody (Sigma-Aldrich;Cat# M7898)were added to eliminate the surviving fibroblasts.The cells were cultured in the mixture of Dulbecco’s minimum Eagle’s medium (Gibco,Grand Island,NY,USA),100 IU/mL streptomycin (Sigma-Aldrich) and 100 g/mL penicillin.Then,the cells were selected using monoclonal mouse anti-Thy1.1 antibody (1:200 dilution;Santa Cruz Biotechnology,Santa Cruz,CA,USA).The final cells consisted of 98% SCs,as determined by immunofluorescence for mouse anti-S100 monoclonal antibody (1:500 dilution;Santa Cruz Biotechnology),which is a specific SC marker and Hoechst 33342 immunocytochemistry.The SC culture was passaged no more than three times.
Immunohistochemistry analysis
The injured sciatic nerves were fixed in paraformaldehyde(4%) and then dehydrated in sucrose (30%).The sciatic nerve samples were divided into thick sections (12 µm) using Research Cryostat Microtome (CM3050 S;Leica,Wetzlar,Germany),mounted onto the slides,and then rinsed in phosphate-buffered saline.Before staining,sample sections were permeabilized in a solution of goat serum (5%,Abcam,St.Louis,MO,USA)),Triton X-100 (0.3%;RichJoint) and bovine serum albumin (1%,RichJoint) in phosphate buffer.Subsequently,the samples were incubated with anti-BIRC3(a mouse monoclonal antibody,Cat# sc-7944,1:50,Santa Cruz Biotechnology),and anti-S100B (a mouse monoclonal antibody,Cat#S2532,1:500,Sigma-Aldrich) at 4°C overnight.Thereafter,the samples were incubated with goat anti-mouse IgG-Cy3 (1:400,Cat#SA00009-2,Sigma-Aldrich) or goat antirabbit IgG-Alexa Fluor 488 (1:400,Cat#SA00006-5,Invitrogen,Carlsbad,CA,USA) for another 2 hours at room temperature.They were also counterstained with the Hoechst 33342(Sigma-Aldrich).All samples were examined with a confocal laser scanning microscope (FV10i-oil,Olympus,Tokyo,Japan).
BIRC3 small interfering RNA transfection and overexpression in cultured SCs
The primary cultured SCs were transfected with three different BIRC3 small interfering RNAs (siRNAs;Ribobio,Shanghai,China) (Table 2) for siRNA interference analysis.According to the manufacturer’s protocol,SCs were transfected with transfection reagent,Lipofectamine RNAi MAX (Invitrogen,Shanghai,China) and overexpressed the BIRC3.Negative and blank control siRNAs (RiboBio,Guangzhou,China) were used.To examine the roles of BIRC3 overexpressionin vitro,purified primary cultured SCs were transfected with a mixture of pcDNA3.1+BIRC3 plasmid (10 µg/µL,RiboBio,Guangzhou,China) and X-treme GENE HP DNA Transfection Reagent(Roche,Mannheim,Germany),or an empty vector and a mixture of DNA Transfection Reagent for 48 hours.Then,the RNA and protein expression levels were detected after transfection.

Table 2|The primers of BIRC3 siRNA
Quantitative real-time polymerase chain reaction
Total RNA from injured sciatic nerves was isolated using RNA extraction reagent (Qiagen,Valencia,CA,USA) to determine theBIRC3expression level.RNA samples were reverse transcribed to cDNA using a cDNA Transcription Kit(Qiagen) following the manufacturer’s instructions (Applied Biosystems® 2720,Foster City,CA,USA).According to the manufacturer’s protocol,we performed quantitative real-time polymerase chain reaction (qRT-PCR) with SYBR Premix Ex Taq(TaKaRa,Shanghai,China) using a polymerase chain reaction(PCR) system (Applied Biosystems® Real-Time PCR System).The forward and reverse PCR primers are listed inTable 3.The relative expressions were calculated using the comparative cycle threshold method.
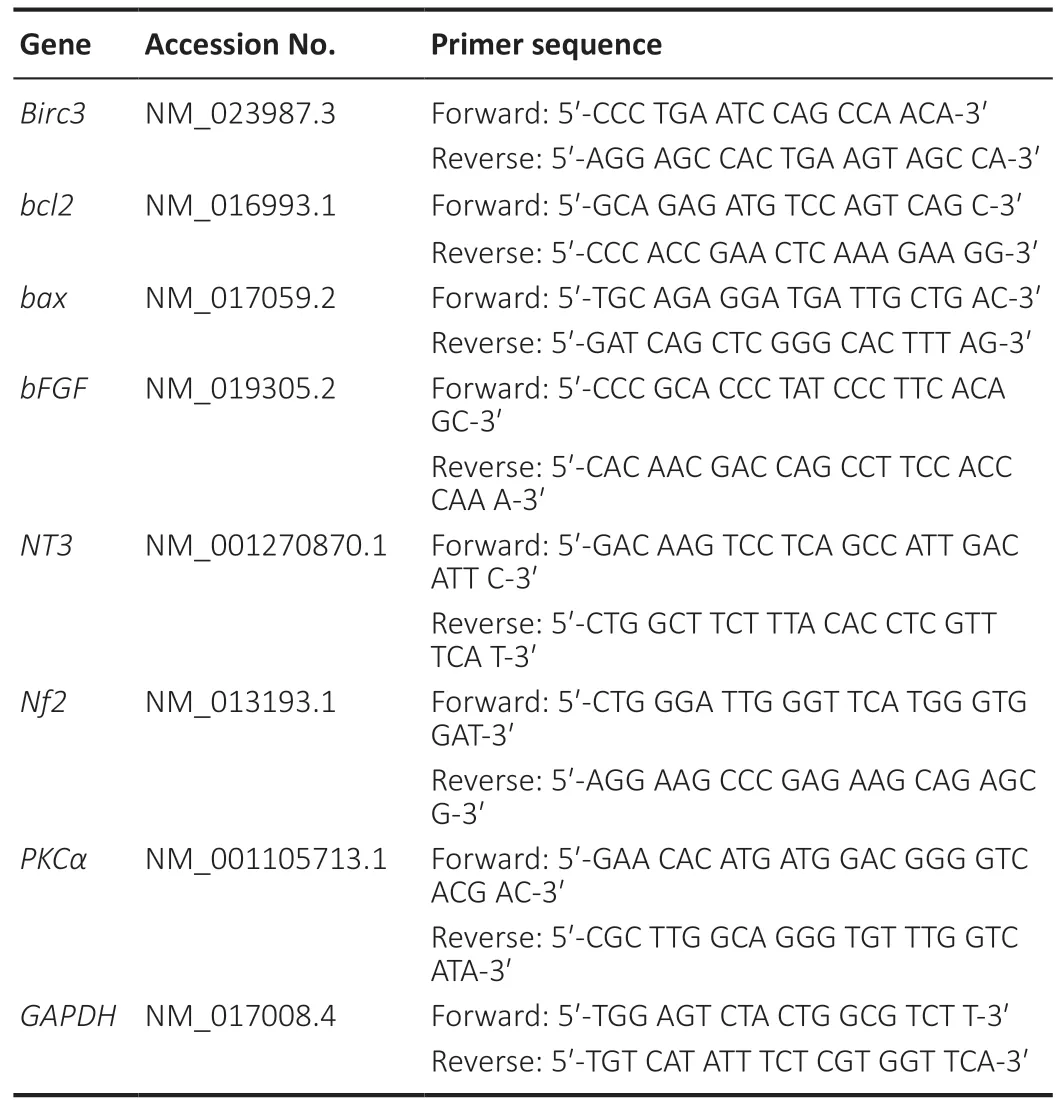
Table 3|Real-time quantitative polymerase chain reaction primers used in this study
Western blot assay
Proteins were extracted from the samples of injured nerves and primary cultured SCs using protein lysis buffer.Equal amounts of protein were separated using 10% protein gel electrophoresis and then the proteins were transferred onto polyvinylidene fluoride membranes (Bio-Rad,Hercules,CA,USA).The membranes were blocked using 3% bovine serum albumin in Tris-buffered saline containing Tween-20.The protein expression levels were detected and the protein samples were incubated with the following primary antibodies at 4°C overnight:BIRC3 (mouse,monoclonal antibody,1:200,Cat# sc-7944,Santa Cruz Biotechnology),c-Fos (rabbit,monoclonal antibody,1:200,Santa Cruz Biotechnology,Cat# sc-253),β-catenin (mouse,monoclonal antibody,1:200,Santa Cruz Biotechnology,Cat# sc-59737),p-AKT/AKT(rabbit,monoclonal antibody,1:1000,CST,Cat# 4060),p-c-Jun/c-Jun (mouse,monoclonal antibody,1:200,Santa Cruz Biotechnology,Cat# sc-822) and phosphorylated extracellular regulated protein kinase (p-ERK)/ERK (rabbit,monoclonal antibody,1:200,Santa Cruz Biotechnology,Cat# sc-7383).Protein samples treated with above mentioned primary antibodies were compared with negative control.Then protein samples were incubated with secondary antibody horseradish peroxidase-labeled goat anti-rabbit IgG (1:1000,Cat# A0208,Beyotime,Nanjing,China) for another 2 hours at room temperature.For this analysis,β-actin (a mouse monoclonal antibody,1:400,Cat# A1978,Sigma-Aldrich) was used as a reference to normalize total protein levels.The images were scanned with Bio-Rad GS800 (Bio-Rad,Hercules,CA,USA) and then the relative expression was shown as optical density ratio relative to β-actin.
Cell proliferation
SC proliferation was assessed after SCs were transfected with BIRC3 plasmid and siRNA for 48 hours.According to the manufacturer’s instructions,Cell-Light 5-ethynyl-2′-deoxyuridine (EdU) DNA Proliferation Kit (RiboBio,Guangzhou,China) was used to measure SCs proliferation.SCs (2 × 105cells/mL) were cultured in Dulbecco’s modified Eagle’s medium,and plated onto plates coated with 0.01% poly-Llysine.EdU was added to the medium for 2 hours and the cells were fixed using formaldehyde.The ratio of EdU-positive cells was determined using randomly selected field images under a fluorescence microscope (Leica DMI 4000B Research Inverted Microscope).
Cell migration
Cell migration was evaluated by Transwell migration assay.As described previously (Weinstein and Wu,2001),6.5-mm transwell chambers with 8-µm pores (Costar,Cambridge,MA,USA) were used to examine SC migration.The Transwell chamber membrane surface was pre-coated with 10 µg/mL fibronectin (Beyotime).SCs were resuspended in Dulbecco’s modified Eagle’s medium (1 × 106cells/mL) and transferred onto the top of the Transwell chamber.After incubation at 37°C in 5% CO2for 24 hours,SCs were then allowed to migrate to the lower chamber.The SCs were then stained with crystal violet (Beyotime) and counted using an inverted microscope(Leica Inverted Microsystems).
Flow cytometry
SC apoptosis was assessed using the Annexin V-FITC Apoptosis detection kit (Invitrogen,New York,NJ,USA) according to the manufacturer’s recommended protocol.The transfected SCs were labeled with annexin V and fluorescein isothiocyanate in binding buffer and collected for flow cytometry (Invitrogen,New York,NJ,USA).The samples were incubated with propidium iodide at 4°C in the dark.The number of apoptotic cells was counted using FACScan flow cytometry (BD,San Jose,NJ,USA) software according to manufacturer’s instructions.The assay was performed three times.
In vivo experiments
Chimeric Rabies Virus Glycoprotein (RVG-9R) Fragments were used as a tool for siRNA delivery to the peripheral nerves(Yao et al.,2012,2013).Adult Sprague-Dawley rats were used.Rat sciatic nerves were exposed and 1cm-long defects were created.To bridge the nerve gaps,1-cm silicone tubes(Invitrogen,New York,NJ,USA) were used.The rats with bridged nerve gaps were randomly divided into two groups(n=3):a control group and an assay group where RVG-9R(RiboBio,Guangzhou,China) and BIRC3 siRNA were injected.The plasmids overexpressing Birc3 (RiboBio,Guangzhou,China) and Matrigel (BD) were used to overexpress BIRC3in vivo.The BIRC3 siRNA (RiboBio,Guangzhou,China) and Matrigel complex were also injected into the tube.The rats were anesthetized using an intraperitoneal injection of complex narcotics for surgery.After 7 and 14 days,the nerves with silicone tubes were collected.Western blot assay,qRTPCR and transmission electron microscopy were performed.
Transmission electron microscopy of nerve structure in vivo
Changes in sciatic nerve microstructure were determined in sections of the sciatic nerves with bridged nerve gaps,control (RVG-9R),blank BIRC3 siRNA,BIRC3 siRNA,BIRC3 plasmid (Birc3 overexpression) and Matrigel groups using transmission electron microscope (CM-120,Philips,Eindhoven Netherlands).
Cell apoptosis in sciatic nerves in vivo
Cell apoptosis in sciatic nerves with bridged nerve gap was assessed using terminal deoxynucleotidyl transferasemediated nick-end labeling (TUNEL) apoptosis kit (Roche Inc.,Basel,Switzerland).The frozen slices were examined using a TUNEL kit.The nuclei were labeled with Hoechst 33342(RiboBio,Guangzhou,China),and the average number of TUNEL-positive apoptotic cells was calculated under M2 fluorescence microscope (Leica,Mannheim,Germany) and a Leica Imager (Leica,Mannheim,Germany) with an objective.
Statistical analysis
SPSS 15.0 for Windows (SPSS,Chicago,IL,USA) and Prism 5 software (GraphPad,San Diego,CA,USA) were used for statistical analysis.Independent samplet-test was used to compare the differences between two groups.All data are presented as the mean ± standard error of mean (SEM).P<0.05 was considered statistically significant.
Results
BIRC3 expression in SCs is up-regulated during early WD(within 1 day)
Since we previously identified BIRC3 as a key regulator in nerve injury and repair by bioinformatics analysis (Li et al.,2014),we further investigated BIRC3 in this study.We determined the expression levels of BIRC3 using western blot assay,qRT-PCR and immunohistochemistry at 3,6,12,18,and 24 hours post sciatic nerve injury.Both western blot assay and qRT-PCR indicated that BIRC3 protein and mRNA expression was already up-regulated 3 hours post-injury.We also used immunohistochemistry to visualize the immunopositivity and localization of BIRC3 and S100B in injured sciatic nerve at 6,12 and 24 hours post-injury and in the primary cultured SCs.BIRC3 and S100B were colocalized in the SCs and their expression in the injured sciatic nerve increased substantially.We immunostained the SCs using anti-S100B in nerves and cultured SCs.The results showed BIRC3 was also expressed in cultured SCs (Figure 1).
BIRC3 affects SC proliferation,migration,and apoptosis in vitro
Three specificBIRC3siRNAs were synthesized.BIRC3mRNA expression was significantly decreased in BIRC3 siRNAs withBIRC3knockdown.ThenBIRC3knockdown was used for subsequent analysis.To explore the effect ofBIRC3on SCsin vitro,primary cultured SCs were transfected with pcDNA3.1+BIRC3plasmid,BIRC3siRNA,or a negative control (control siRNA) vector and screened for proliferation,migration,and apoptosisin vitro.Cell proliferation was expressed as the ratio of EdU-positive cells.Compared with control siRNA,SC proliferation was significantly reduced byBIRC3siRNA transfection,while transfection with pcDNA3.1+BIRC3increased proliferation (Figure 2).Transwell migration assays showed that silencingBIRC3expression in SCs significantly decreased SC migration compared with the siRNA controls,while the pcDNA3.1+BIRC3plasmid increased SC migration compared to the control siRNA (Figure 3).The apoptotic cells were determined using flow cytometry analysis by detection of Annexin V.Silencing BIRC3 expression increased SC apoptosis,and up-regulated BIRC3 expression decreased SC apoptosis compared to the blank control cells after transfection (Figure 4).The data indicated that changes in BIRC3 expression significantly affected SC proliferation,migration and anti-apoptosisin vitro.
BIRC3 up-/down-regulates the expression of other genes in SCs in vitro
To further explore the potential effects of BIRC3in vitro,we then analyzed the expression of several nerve degeneration and/or regeneration related genes afterBIRC3overexpression or knockdown in transfected SCs.qRT-PCR results indicated compared with black group,neurotrophin 3 (NT3) (P<0.001),neurofibromin 2 (NF2) (P<0.001) and basic fibroblast growth factor (bFGF) (P<0.001) mRNA expression levels were downregulated and protein kinase C α (PKCα) (P<0.001) was upregulated in cultured primary SCs followingBIRC3knockdown.BIRC3 overexpression exhibited the opposite effect on these mRNA expression levels (P<0.05 orP<0.001;Figure 5).Therefore,altered BIRC3 expression could regulate gene expression levels of nerve degeneration and/or regeneration related factors in SCsin vitro.
BIRC3 affects the c-fos and p-ERK/ERK pathways in vitro
After we assayed the potential roles of BIRC3in vitrothrough how changes in its expression affected the release of related factors,we examined whether BIRC3 affects cell signaling pathways in cultured SCsin vitro.Therefore,we detected the expression levels of BIRC3,c-Fos,β-catenin,p-AKT/AKT,p-c-Jun/c-Jun and p-ERK/ERK.Compared with negative control,the c-Fos expression and ERK phosphorylation were significantly increased after BIRC3 siRNA or pcDNA3.1 (+)-BIRC3 plasmid transfection (P<0.05 orP<0.01),while β-catenin expression,AKT phosphorylation and c-Jun phosphorylation were almost unchanged (Figure 6).These results indicated that BIRC3 may activate the c-Fos and ERK signaling pathways in cultured SCsin vitro.
BIRC3 expression affects the pathological morphology of injured sciatic nerve
To further analyze the role of BIRC3 in injury and/or repair after sciatic nerve injury in rats,the effects of BIRC3 on nerve injury were analyzed after 7 and 14 days.We exposed the SD rat sciatic nerves and created 1-cm gaps,and then implanted silicone tubes to bridge the nerve gaps.We injected RVG-9R andBIRC3siRNA to the bridged nerve gaps.TheBIRC3siRNA and Matrigel complex was also injected into the tube.We exposed the injured sciatic nerves to pcDNA3.1(+)-BIRC3,BIRC3siRNA,and negative control.The qRT-PCR results revealed that pcDNA3.1+BIRC3andBIRC3siRNA had special effects on injured sciatic nervesin vivo(Figure 7).The mRNA expressions ofBIRC3were downregulated after being silenced byBIRC3siRNA and upregulated after being overexpressed by pcDNA3.1+BIRC3on days 7 and 14in vivo.Immunohistochemistry (Figure 7) and transmission electron microscopy (Figure 8) also revealed morphological and microstructure changes in the injured sciatic nerves.The myelin and axonal debris were better cleared after being silenced byBIRC3siRNA and upregulated after being over-expressed by pcDNA3.1+BIRC3on day 14 than on day 7in vivo.
BIRC3 expression affects nerve degeneration and regeneration in vivo
We used a TUNEL assay to explore the cell apoptosis effects of BIRC3 on injured sciatic nervesin vivo.The apoptotic cell number was reduced after BIRC3 was over-expressed,while apoptosis was induced when BIRC3 expression was silenced (Figure 4).The data was consistent with the mRNA expression results.Further,we explored whether BIRC3 affected signaling pathwaysin vitrowere also alteredin vivo.The western blot data showed that β-catenin,c-Fos and c-Jun expression and ERK and AKT phosphorylation were increased by BIRC3 silencing on days 7 and 14 post-injury (Figure 9),especially on day 7.The β-catenin and c-Fos expression and ERK phosphorylation were also affected following BIRC3 overexpression (Figure 9).These results suggest that post-injury BIRC3 plays important anti-apoptotic roles in peripheral nerve degeneration and regeneration during early WDin vitroandin vivo.
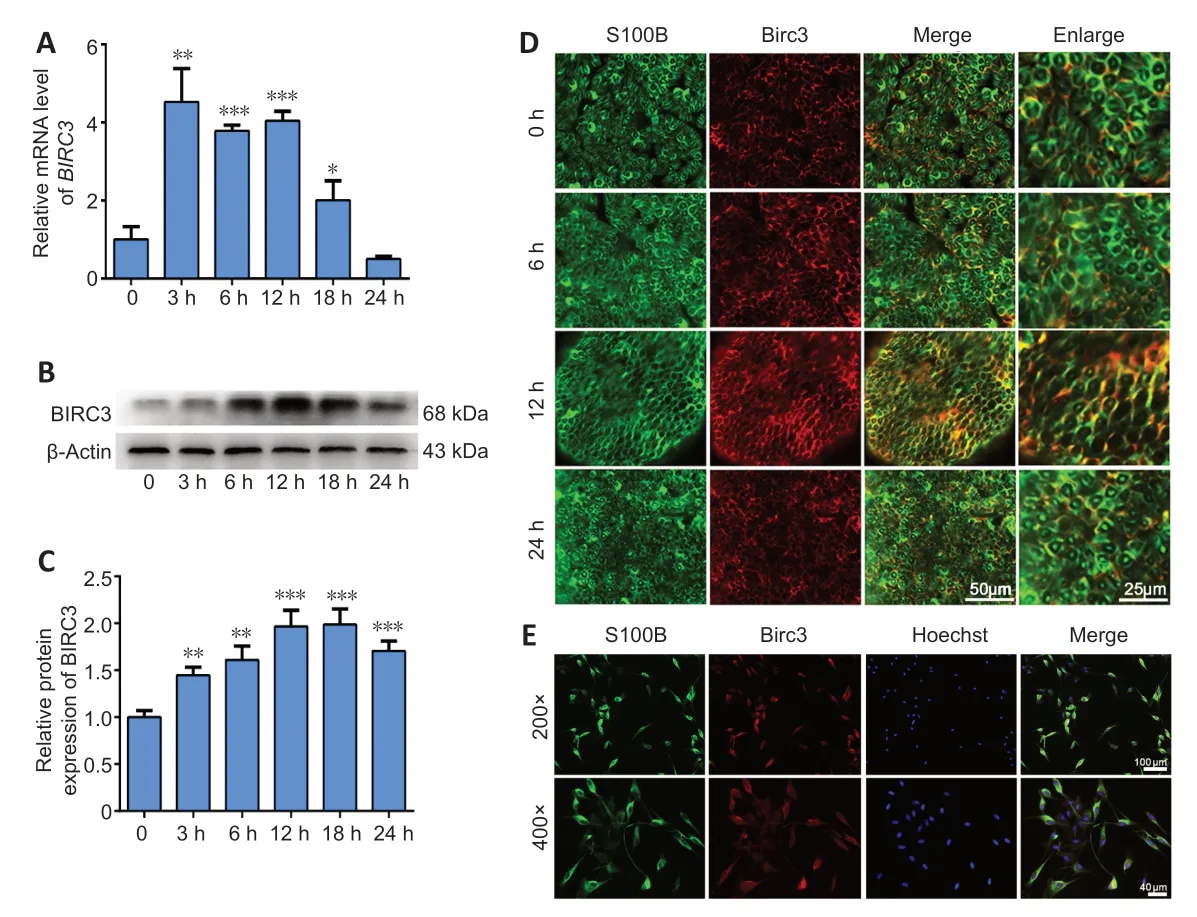
Figure 1|BIRC3 expression in cultured Schwann cells and injured sciatic nerves.
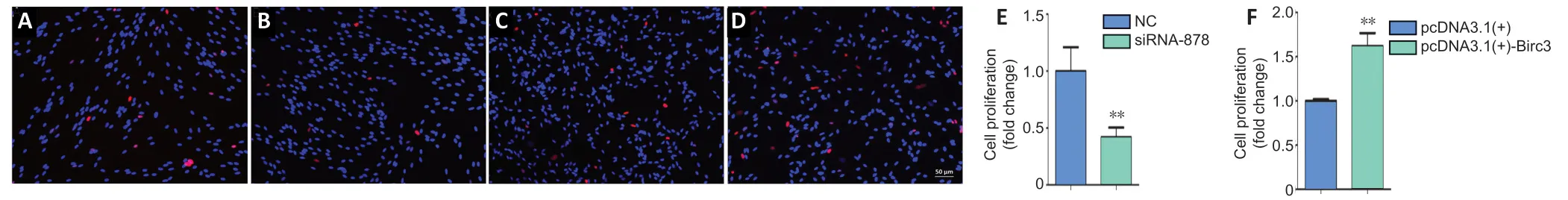
Figure 2|BIRC3 expression affects Schwann cell proliferation in vitro.
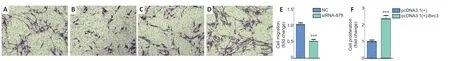
Figure 3|BIRC3 expression affects Schwann cell migration in vitro.

Figure 4|BIRC3 expression affects Schwann cell apoptosis in vitro.
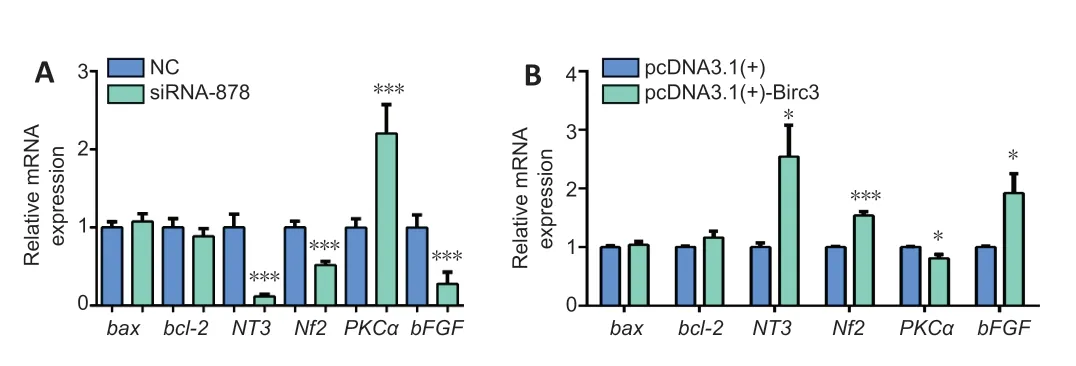
Figure 5|BIRC3 knockdown and overexpression affect the expression of related factors in SCs in vitro detected by quantitative real-time polymerase chain reaction.

Figure 6|C-Fos,p-c-Jun/c-Jun,p-AKT/AKT,p-ERK/ERK and β-catenin expressions in Schwann cells after BIRC3 overexpression and knockdown.

Figure 7|BIRC3 knockdown or overexpression in injured rat sciatic nerve in vivo.

Figure 8|BIRC3 siRNA knockdown and overexpression in the injured rat sciatic nerves cause morphology changes on days 7 and 14 in vivo.
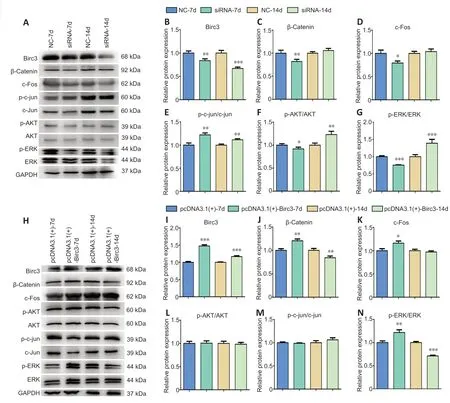
Figure 9|Altered BIRC3 expression affects the different signaling pathways in injured sciatic nerves in vivo.
Discussion
Nerve injury and repair is an important field of study in modern neuroscience.WD in the PNS is a process of nerve degeneration and regeneration after injury.It can be caused by inflammatory injuries to the axon,which begins from the disintegration of the axolemma and axoplasma within 24 hours.Studies on WD have explored its molecular mechanisms (Yao et al.,2012,2013;Gong et al.,2014;Li et al.,2015;Liu et al.,2019,2020;Rassu et al.,2020).The results showed that it is essential to clean and reinnervate the damaged distal stump.The axonal debris and myelin are cleared,which require a macrophage response within 1–2 weeks (Locksley et al.,2001;Hirakawa et al.,2003;Chen et al.,2007;Griffin et al.,2010;Gong et al.,2014;Li et al.,2015;Liu et al.,2017).The macrophage activation is an important aspect of this rapid response in the PNS that leads to myelin and axonal debris phagocytosis and clearance.The rapid myelin clearance contributes to peripheral nerve regeneration after injury.Macrophages in degenerating nerves are mainly of hematogenous origin (Weinstein and Wu,2001;Kumar et al.,2007;Yao et al.,2012,2013;Wu et al.,2013;Jung et al.,2014;Rassu et al.,2017).Activation of SCs during WD includes macrophage invasion,cellular alteration,and neurotropic regulation.The response of SCs is estimated earlier to the onset of axonal degeneration.SCs release cytokines and chemokines to recruit macrophages which could be mediated by the actin cytoskeleton in response to various stimuli (Yao et al.,2012,2013;Wu et al.,2013;Jung et al.,2014;Rassu et al.,2017).Therefore,understanding the regulators that activate the rapid macrophage responses during WD and their molecule mechanism in the PNS may provide new insights into the strategies for peripheral nerve repair and regeneration.
Studies have reported that BIRC3 exhibits a pivotal effect on the immune system.It may also play important roles in nerve injury and/or repair (Wang et al.,2012;Asmal et al.,2013).However,the molecular mechanism of BIRC3 remains largely unknown and has not been exploredin vitroandin vivo(Wang et al.,2012;Li et al.,2014).The main purpose of this study was to elucidate these mechanisms.In this study,we analyzed the change in BIRC3 expression during early WD after sciatic nerve transection in rats.We found that BIRC3 expression was up-regulated from 3 hours post nerve injury and reached its maximum level during early WD.In this study,we investigated the roles of BIRC3,which might delay early WD via anti-apoptosisin vivoandin vitro.The data indicated that BIRC3 expression was significantly up-regulated in injured sciatic nerves.Functional analysis showed that altered BIRC3 expression affected SC migration,proliferation,apoptosis and the expression of some related factors through activating the c-fos and p-ERK/ERK pathways.BIRC3 might be the most viable potential target for anti-apoptotic protection mediated by inflammatory cytokines.
The BIRC family is composed of eight members.The majority of BIRCs serve as endogenous inhibitors of apoptosis.All BIRC family members contain a motif termed the baculovirus IAP repeat,which is required for their cytoprotective function.Several studies have focused on the effect of BIRCs in various types of neoplasms (Piro et al.,2015;Gressot et al.,2017).BIRC1 is involved in neurodegenerative disorders.The resistance of BIRC3 to fludarabine results in truncated protein expression.BIRC5 has been shown to be expressed in tumors.Finally,BIRC3 is an important regulator of the TNF-α proapoptotic pathway (Wang et al.,2012;Li et al.,2014;Piro et al.,2015;Gressot et al.,2017).It suppressed TNF-α pathway signaling by preventing the formation of TNF receptor proapoptotic signaling complex.TNF receptors are able to activate the NFkB signaling pathway and TNF-α is one of the primary initiators of the inflammatory cascade.In the PNS,TNF-α may induce mature SCs toward an immature state.This phenotype reversion increases SC susceptibility to pro-apoptotic molecules (Asmal et al.,2013;Tan et al.,2013;Glodkowska-Mrowka et al.,2014;Piro et al.,2015;Gressot et al.,2017;Jiang et al.,2017;Lee et al.,2017).As major glia,SCs play key roles in nerve repair and regeneration after peripheral nerve injury.They prevent damage from inflammatory cytokines and maintain functional and structural integrity.SCs can initiate a self-protective response to the excessive inflammatory cytokines that are responsible for turning off pro-inflammatory cytokines.Thus,they might be activated through the c-jun/p-c-jun or NFkB signaling pathways by exerting anti-apoptotic functions (Adalbert et al.,2006;Mietto et al.,2015;Pan et al.,2017;Shen et al.,2020).
In this study,we investigated the role of BIRC3,one of the major regulators,in the early WD following sciatic nerve injury.It has been reported that the delay in WD after injury is due to a delay in the axonal degeneration and the clearance rate of myelin.Our results indicate that BIRC3 has an effect on SC proliferation,migration,and apoptosis.BIRC3 might delay early WD via anti-apoptotic effectsin vivoandin vitro.We speculate that BIRC3 is likely to produce an anti-apoptotic response and might therefore play key roles during the activation of early signaling pathways.Understanding the molecular mechanisms underlying anti-apoptotic effects in sciatic nerve injury would open the possibility of therapeutic interventions for peripheral nerve degeneration and regeneration.
In summary,based on previous studies,this study explained the mechanism behind the effects of BIRC3 on nerve degeneration and/or regeneration.These findings may prove beneficial for the use of cell and gene therapy or other basic medical methods in the treatment of peripheral nerve injury.Collectively,in this study,we found that BIRC3 affected the migration,proliferation,and apoptosis of SCs through activating the c-fos and p-ERK/ERK pathways.BIRC3 may delay early WD via anti-apoptotic pathways following nerve injury.WD is of great importance for nerve injury,repair,and regeneration.In this study,we explored the role of BIRC3 in early WD.The study demonstrated that BIRC3 expression was significantly upregulated in injured rat sciatic nerves.BIRC3 may delay early WD through an anti-apoptotic mechanism in affected SCs following nerve injuryin vivoandin vitro.The study provides insights into the role of BIRC3 in early WD during peripheral nerve degeneration and/or regeneration.
Acknowledgments:We greatly appreciate the editorial assistance of Ian Haigler from Jiangsu Key Laboratory of Neuroregeneration‚Nantong University‚China.
Author contributions:Study design and manuscript writing:DBY;experiment coordination:MC‚JS‚YW;gene expression analysis:BY‚DBY;immunohistochemical experiments:YMF;functional and biochemical data analysis:XX;in vivo study:NNG;data analysis:HHZ‚XX‚DBY.All authors read and approved the final manuscript.
Conflicts of interest:The authors declare that they had no competing interests.
Financial support:This work was supported by the National Natural Science Foundation of China‚Nos.31971277‚31950 410551;Scientific Research Foundation for Returned Scholars‚Ministry of Education of China;Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD);and the Postgraduate Research &Practice Innovation Program of Jiangsu Province‚China‚No.KYCX 19-2050(all to DBY).The funders had no roles in the study design‚conduction of experiment‚data collection and analysis‚decision to publish‚or preparation of the manuscript.
Institutional review board statement:The study was approved by the Institutional Animal Care and Use Committee of Nantong University‚China (approval No.2019-nsfc004) on March 1‚2019.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal‚and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License‚which allows others to remix‚tweak‚and build upon the work non-commercially‚as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Ernesto Doncel-Pérez‚Hospital Nacional de Parapléjicos‚Spain.
- 中国神经再生研究(英文版)的其它文章
- Towards a comprehensive understanding of p75 neurotrophin receptor functions and interactions in the brain
- Microglia regulation of synaptic plasticity and learning and memory
- Stroke recovery enhancing therapies:lessons from recent clinical trials
- Functional and immunological peculiarities of peripheral nerve allografts
- MicroRNA expression in animal models of amyotrophic lateral sclerosis and potential therapeutic approaches
- Significance of mitochondrial activity in neurogenesis and neurodegenerative diseases

