Visual system repair:what’s next?
Noemie Vilallongue,Homaira Nawabi
One of the hallmarks of the mature neurons from the central nervous system(CNS) is their inability to grow their axons after an insult.In turn,whenever a neuronal circuit is damaged,either by a neurodegenerative disease or a traumatic injury,the motor and/or cognitive functions associated with it are lost permanently.Therefore,understanding the molecular and cellular barriers to CNS repair is a major challenge not only for Neurosciences but also for public health.
Regrowing mature axons has been the limiting step in the field of CNS regeneration for many years.Indeed,for a long time,studies focused only on the inhibitory role of the environment(glial scar and myelin debris),but its manipulation resulted on moderate growth (He and Jin,2016).Ten years ago,the discovery of the critical role of neurons themselves has been a game changer leading to extensive axonal regeneration:from the eye to the brain in the visual system (Belin et al.,2015;He and Jin,2016).In addition,it has been shown recently that regenerating axons can be myelinated using a combination of drugs (Wang et al.,2020).This is a critical milestone to reach the functionality of the regenerating circuit.
Despites all those achievements,functional recovery remains challenging.As a matter of fact,the huge diversity of neuron populations as well as their different capabilities to survive,to regenerate,to reach their targets after the injury represent a major roadblock to build functional circuits (Figure 1).We will discuss all these remaining challenges in this perspective.
Diversity of neuronal populations:In the nervous system,many different types of neurons co-exist and interact in a tight coordination in order for the body to function smoothly.Interestingly recent data reveal that each neuronal population will show a specific response to injury and might need its own molecular cocktail to be able to grow axons.
Neurons show different rates of survival after the injury,even within the same region of the nervous system.A detailed analysis of neuronal behavior upon axon lesion shows that in the retina some neurons are more resilient than others(Figure 1).The retina,that belongs to the CNS,is the organ that perceives the visual stimuli.Visual information is conveyed by axons of the retinal ganglion cells(RGC) that form the optic nerve,the only bridge between the eye and the brain.At least 30 subtypes of RGC have been described so far,characterized by the specific expression of molecular markers,anatomical localization,specificity of neuronal target or visual function (Figure 1) (Sanes and Masland,2015).
Indeed,RGC can be classified according to their function.In one hand,specific subtypes of RGC support each aspect of the vision (image-forming function):light intensity,motion rate,direction.For example,the ON-OFF directionally selective ganglion cells (ooDSGC),expressing specifically the marker CART(cocaine-and amphetamine regulated transcript) respond to both increase and decrease of light intensity but also have directional preference in motion.In another hand,other RGC are specialized in non-image forming functions.In this regard,the ipRGC (intrinsically photosensitive RGC) express the melanopsin photopigment allowing them to naturally detect light and regulate the photoentrainment of circadian rhythms(Figure 1).
Interestingly,each RGC subpopulation responds differently to axon injury.Indeed,within only 2 weeks after optic nerve injury,as much as 80% of RGC undergo apoptosis.Duan and colleagues(Duan et al.,2015) used reporter lines and immunostaining to follow the fate of several RGC subtypes after axon lesion.The alpha-RGC that specifically innervate the dorsal lateral geniculate nucleus(dLGN) and the superior colliculus at eye opening,are surviving extremely well even 28 days post optic nerve lesion(around 80%).The M1 subtype of ipRGC that project to the suprachiasmatic nucleus (SCN),ensuring its role of master regulator of circadian rhythms,are also able to bear very well axon lesion as 70%of injured M1-RGC are surviving.A recent single cell profiling study addressed the resilient potential to injury of different RGC subpopulations.With this approach,authors were able to distinguish 46 transcriptionally different subtypes of RGC(Tran et al.,2019).Using this atlas,they confirmed that 2 out of 4 subtypes of alpha-RGC and all ipRGC are resilient upon injury.In contrast to dying RGC,resilient RGC keep their dendritic morphology and complexity,suggesting that their functions are unaltered even if their axons are lesioned.Another remarkable feature of resilient neurons comes from their firing rate:indeed,those neurons present a sustained response as it has been already reported for surviving motoneurons in the spinal cord,in the context of amyotrophic lateral sclerosis.
However,some other RGC subpopulations are most sensitive to the lesion,such as the W3-RGC that are among the smallest in terms of soma size but among the most abundant RGC that show only 10% of survival.Moreover,a novel class of RGC called N-RGC which expresses transcription factors Neurod2 and Satb2 also seems to be highly susceptible to injury (Tran et al.,2019).Finally,ooDSGC present few if any survival even 14 days after injury (Duan et al.,2015).
Besides their survival potential,neurons also show different rates of regeneration after the injury:mostly alpha-RGC and ipRGC are believed to regenerate well(Bray et al.,2019).Molecular wise,it is rather difficult to pinpoint precisely universal molecules that could predict resilience and regeneration rates.However,overexpression of some molecules such as Thrombospondin (as it was already been reported in Bray et al.,2019),urocortin (Ucn),Timp2 or Mmp12 globally improve the survival of RGC that were not expressing them originally.Strikingly,not all the RGC respond to the molecular manipulations (Duan et al.,2015;Tran et al.,2019).It appears that each subpopulation of neuron has its own degree of molecular flexibility that allows it to launch survival and even growth programs.One elegant demonstration comes from the manipulation of the transcription factor Sox11 (Norsworthy et al.,2017) expressed in differentiating retinal progenitors.Overexpression of Sox11 induces axon regeneration of some populations of mature neurons while killing others.Indeed,the forced increase of Sox11 levels is toxic for alpha-RGC.Interestingly,alpha-RGC are among the few in the retina that respond positively to the mTOR pathway activation (either by Pten deletion or the overexpression of IGF-1 (Duan et al.,2015).The combination of mTOR activation and Sox11 induces some long-distance regeneration from RGC subpopulations that were originally insensitive to mTOR activation (Figure 1)(Norsworthy et al.,2017).
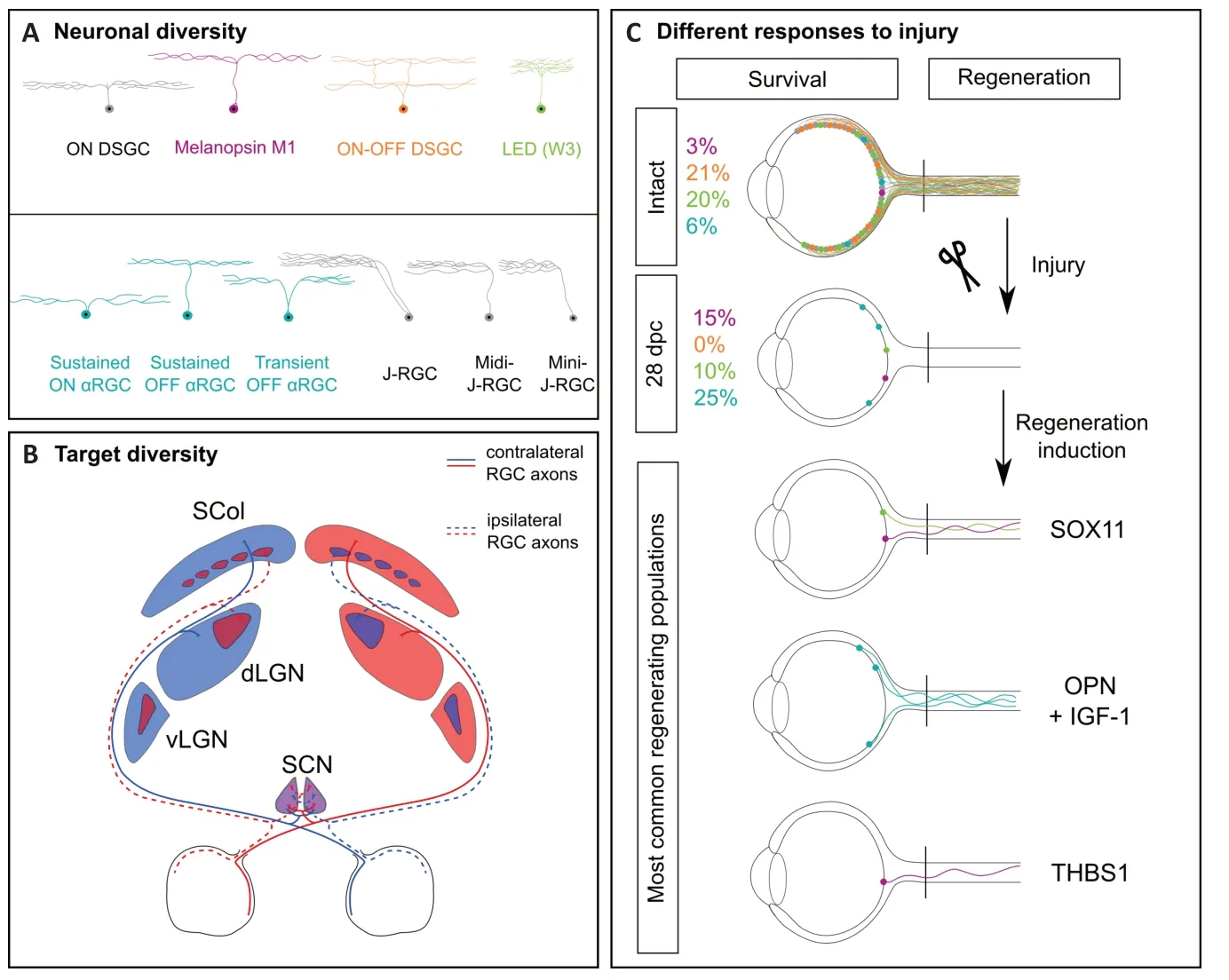
Figure 1|Roadblocks to achieve functional recovery after axon regeneration.
Altogether,these findings shed the light on the nervous system complexity in terms of heterogeneity.So far,it is not clear how many neurons are needed in terms of survival and regeneration in order to reach the threshold for sufficient activation of their post-synaptic partners and ensure visual functions.Moreover,the wide range of functions provided by the different RGC subpopulations has to be operational to allow functional recovery.To this end,it is essential to find ways to allow the survival and the growth of most RGC subtypes.The next step would be to ensure that growing axons form the original topographic maps.
Connectivity within the brain targets:The visual system is formed by the axonal projections of both eyes.During development,the connectivity between the eyes and the brain is tightly regulated in order to draw stereotypical topographic maps that allow binocular vision.Indeed,each eye will project to both sides of the brain and the degree of 3D vision is correlated with the percentage of segregation between crossing and noncrossing RGC axons.When axons reach the distal part of the optic nerve,they face a choice point called the optic chiasm:in mice,10% of axons stay in the ipsilateral side,while almost 90% will cross the midline.In human,50% of axons cross the midline.After the optic chiasm most of the axons will travel within the optic tracts to reach one of the 13 main brain targets.
The SCN is located in the ventral part of the hypothalamus,just above the optic chiasm,and is the closest visual target to the eye.As described before,the SCN is innervated by the M1-ipRGC that represent about 3% of the RGC neurons.Unlike other brain regions,each SCN is innervated equivalently by both eyes:indeed,each ipRGC axon will be divided into two branches to innervate ipsilateral and contralateral SCN (Fernandez et al.,2016).Thus,if ipRGC are able to regenerate,one must verify whether the programs that drive axon branching during development are still effective in the mature regrowing axons and whether the patterns of SCN reinnervations correspond to the ones establish during development.
For the other targets,the most studied ones are the Thalamus (mostly the dLGN)and the superior colliculus.Those regions are located deeper into the brain and axons need to travel within the optic tract to reach them.They receive also projections from both eyes but here the RGC projecting ipsilaterally are less abundant than the contralateral RGC and there is a segregation of the territories they occupy.At birth the projections from both eyes overlap in those brain targets.However,within few days after eye opening (around post-natal day 12 in mouse),the dLGN peripheral domain is only connected by the contralateral eye and the central domain by the ipsilateral eye (Figure 1B).The pruning of those unwanted connections is regulated by neuronal activity and mediated by microglia (Schafer et al.,2012).Therefore,it would be interesting,for example,to address how the innervation of dLGN occurs during regeneration:will the projections be segregated directly or whether as during development,a pruning step would be needed?
Moreover,it is now well documented that axon lesion induces changes within the glia.For example,it has been recently shown that microglia is transcriptionally different in immatureversusmature CNS and regulates differently the regenerative outcome (Li et al.,2020).Indeed,while glia is responsible for a scar formation in the adult,inhibiting regeneration (physical barrier and secretion of inhibitory factors),a scar-free wound-healing response takes place two weeks after the injury in P2 animals.In this context,one question is whether microglia is still able to resume its activities in denervated regions of the brain to the same extent as in young animals.
Finally,the guidance of regenerative fibers is still an open question in the field.During development,neurons are paired to their proper partners through a mechanism called axon guidance.In this time window,the environment expresses diverse molecules that give topographic information to growing axons and help them to travel over long distances to reach their final targets.In mature nervous system this road map is completely reset.Therefore,it came with no surprise that growing axons in the mature nervous system present strong guidance defects.It is now very commonly described that a large number of regenerative axons displays misprojections:within the optic nerve,some will turn towards the eye ball,stall at the optic chiasm,won’t respect the ipsilateral-contralateral segregation or will enter into the brain randomly (Belin et al.,2015).Those axons compromise strongly any attempt for functional recovery.Not only do they fail to reform the original neuronal circuit but they might also form aberrant circuits that will interfere with functional recovery.For instance,Sox11 overexpression in the somatosensory cortex induces the regeneration of the corticospinal axons after spinal cord injury.However,when the behavior outcome was tested,these animals were less performant than the controls (Wang et al.,2015).Thus,axon regeneration is not sufficient by itself to promote CNS repair.
Conclusion:Great progress has been accomplished to unlock molecular and cellular mechanisms of CNS regeneration.The visual system has been an important study model to identify those players.However,there are still many challenges ahead of us in order to reach the ultimate goal in the field:sustained functional recovery.To this end,helping axons to grow is not enough anymore.The next step would be to induce the growth of most of neurons and this regeneration should be controlled by considering the heterogeneity in neuronal injury response and potential for survival and regeneration.Regenerative axons should be paired with their correct targets and those circuits should be consolidated through time to ensure long-lasting functional recovery.
We would like to apologize for authors whose work could not be included due to space limitation.
We thank Julia Schaeffer and Stephane Belin for their critical reading of the manuscript.
The present work was supported by ERCSt17-759089-DRIVE and NRJ Foundation(to HN).
- 中国神经再生研究(英文版)的其它文章
- Towards a comprehensive understanding of p75 neurotrophin receptor functions and interactions in the brain
- Microglia regulation of synaptic plasticity and learning and memory
- Stroke recovery enhancing therapies:lessons from recent clinical trials
- Functional and immunological peculiarities of peripheral nerve allografts
- MicroRNA expression in animal models of amyotrophic lateral sclerosis and potential therapeutic approaches
- Significance of mitochondrial activity in neurogenesis and neurodegenerative diseases

