Potential use of lactate for the treatment of neonatal hypoxicischemic encephalopathy
Isadora D’Ávila Tassinari,Luciano Stürmer de Fraga
Function of lactate:Lactate is a three-carbon molecule produced by glycolytic metabolism that is a metabolic waste product with no known use in clinical therapy.Conversely,it is a metabolite that the body should quickly guarantee the clearance.However,lactate is now recognized as a potential energy substrate,as well as an anti-inflammatory signaling molecule.These actions were first reported in adult animal models with a brain injury,including a traumatic brain injury and cerebral ischemia,and have also been observed in human patients (Magistretti and Allaman,2018).Recently,however,two studies by independent research groups described promising neuroprotective results from the use of lactate in animal models with neonatal hypoxia-ischemia (Roumes et al.,2021;Tassinari et al.,2020).Both studies suggested that lactate administered intraperitoneally was able to reach the brain and contribute to the reduction of brain injury,as well as improve behavioral parameters.Despite that the pre-clinical studies were performed using neonatal rats,they suggest the potential use of lactate as clinical therapy for the treatment of hypoxicischemic encephalopathy (HIE),a disorder still affecting a high number of newborns.
Neonatal hypoxic-ischemic encephalopathy:Babies may undergo impairments around the time of birth,leading to HIE,a type of neonatal encephalopathy due to hypoxic-ischemic (HI)events.HIE remains a public health concern worldwide,affecting a significant percentage of newborns in high-,medium-,and lowincome countries (Gunn and Thoresen,2019).
Any situation that compromises the delivery of oxygen and glucose to a newborn’s brain during the perinatal period is a risk factor for HIE,which contributes to the long-term,severe damages,such as motor and cognitive deficits,epilepsy,and cerebral palsy,observed after HI events.Even if the event affects the cardiovascular system and other systemic responses,the brain is the most injured organ.The pathophysiology of HIE can be divided into phases,with the acute phase shaped by energy depletion,hindering electrochemical gradient homeostasis(Gunn and Thoresen,2019) and leading to cell death.The succeeding phase of brain injury evolution involves inflammation and oxidative stress.To attenuate the deleterious effects of HIE and reduce poor long-term neurological outcomes,therapeutic hypothermia (TH) has been the standard approach in newborns suffering from HIE (Gunn and Thoresen,2019).However,TH has some limitations,such as a short therapeutic window.Moreover,in severe cases of HIE,TH appears not to be neuroprotective.Since the neuroprotection offered by TH alone is variable and presents pronounced limitations,several potential adjunctive therapies have been tested in clinical trials,such as erythropoietin and melatonin (Gunn and Thoresen,2019).Focusing on energy depletion caused by HI events,energy substrates could also be tested as a neuroprotective therapy.Once considered as a waste product of glucose metabolism that should be quickly removed from the organism,lactate has demonstrated promising neuroprotective effects on the brain,even in adult animal models with cerebral ischemia.Previously,lactate was believed to only be produced during exercise and low oxygen conditions.However,it is now known that lactate is continuously produced,even during aerobic conditions in glycolytic cells.Lactate is transferred to and used by oxidative cells,either in muscles between different types of fibers (Brooks,1985) or in the brain between astrocytes and neurons (Pellerin and Magistretti,1994;Magistretti and Allaman,2018).
Metabolic switch over time:The preference for specific metabolic substrates differs between the developmental and adult brain.Since metabolic patterns during brain maturation can quickly change in the first days of life,careful attention should be given to the metabolic preference of each substrate as it could be related to the presence of monocarboxylate transporters(MCTs),which are in charge of lactate transport,in the neonates’ brains (Pellerin et al.,1998).During the suckling period,lactate and ketone bodies are the preferential metabolic substrates of the brain,however,after weaning,their transport through the blood-brain barrier (BBB) is reduced.Nonetheless,MCTs are still present in the brain parenchyma,suggesting that these substrates no longer come from blood,although they can originate within the braintissue (Pellerin et al.,1998).This corroborates the existence of a lactate transport system,also known as the astrocyte-neuron lactate shuttle (ANLS) (Pellerin and Magistretti,1994;Magistretti and Allaman,2018),inside the central nervous system (CNS),in which MCTs play a crucial role (Magistretti and Allaman,2018).
This shuttling system was first reported in skeletal muscle cells (Brooks,1985).However,there is compelling evidence that there is a similar system between CNS cells(Pellerin and Magistretti,1994;Magistretti and Allaman,2018;Figure 1A).Briefly,ANLS allows cells with a high NADH:NAD+ratio,such as astrocytes,to support oxidative cells with a lower NADH:NAD+ratio,such as neurons,via lactate exportation,ensuring mitochondrial adenosine triphosphate(ATP) production during neuronal activation under physiological or pathological conditions.Lactate is produced in a redox reaction mediated by isoform 5 of enzyme lactate dehydrogenase (LDH5,abundant in glycolytic cells,such as astrocytes,with high amounts of reducing equivalents,i.e.,NADH),guaranteeing the regeneration of NAD+,which is important to glycolytic flux maintenance.Lactate is exported through MCT4 from astrocytes to neurons,in which it is taken up by MCT2 and oxidized into pyruvate,through LDH1.This mechanism is useful for neurons suffering from oxygen deprivation,since importing lactate means that neurons do not need to use two ATP molecules in the investment phase of glycolysis (Magistretti and Allaman,2018).
The existence of machinery to oxidize lactate in neurons and the presence of transporters that allow lactate to cross the BBB in the neonatal brain reinforces the idea of using lactate administered peripherally as a possible neuroprotective agent following hypoxia-ischemia.
A route into the CNS:Neonatal hypoxiaischemia leads to a transient opening of the BBB (Lee et al.,2017).While this process allows the entrance of cytokines and large molecules into the CNS,which contributes to the extent of brain damage,it may also enable therapeutic molecules to reach different compromised areas of the brain(Lee et al.,2017).The post-HI enhancement of BBB permeability for lactate,may be due to the high expression of MCTs in the developmental brain (Pierre and Pellerin,2005) or BBB disruption following an HI event (Lee et al.,2017).Accordingly,an understanding of the time-frame when BBB permeability is increased may streamline the delivery of therapeutic molecules for neuroprotection following an HI event.Thus,evaluating lactate uptake and transport through and in view of increased BBB permeability would provide new insights into HIE treatments.These analyses could uncover different therapies for the management of HIE,including lactate.
Lactate as an energy booster:ATP deficiency stimulates lactate production in astrocytes and oligodendrocytes (Magistretti and Allaman,2018).Furthermore,lactate has neuroprotective effects in pathological conditions,such as brain ischemia and psychiatric disorders in adult mice and traumatic brain injury in humans (Magistrettiand Allaman,2018).There are only two studies in the literature evaluating the effect of lactate therapy on HIE harmful outcomes,both of which studied a rat model with neonatal HI (Roumes et al.,2021;Tassinari et al.,2020).Neither of these studies evaluated MCTs expression;however,peripheral administration of lactate increased lactate levels in the hypothalamus(Tassinari et al.,2020) and allowed the13C of13C-lactate to be incorporated in brain metabolites in both hemispheres (Roumes et al.,2021).Altogether,these findings suggest that exogenous lactate was taken up and utilized by the neonatal brain,which showed high levels of MCTs (Pellerin et al.,1998).Therefore,we presume that ANLS is greatly involved in neuroprotection,which was observed after exogenous lactate administration (Roumes et al.,2021;Tassinari et al.,2020).Moreover,when lactate metabolism was inhibited through the LDH inhibitor oxamate,neuroprotection was not observed,suggesting that protection depends on lactate metabolism (Roumes et al.,2021).
Moreover,since the harmful effects of neonatal hypoxia are systemic,organs other than the brain could take advantage of the glucose-lactateshuntto fulfill energy requirements as thelactate-shuttlehas already been described in skeletal muscle(Brooks,1985),the heart,liver,and kidneys(Pierre and Pellerin,2005).Although there is no detailed investigation into these aspects in neonates,this would be one more advantage of lactate administration after neonatal HIE.
Damage to the white matter:HIE also damages the white matter,which is plentiful in oligodendrocytes,the myelin-producer cells of the CNS.These cells are injured due to excitotoxicity and oxidative stress caused by HIE,and white matter damage can lead to mental and motor detriments.Nevertheless,oligodendrocytes may utilize lactate as an energy substrate (Pierre and Pellerin,2005) and precursor for lipid synthesis enrolled in myelin sheet formation.Likewise,energy deprivation can cause a delay in myelinization and degeneration of white matter structures.This process constitutes an elementary feature in motor deficits found in cerebral palsy and other neurological disabilities (Gunn and Thoresen,2019).Similar to ANLS,oligodendrocytes appear to present the machinery required to produce and export lactate through MCT1,which is rapidly taken up from axons through MCT2.The existence of a putative lactate transfer from oligodendrocytes to active axons has also been proposed (Magistretti and Allaman,2018;Figure 1B).
Due to the presence of MCTs in astrocytes,oligodendrocytes,and neurons,ANLS and axo-myelinic transmission constitute the foremost,tightly regulated mechanism to fulfill neurons’ energy demands,and lactate is produced in and exported from these cells.Nevertheless,white matter maturation in neonates is still a matter of debate,but it could be another therapeutic target for lactate and deserves further investigation in the neonatal brain.
Anti-inflammatory effects of lactate:Elevated plasma lactate levels during HIE treatment may also produce peripheral effects related to the reduction of brain injury,such as attenuating immune response(Hu et al.,2020;Figure 1C).Indeed,it has been demonstrated that lactate treatment in a cell line of macrophages and a model of acute pancreatitis and hepatitis causes an immunomodulatory response viaG protein-coupled receptor 81(GPR81),also known ashydroxy-carboxylic acid receptor 1(For review,see Hu et al.,2020;Figure 1D).Lactate reduces inflammasomes’activation,especially that ofnod-like receptor protein 3(NLRP3),and consequently,the production of pro-inflammatory cytokines is also reduced via GPR81 signaling (Hu et al.,2020).Moreover,lactate interferes in classical microglial polarization induced by lipopolysaccharidein vitroandin vivo,reducing neuroinflammatory parameters and sickness behavior (Kong et al.,2019).Since lactate appears to play a role in the immune system,it could mediate microglial polarization (Figure 1E).Therefore,immune organs,such as the spleen,thymus,and liver,require further investigation in the context of lactate employment to reduce peripheral and central inflammation.
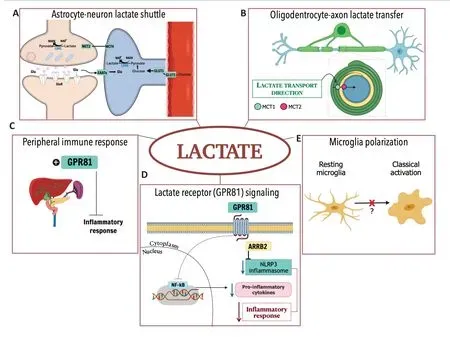
Figure 1|Hypoxic-ischemic encephalopathy is one of the main causes of mortality and disabilities in newborns,and therapeutic hypothermia (TH) is the only standard clinical treatment used to date.
Moreover,since the effects of neonatal hypoxia are systemic,the advantageous outcomes on different organs other than the brain,in which lactate may attenuate inflammatory responses,are another reason that lactate could be therapeutic for newborns after an HI event.
Lactate-mediated neuroprotection following HIE:There are important steps to be followed before using lactate for the treatment of HIE in human babies.Before testing lactate therapy in clinical trials,preclinical studies with large animals,such as piglets and ewes,and testing of different doses and schedules of administration should be conducted.Additionally,studies combining lactate administration with TH(the standard clinical treatment for HIE)in animal models would shed light for further analysis.Notably,recent animal studies combining two therapies,such as mesenchymal stem cells with TH,present conflicting results (Thoresen,2018).Thus,the timing to integrate treatments with TH is still a topic worthy of discussion for HIE care (Thoresen,2018).A meticulous diagnosis is also important for appropriate neuroprotective therapy (Gunn and Thoresen,2019).Furthermore,investigations regarding lactate’s mode of action in different brain cells would lay the foundations for HIE treatment.
TH is an example of translational research from animal models to the clinic,frombench-to-bed.Even though it presents some limitations,TH has already saved millions of lives.As animal models have successfully indicated the use of TH as a therapy for HIE,these two new lactate studies (Roumes et al.,2021;Tassinari et al.,2020) may complement TH therapy in the future.
The brain is an energy-demanding organ that requires careful organization within its structures and physiological processes,with many different cell types and metabolic profiles.Moreover,HIE is a condition that leads to neurological impairment associated with the early stages of asphyxia,and therefore on energy failure mechanisms.HIE affects many newborns,but it also affects their families and lifestyle.Lactate could be a promising strategy for HIE treatment considering the improvements it could have on newborns’ and their families’ health and quality of life.
We apologize to those authors that have been developing research on this topic‚whose original works were not referenced due to the limited number of references.
Studies from our research group over the years have received funding from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-Brazil)‚Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES-Brazil)‚Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul(FAPERGS-Brazil) and Fundo de Incentivo à Pesquisa e Eventos do Hospital de Clínicas de Porto Alegre (FIPE/HCPA-Brazil) (to LSdeF).
- 中国神经再生研究(英文版)的其它文章
- Towards a comprehensive understanding of p75 neurotrophin receptor functions and interactions in the brain
- Microglia regulation of synaptic plasticity and learning and memory
- Stroke recovery enhancing therapies:lessons from recent clinical trials
- Functional and immunological peculiarities of peripheral nerve allografts
- MicroRNA expression in animal models of amyotrophic lateral sclerosis and potential therapeutic approaches
- Significance of mitochondrial activity in neurogenesis and neurodegenerative diseases