Clinical potential of tensionlengthening strategies during nerve repair
Stanley Bazarek,Justin M.Brown,Sameer B.Shah
A (very) brief history of tension in nerve repair:Successful nerve repair is achieved by conveying as many axons successfully to their targets as possible.Typically,this is best achieved through a direct end-to-end repair under minimal tension (Millesi,1986).However,this is not feasible in most cases of trauma,where a segment of tissue damage must be excised and overcome.This has most commonly been addressed with the use of nerve grafts to bridge the gap.Autologous nerve grafts are considered the gold standard,with allograft or synthetic substitutes demonstrating some success over shorter distances.Despite their utility,autologous grafts pose challenges of their own.These include functional deficit in the donor distribution (typically sensory),extended operative duration,additional scarring,and a lack of intrinsic blood supply.They are also a poor anatomical match for the stumps being bridged,both internally (disparate neuronal size and composition) as well as externally(often requiring cabled bundles to approximate the caliber of the nerve being repaired).Finally,unlike end-to-end repairs,autologous grafts also require axons to traverse a second repair interface,where a large proportion of axons are lost across the anatomical discontinuity.
Given the challenges associated with grafting,there is incentive --and historical precedent --for enabling end-to-end repairs,with tension as a major player.During World War I,for example,nerves were often repaired under tension to enable an end-to-end repair (Sanders,1942).Typically,this involved immobilizing a joint in a position favorable for initial repair (i.e.,a configuration in which nerve ends are closer together),followed post-operatively by a gradual increase in range of motion.Given that such cases yielded mixed outcomes,a fact exacerbated by poor documentation,this approach was largely abandoned.Rationale cited to avoid tensioned repairs include the catastrophic consequences of a possible rupture at the repair site,as well as reports suggesting that tension-induced scarring and ischemia were detrimental to regeneration across the repair site (Millesi,1986).Thus,it has become dogma in the peripheral nerve field to avoid repair under tension.
On the other hand,there is increasing evidence for a positive role for tension in nerve repair and regeneration.Nerves are inherently a mechanical tissue,tolerating multi-directional loads during skeletal growth,joint movement,and surgical manipulation.The concept of rapid nerve growth under moderate tension is also well-demonstrated clinically by limb lengthening and tissue expansion procedures by neuro-,orthopedic,and plastic surgeons,where nerve function is maintained despite significant elongation (Vaz et al.,2014).Such findings demonstrate that nerves have the capacity for substantial non-elastic growth(i.e.,material addition,not simply reversible stretch) under tension.They also provide clues as to possible deformation thresholds beyond which nerve stretch is not advisable.These clinical studies are supported by a number of basic science reports,which demonstrate an incredible capacity for stretch-mediated axonal growth at moderate strain-rates (i.e.,tension applied along the shaft of the axon (Pfister et al.,2004)),and increased protein synthesis to meet the material demands of elongation(Love et al.,2017).In short,nerves,like many musculoskeletal and other soft tissues,respond favorably to tension,provided that loading conditions are controlled.
In subsequent sections,we briefly summarize positive clinical and pre-clinical outcomes of tension applied to nerve repair.We also provide rationale underlying the safe use of tension in neuro-regenerative strategies.References within this perspective have been selected judiciously;they contain more comprehensive supporting literature and rationale for deploying tension in nerve repair strategies.
Protected repairs under tension:Laboratory work and a recent clinical case series (Bhatia et al.,2017) have re-introduced the abandoned concept that nerve tension may be used to achieve direct,graft-free repair across a tissue gap with improved outcomes (Hentz et al.,1993).Our team has now performed a number of repairs utilizing limb positioning to overcome a gap followed by progressive opening of the joint until full range of motion is recovered.A critical component of our repair strategy is protection of the repair interface.In order to avoid historical problems created by high tension at the site of repair,we have employed nerve tubes to redistribute tension away from the site of axon crossing towards the more robust epineurium and structurally sound intra-epineurial tissue proximal and distal to the repair site.This approach both reinforces the tensile strength across the repair site and reduces the complex forces seen by the regenerating axons across the gap (cf.analogous approaches in (Kechele et al.,2011;Howarth et al.,2019a).Two cases suggest the potential utility of this strategy,which is summarized inFigure 1AandB.
Case 1 (repair of a large nerve gap):An 18 year-old female suffered a gunshot wound through the mid-humerus,resulting in a complete radial nerve palsy distal to the branches to the triceps with complete loss of wrist and finger extension.Upon exploration,3.5 cm of scarred nerve was removed.An end-to-end repair was achieved by flexion of the elbow less than 90°.A nerve conduit was slid over the anastomosis and sutured to the epineurium distally and proximally to the repair site,to protect the primary repair from full tension.Post-operatively,her arm was immobilized at 45° for 3.5 weeks to allow initial healing of the repair site,followed by a 10° increase in range of motion every 2 weeks thereafter until full extension was achieved.At 4 months,she demonstrated good brachioradialis and wrist extension against gravity with the beginning of weak finger extension.By 6 months,finger extension was more evident and at one year follow up she had gained near full strength throughout all muscles innervated by the radial nerve.
Case 2 (nerve transfer):In a typical spinal cord injury (SCI) at the C5-level,elbow flexion is spared,but hand function is completely lost.The primary flexors of the elbow are the biceps brachii and the underlying brachialis muscles.Transfer of the brachialis branch of the musculocutaneous nerve to the anterior interosseous nerve (AIN) is used to restore finger flexion in this patient population.Elbow flexion is preserved by leaving the biceps muscle intact.A meticulous neurolysis of the AIN away from the main trunk of the median nerve in addition to positioning the elbow in flexion can enable a direct repair of the brachialis branch to the AIN without an intervening graft.Our team performed this procedure bilaterally on a 21-year-old patient who experienced C5 SCI.Following dissection of the AIN proximally,donor and recipient nerves could only be approximated with 90°of elbow flexion.Thus,in similar fashion to Case 1,after performing these nerve repairs in both arms with the elbow in flexion,the arms were progressively extended by 20° every 2 weeks.At 18 months,the resulting grip could overcome resistance with MRC values of 4/5 on the right and 4–/5 on the left.Of note,the leftarm was extended more rapidly than the right,which may account for the decreased strength in the final result.
Additional details on these and additional cases will be provided in a subsequent series;however,our clinical experience illustrates proof of concept and potential efficacy of protected direct end-to-end repair followed by post-operative nerve lengthening via progressive joint remobilization.Optimal stretch parameters remain to be determined,but are likely influenced by the rate of remobilization,location of the repair relative to articulating joints (potentially higher strains),proximality of the repair (more proximal may require more gradual mobilization/slower lengthening),and quality of the nerve (more fibrotic or aged nerves may be more stiff/brittle).
Progressive lengthening prior to repair:An intriguing clinical application of stretch-growth would be to pre-elongate the proximal nerve stump/donor nerve prior to more distal and direct graft-free anastomosis to the distal recipient (Vaz et al 2014,summarized inFigure 1C–E).A major barrier to nerve repair or reconstruction efforts is the neuromuscular degeneration following denervation.There is a critical window of about approximately 18–24 months for restoration of the neuromuscular connection before the muscle is irreversibly lost due to fatty and fibrotic replacement.Nerve stretching and subsequent distal repair would maximize the number of axon donors that traverse a single interface,with the potential to bypass large swaths of a degenerating distal stump.In addition,such an approach could accelerate growth of the intact nerve shaft to bridge a large gap,as opposed to being limited by regenerative outgrowth of growth cones across a grafted gap.Laboratory stretching of sensory axons of 8 mm/day was effected with retained function,which is approximately 8 times faster than that of endogenous regeneration (Pfister et al.,2004).Biomechanically,the efficacy of such stretch is impacted both by deformation rate (higher strain rates being more damaging) as well as magnitude of deformation (a given deformation may be better tolerated by a longer nerve stump;i.e.,a lower strain).
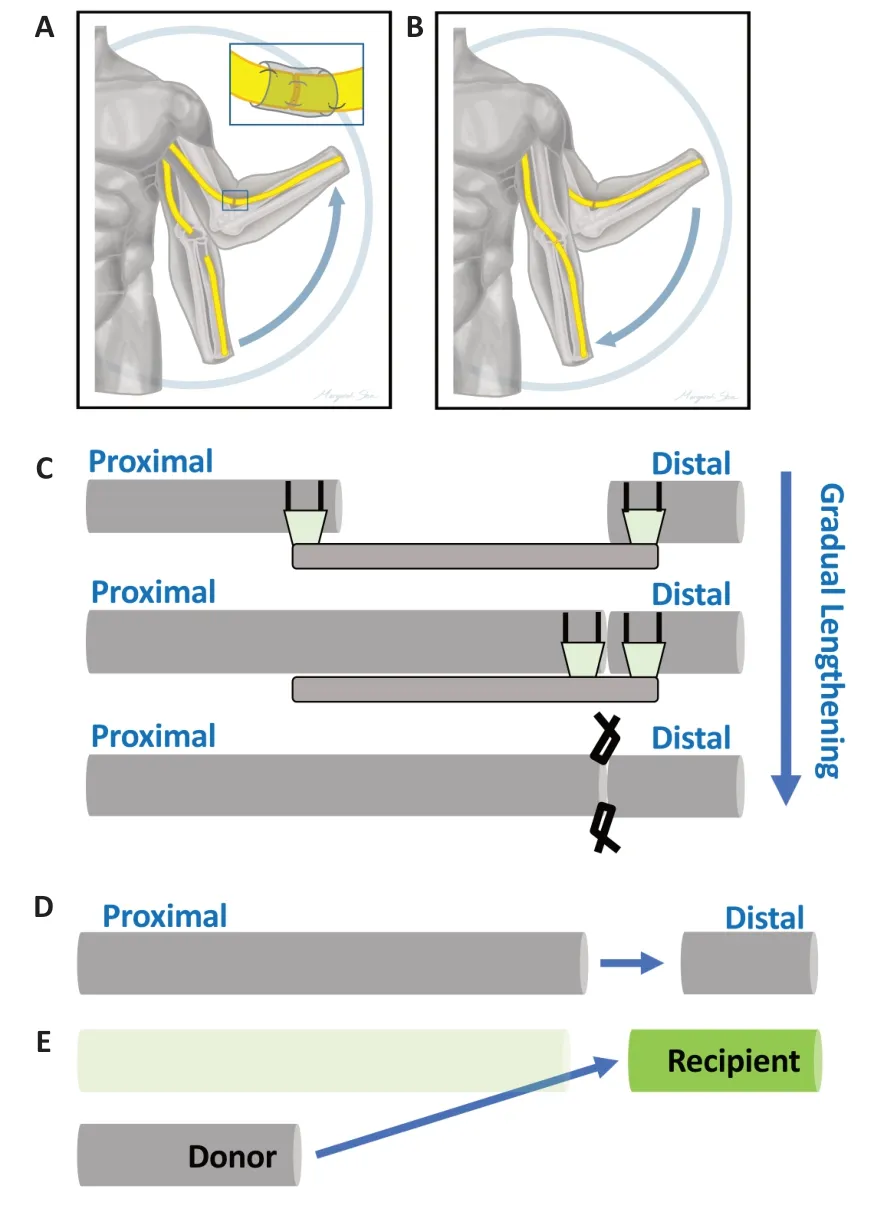
Figure 1|Dynamic mobilization (A,B)and lengthening followed by end-toend repair (C–E).
Preclinical innovation:While pre-clinical innovation has been primarily directed towards increasingly elegant engineered nerve grafts,there is significant potential for pre-stretching of proximal nerve stumps for nerve repair or reconstruction (Vaz et al 2014).Proof of principle has been established in our laboratory,as we have shown that following acute sciatic nerve transection in the adult rat,the proximal transected nerve end could be elongated by an implanted device past the distal target to allow repair over a 10 mm gap,which corresponds to a 2–3 cm gap in humans.Both structurally and functionally,lengthening followed by endto-end repair showed improved outcomes when directly compared with a gold standard autograft (Howarth et al.,2019b).Such approaches are now being successfully performed in larger animal model to evaluate the potential of lengthening to span clinically relevant gaps,thereby establishing nerve stretching as a powerful strategy for nervous system reconstruction.
Case 3 (clinical potential of ner ve lengthening):An 8-year-old girl presented with foot drop and was found to have an infiltrative nerve lesion (intraneural perineuroma) along her common peroneal nerve.During the operation,it was determined that complete tumor resection would preclude direct repair or require a long graft.Therefore,only 4 cm of tumor was excised,leaving some residual tumor on both ends.Tumor-ensheathed nerve ends were sutured together directly,and the nerve was gradually stretched,with anticipated complete resection and direct nerve repair within 6 weeks.The knee was extended by 20° each week,and by 6 weeks she was~20°short of full extension.Upon exploration,we found the original anastomosis intact with good vascularization.Unfortunately,the tumor had infiltrated further than expected,requiring us to forego direct repair a second time.However,significant and rapid lengthening over several centimeters was successful;this eliminated the need for a 10 cm graft,which would be required in the absence of stretch,and enabled the deployment of a 6 cm graft (40% shorter)into clean margins.
Conclusions:The previous sections provide examples and underlying rationale for a spectrum of applications related to the use of tension in nerve repair.The fact that some of these approaches have already been deployed clinically bodes well for more widespread integration of more complex strategies into clinical practice.There are a number of indications for which such approaches may additionally be useful.Pre-lengthening may be especially useful for nerve transfers following SCI.For example,the target muscles of the hand are often below the injury site after SCI,thus structurally preserving the lower motor neurons and neuromuscular viability,albeit with loss of cortical input.Pre-stretching would permit the recipient targets to remain structurally innervated until donor axons have reached a more distal location for an eventual delayed anastomosis.Even if the recipient is denervated,the accelerated nature of stretch growth may still permit reanimation of previously unreachable distal targets within the critical time frame.Current waiting times of a year to realize new function could be reduced to few months,allowing repair of the lower extremities following spinal injuries to the conus or cauda equina,or providing additional nerve transfer options for reanimation of the hand.Supplemental techniques such as electrical stimulation or pharmacological enhancement of nerve regeneration and muscle preservation may further enhance regenerative outcomes.The present work was supported by Department of Defense/CDMRP Award# W81XWH2010510(to SBS).
- 中国神经再生研究(英文版)的其它文章
- Towards a comprehensive understanding of p75 neurotrophin receptor functions and interactions in the brain
- Microglia regulation of synaptic plasticity and learning and memory
- Stroke recovery enhancing therapies:lessons from recent clinical trials
- Functional and immunological peculiarities of peripheral nerve allografts
- MicroRNA expression in animal models of amyotrophic lateral sclerosis and potential therapeutic approaches
- Significance of mitochondrial activity in neurogenesis and neurodegenerative diseases

