Induced pluripotent stem cells for defining Parkinsonian patient subtypes:a further step toward precision medicine
Federica Bono,Cristina Missale,Chiara Fiorentini
Introduction:Parkinson’s disease (PD) is a common neurodegenerative disorder mainly characterized by the progressive decline of motor function with a prevalence that is greatly increasing since the pathology is principally driven by aging.The currently available treatments only mitigate motor symptoms;therefore,a great deal of studies are aimed at finding novel drugs that are able to arrest the evolution of the disease.
The progressive death of nigrostriatal dopaminergic (DA) neurons and the formation of the typical Lewy bodies neuronal inclusions containing misfolded alpha-synuclein are two key histopathological features of the disease.However,it is now evident that PD has to be considered as a heterogeneous disorder that includes subtypes with distinctive clinical and biological characteristics(Ryden and Lewis,2019).Therefore,the assumption that PD is a unique entity to be treated with symptomatic strategies needs to be reconsidered in light of the fact that there exist various subtypes that might differentially respond to a given therapeutic approach.For these reasons,it is imperative to stratify PD patients into recognizable subgroups with regard to the many aspects of the disease,including age of onset,genetic mutations,clinical phenotypes,and disease progression with a combination of clinical,imaging,and basic molecular approaches.
Modeling LRRK2 mutation-associated PDwith induced pluripotent stem cell (iPSC)technology:Over the last decade,the improvement in iPSC technology allowed us to easily obtain patient-derived iPSCs from somatic cells and to differentiate iPSCs into neurons for the development of“in vitro”models of many neurologic and neuropsychiatric diseases,including PD.The possibility of deriving DA neurons from PD patient-derived iPSCs thus represents a unique experimental tool to characterize and classify PD subgroup patients from a precise molecular point of view and to define whether specific alterations may account for the vulnerability of DA neurons.Moreover,investigating cellular and molecular abnormalities that likely precede neuronal death may help in identifying,in specific subtypes of PD patients,the best therapeutic strategy useful for protecting DA neurons from neurodegeneration or slowing the neurodegenerative processes.
On these bases,we recently investigated the characteristics of DA neurons derived from iPSCs obtained from two PD patients harboring the G2019S LRRK2 mutation(Bono et al.,2021).Mutations in the LRRK2 gene are the most frequent cause of lateonset familial PD and a major risk factor for idiopathic PD,with the G2019S mutation accounting for up to 6–40% of familial cases and up to 2% of all sporadic cases(Ryden and Lewis,2019).Many different experimental approaches,including iPSCderived neurons,have been used to clarify the role of the G2019S LRRK2 mutation in DA neuron degeneration.In particular,iPSC-derived DA neurons carrying the G2019S LRRK2 mutation have been described as characterized by reduced neurite outgrowth,increased levels of alpha-synuclein,and alterations in mitochondrial morphology with impaired mitophagy and increased sensitivity to stress (Weykopf et al.,2019).We identified additional neuronal alterations that likely represent pre-degenerative features associated with the mutation in the LRRK2 gene,regardless of age-and environmentrelated events that might significantly contribute to the selective vulnerability of DA neurons (Bono et al.,2021) (Figure 1).
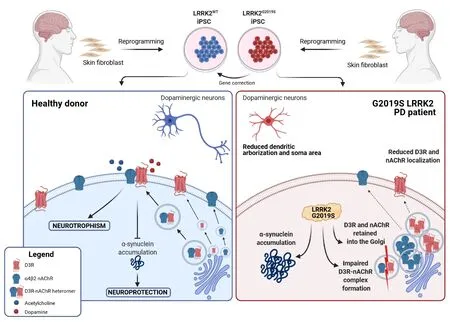
Figure 1|Modelling the G2019S LRRK2-induced molecular alterations with iPSC technology.
Altered D3 receptors (D3R)-nAChR heteromer formation in iPSC-derived DA neurons with PD-related G2019S LRRK2 mutation:We recently reported that the nicotinic acetylcholine receptors (nAChR),in particular those containing the alpha4 and beta2 subunits and the D3R,both localized on DA neurons (Exley and Cragg,2007;Beaulieu and Gainetdinov,2011),are two key elements deeply involved in neuronal homeostasis and health (Bono et al.,2020).In particular,the role of D3R and nAChR was investigated in human DA neurons differentiated from a healthy iPSC line.Interestingly,we found that chronic treatment with nicotine significantly induced DA neurons’ structural remodeling by increasing soma size,dendritic length,and dendritic arborization (Bono et al.,2018).Moreover,using the glucose deprivation model of neuronal injury,we found that nicotine stimulation prevented the formation of alpha-synuclein inclusions and the subsequent morphological defects with a mechanism that depends on phosphoinositide 3-kinase activity(Bono et al.,2019).Intriguingly,both the neurotrophic and the neuroprotective effects sustained by nAChR stimulation required D3R activity (Bono et al.,2018,2019).The synergy between nAChR and D3R in exerting both of these effects has been explained by the concept of heteromerization,defined as the capability of G protein coupled receptors to directly interact with other receptors in order to form heterodimers or high-order oligomers that act as novel receptor entities with unique pharmacological,signaling,and trafficking properties.Of relevance,the various receptor heteromers could have specific distribution in cells and tissues restricted to those cells where the two interacting receptors are co-expressed,and,therefore,they could have specific physiological and physio-pathological roles(Bono et al.,2020).Among the various heteromers formed by DA receptors,that composed by D3R and nAChR (D3R-nAChR)derives from the interaction between the D3R and the beta2subunit of nAChR(Bontempi et al.,2017).The physiological expression of the D3R-nAChR heteromer in both mouse and human DA neurons has been disclosed by using proximity ligation (PLA) (Bontempi et al.,2017;Bono et al.,2019).Remarkably,this heteromer represents the key molecular unit mediating nicotine-induced neurotrophic and neuroprotective effects (Bontempi et al.,2017;Bono et al.,2019).
While the precise role of LRRK2 remains largely unknown,recent studies have indicated that LRRK2 is involved in various cellular functions,including vesicle trafficking that allows neurotransmitter release and receptor localization at the plasma membrane (Weykopf et al.,2019);interestingly,an association of the G2019S LRRK2 mutation with abnormalities in DA receptor trafficking in various cell models has been described (Weykopf et al.,2019).Therefore,the expression and function of the D3R-nAChR heteromer were analyzed in human DA neurons derived from iPSCs of PD patients carrying the G2019S LRKK2 mutation (LRRK2-PD).Morphologically,LRRK2-PD DA neurons were characterized by reduced dendritic arborization and a reduced soma area and exhibited a spontaneous accumulation of non-fibrillary alpha-synuclein.Moreover,while the LRRK2-PD neuronal cultures retained the ability to release DA both in basal conditions and after non-specific depolarizing stimuli,DA release evoked by nAChR stimulation was abolished,likely reflecting an altered function of the acetylcholine receptor system (Bono et al.,2021).In contrast,DA neurons derived from the corresponding gene-corrected iPSCs (LRRK2-ISO) displayed a normal phenotype,thus suggesting that these alterations were specifically associated with G2019S LRRK2 mutation (Bono et al.,2021).
Interestingly,LRRK2-PD DA neurons,but not LRKK2-ISO,were almost completely resistant to the neurotrophic effects of nicotine likely mediated by activation of the D3R-nAChR heteromer,suggesting a close correlation between G2019S LRRK2 mutation and the inability of nAChR agonists to exert remodeling properties on DA neurons (Bono et al.,2021).The immunofluorescence analysis of D3R and the alpha4 subunit of nAChR showed that while in LRRK2-ISO DA neurons,D3R and nAChR were mostly co-localized at the plasma membrane of both soma and neurites,an evident decrease in cell surface fluorescence signal was observed in LRRK2-PD DA neurons,with a significant accumulation of both the receptors in the intracellular compartments;costaining with the RCAS1 Golgi marker also showed that D3R and nAChR were mainly accumulated in the Golgi compartment(Bono et al.,2021).Using the iPSC model,translational abnormalities have been recently associated with LRRK2 G2019S mutation (Kim et al.,2020);however,the observations that LRRK2-PD and LRKK2-ISO DA neurons exhibited similar levels of mRNA encoding for D3R and the alpha4 subunits (Bono et al.,2021) and that the immunopositive signal for D3R and alpha4 subunit did not show any difference between LRRK2-PD and LRRK-ISO DA neurons (unpublished result) may indicate that the G2019S mutant LRKK2 does not affect D3R and nAChR mRNA transcription and translation but has an impact on receptor trafficking between intracellular sites and the cell surface (Bono et al.,2021).Remarkably,the results obtained by PLA,used to detect D3R-nAChR heteromer localization,showed that while in LRRK2-ISO DA neurons,the signal was detected at the cell membrane,in LRRK2-PD DA neurons,the PLA signal was almost totally absent (Bono et al.,2021).Therefore,the alteration of vesicle dynamics induced by the G2019S mutant LRRK2 not only perturbs D3R and nAChR trafficking to the plasma membrane but also abolishes their capability to interact and to form the D3RnAChR heteromer.
Together,these results suggest that in the G2019S LRRK2-derived DA neurons,D3R and nAChR trafficking to the plasma membrane is impaired as well as D3R-nAChR heteromer formation;consequently,we could speculate that in individuals bearing this mutation,DA neurons of the substantia nigra might be unresponsive to the neurotrophic and neuroprotective effects physiologically sustained by DA and acetylcholine and,therefore,might be more susceptible to developing PD when aging or other environmental stress occur.
The G2019S mutation has been associated with LRRK2 abnormal kinase activity as the likely cause of pathogenicity (Soliman et al.,2020).Therefore,LRKK2 kinase inhibition is becoming a promising therapeutic strategy for genetic PD expressing mutant LRRK2;several LRRK2 kinase inhibitors have been tested in differentin vitroandin vivomodels of PD and are currently in clinical development(Soliman et al.,2020).In particular,in iPSCderived DA neurons carrying the LRRK2 mutation,treatment with LRRK2 inhibitors has been shown to rescue neuronal structural defects (Weykopf et al.,2019).Similarly,we found that treatment with the GSK2578215A LRRK2 inhibitor strongly increased dendrite length,dendrite number,and soma size and led to a significant reduction in alpha-synuclein levels in LRRK2-PD DA neurons (Bono et al.,2021).More interestingly,inhibiting LRRK2 abnormal activity was sufficient to recover the D3R and nAChR localization at the plasma membrane and to restore the formation of the D3R-nAChR complex and its membrane localization (Bono et al.,2021).Intriguingly,LRRK2-PD DA neurons previously exposed to GSK2578215A regain the ability to respond to nicotine with increased dendritic arborization and soma size induced by D3R-nAChR heteromer stimulation (Bono et al.,2021).
Conclusions and perspectives:Personalized medicine is a concept very suitable for PD,a disease that includes a highly heterogeneous group of patients.Understanding the basis of the large diversity of phenotypes with appropriate clinical,imaging,and biochemical measures is an important step to guide therapy toward its greatest precision and safety.Alongside these key features,the chance to investigate DA neurons from sporadic or familial PD patients at the molecular level with iPSC technology,especially in regard to receptors and signaling pathways involved in neuroprotection,can make a strong contribution not only to clarifying pathogenic mechanisms underlying neurodegeneration but also to developing the most appropriate therapeutic approach for each PD patient and patient subgroups.Moreover,since neuroinflammation is a common feature of many neurodegenerative diseases,iPSC technology may also offer the opportunity to investigate non-neuronal cells,such as microglia and astrocytes,which are known to highly contribute to the progression of neurodegenerative disorders,including PD (Booth et al.,2019).Stratification of PD patients from a molecular point of view could be relevant also in terms of the selection of study participants in clinical trials;otherwise,compounds occasionally identified as possible drugs for PD could fail to demonstrate their efficacy (Espay et al.,2020).Keeping in mind these observations,studies on the role of D3R preferring agonists as neuroprotective drugs in PD patients have failed,while ongoing trials using nicotine as neuroprotective drugs are suggesting a possible benefit that needs to be confirmed (Kalia et al.,2015).The data obtained in our studies imply that,at least in PD patients carrying the G2019S LRRK2 mutation,nicotine or D3R preferring agonists could be ineffective since both nAChR and the partner D3R,as well as the D3R-nAChR heteromers,are not expressed at the plasma membrane.Therefore,for these patients,nicotine or D3R-preferring agonists could be ideally used in combination with LRRK2 inhibitors,the latter used to escape from receptor abnormalities induced by the mutation;if this is not carried out,a clinical trial designed to investigate the disease-modifying activity of both of the compounds is doomed to failure.
In order to stratify PD patients taking advantage of iPSC technology,we are extremely interested in defining whether altered expression of the D3R-nAChR heteromer in DA neurons represents a distinctive feature of the G2019S LRRK2 mutation.Otherwise,in the event that this molecular occurrence is also observed in DA neurons derived from patients with other genetic forms of PD,it will be considered a pathogenic cause of extreme significance for clarifying the molecular basis underlying the selective death of DA neurons in PD,including the sporadic forms of PD.
The present work was supported by grants from the University of Brescia.
- 中国神经再生研究(英文版)的其它文章
- Towards a comprehensive understanding of p75 neurotrophin receptor functions and interactions in the brain
- Microglia regulation of synaptic plasticity and learning and memory
- Stroke recovery enhancing therapies:lessons from recent clinical trials
- Functional and immunological peculiarities of peripheral nerve allografts
- MicroRNA expression in animal models of amyotrophic lateral sclerosis and potential therapeutic approaches
- Significance of mitochondrial activity in neurogenesis and neurodegenerative diseases

