Three-dimensional in vitro models of neuromuscular tissue
Paolo Raffa ,Maria Easler ,Anna Urciuolo
Abstract Skeletal muscle is a dynamic tissue in which homeostasis and function are guaranteed by a very defined three-dimensional organization of myofibers in respect to other nonmuscular components,including the extracellular matrix and the nervous network.In particular,communication between myofibers and the nervous system is essential for the overall correct development and function of the skeletal muscle.A wide range of chronic,acute and genetic-based human pathologies that lead to the alteration of muscle function are associated with modified preservation of the fine interaction between motor neurons and myofibers at the neuromuscular junction.Recent advancements in the development of in vitro models for human skeletal muscle have shown that three-dimensionality and integration of multiple cell types are both key parameters required to unveil pathophysiological relevant phenotypes.Here,we describe recent achievement reached in skeletal muscle modeling which used biomaterials for the generation of three-dimensional constructs of myotubes integrated with motor neurons.
Key Words:3D organization;bioengineering;biomaterials;motor neurons;neuromuscular junction;skeletal muscle models
Introduction
In the past years,the development ofin vitromodels of skeletal muscle has seen tremendous improvements thanks to the integration of biomaterials within threedimensional (3D) culture systems.As better presented within the following chapters,such 3D fabrications,in contrast to their bidimensional (2D) counterparts,have been shown to enhance differentiation of myogenic cells,preservation and maturation of myotubes together with sustainability of their contraction (Bakooshli et al.,2019;Natarajan et al.,2019;Urciuolo et al.,2020b).To date,several tissue engineering strategies have been developed to generate 3D skeletal muscle cultures by using both natural and/or synthetic biomaterials.Improvements in biomaterial formulation and 3D bioprinting technologies also have opened the possibility to develop innovative biofabricated constructs with tunable mechanical properties and complex 3D architecture for the generation of skeletal musclein vitromodels (Derakhshanfar et al.,2018;Murphy et al.,2020).
Despite these important technical and technological progresses,only few studies succeeded in generation of tissueengineered constructs that integrate different cell types along with the myogenic compartment (Baudequin and Tabrizian,2018;Gilbert-Honick and Grayson,2020).In particular,innervation has generally been overlooked in most non-neural tissue engineering applications,in part due to the intrinsic complexity of building organs containing heterogeneous native cell types and structures (Das et al.,2020).However,it has recently become clearer that the reproduction of relevant pathophysiological responses ofin vitroskeletal muscle models could strongly benefit by the integration of the nervous system in the 3D myogenic constructs.Here,we aim to highlight recent achievements in skeletal muscle tissue modeling integrated with neural system,discussing (i) the structural and functional organization of innervated skeletal muscle;(ii) bioengineering 3D skeletal muscle models;(iii) integration of myogenic and neural cultures for 3D neuromuscular tissue modeling;(iv) limitations and future perspectives.
Search Strategy and Selection Criteria
Studies cited in this review published from 2010 to 2020 were searched on the PubMed database using the following keywords:in vitroskeletal muscle,skeletal muscle 3D model,NMJ,NMJ 3D model.
The Structural and Functional Organization of Innervated Skeletal Muscle
Skeletal muscle is a complex tissue in which the 3D architecture and function of myofibers is intimately linked to other tissue components (Yin et al.,2013).The biochemical and spatial 3D interactions existing between the myofibers and other components such as extracellular matrix (ECM),vascular and nervous networks are all essential players for overall tissue homeostasis,and for the correct contraction,force generation and transmission.The aligned and correctly orientated myofibers are disposed in a precise 3D organization also thanks to the network of ECM deposited around them(Gillies and Lieber,2011).Thousands of muscle fibers form together an individual skeletal muscle enwrapped by the connective tissue,called epimysium.The epimysium extends internal processes forming fascicles of ECM by assembling bundles of myofibers within the perimysium.In turn,each single myofiber is further surrounded by the endomysium,composed of basement membrane in contact with connective tissue and characterized by a peculiar ECM,capillaries and nerve terminals (Csapo et al.,2020).The basic unit of contraction in skeletal muscle is the motor unit,composed of a somatic motor neuron (MN) that innervates multiple myofibers at the neuromuscular junction (NMJ) level (Buchthal and Schmalbruch,1980).The NMJ is the anatomical 3D folded synapsis at which MNs and myofibers interact and communicate via molecular signals of soluble factors and of ECM finely disposed into the 3D spatial organization of the synapsis (Tintignac et al.,2015;Rogers and Nishimune,2017;Homan and Meriney,2018;Li et al.,2018).However,during neuromuscular development and regeneration,cells secrete and deposit ECM proteins that ultimately form a basal lamina,with unique composition and containing factors sufficient to induce the differentiation of both pre-and postsynaptic membranes (Singhal and Martin,2011).Moreover,the NMJ is formed within a dynamic and defined path,which is orchestrated in a specific window of time by 3D spatial organization of ECM,MNs and myofibers (Bonanomi and Pfaff,2010).MNs are among the longest projecting neurons in the body.MN axons run from the MN bodies located within the spinal cord in organized structures (nerves) to reach the target muscle.As the nerve terminals innervate muscle fibers during development or regeneration,MN axons make contact at multiple sites along the myofiber generating extra-synapses.However,the differential activity of MNs leads to the selection of a single nerve terminal establishing a NMJ,which can then undergo maturation and stabilization for proper MN-muscle communication (Bonanomi and Pfaff,2010;Burden et al.,2018;Li et al.,2018) (Figure 1).
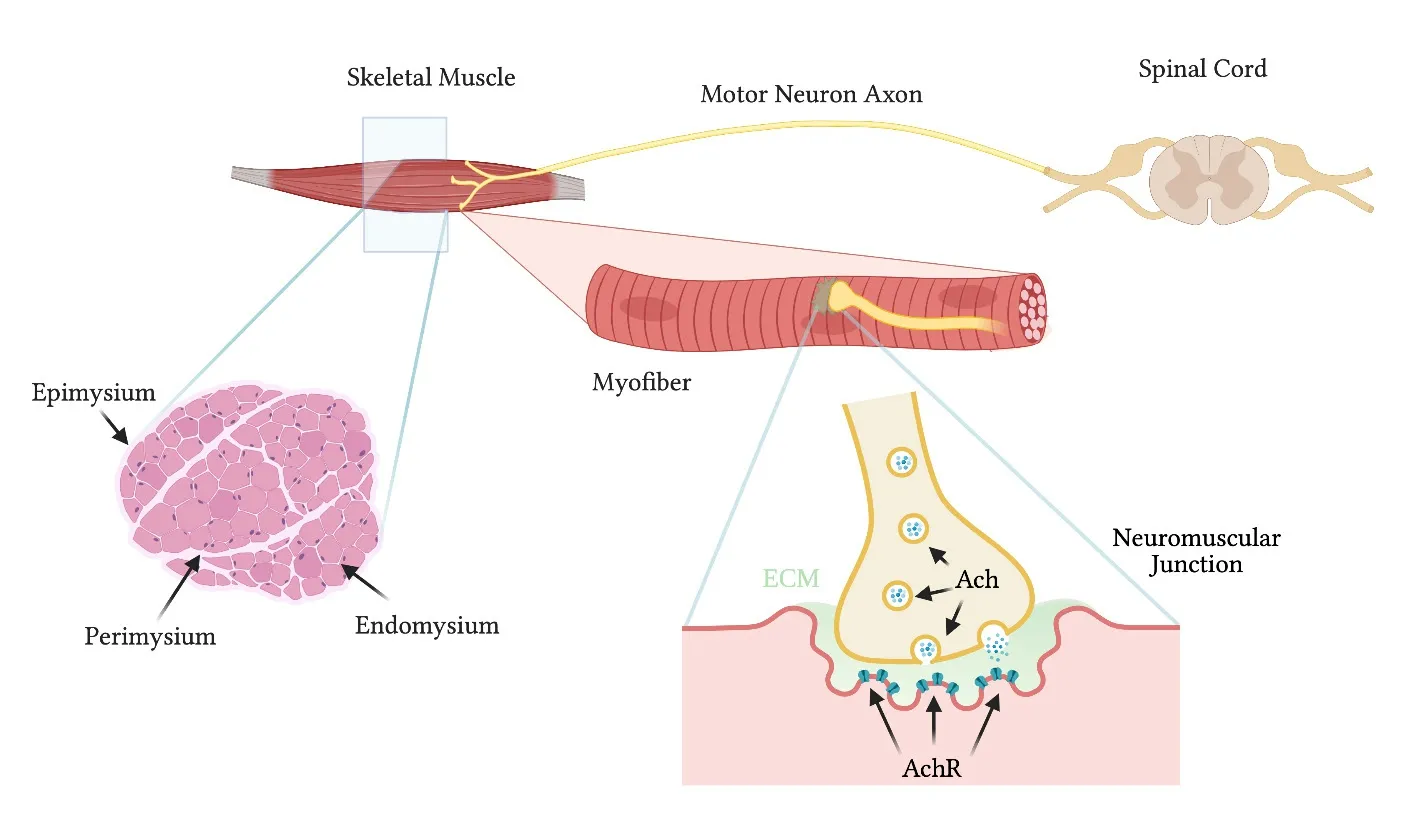
Figure 1|General structure of the skeletal muscle and the neuromuscular junction.
Deficits in NMJ formation and maintenance are associated with neuromuscular disorders characterized by alteration of the muscle function (Li et al.,2018;Gromova and Spada,2020).Innervation from MNs not only allows the control of myofiber contraction,but such interaction also permits the communication between muscular and nervous systems required for the homeostasis,development and regeneration of both myofibers and MNs (Singhal and Martin,2011;Burden et al.,2018;Li et al.,2018;Gromova and Spada,2020).Therefore,the presence of appropriate neurons during thein vitrobiofabrication process should ensure amelioration in development,maturation,and functionality of tissue-specific cells and their functional interaction (Das et al.,2020).Finally,incorporation of innervation in 3D skeletal muscle models allow to better mimic native environment of the organ,in terms of both biochemical and biophysical cues (Struzyna et al.,2015,2017).
Bioengineering Three-Dimensional Skeletal Muscle Models
The combination of extracellular natural and/or synthetic scaffolds (biomaterials) with stem cells and growth factors represent a new frontier for the development of complex 3D constructs for regenerative medicine strategies and forin vitrotissue modeling (Atala et al.,2018).The reproduction of peculiar and complex biochemistry,biomechanics and 3D organization specific to the tissue is the main goal of tissue engineering.In this context,the availability of a good 3Din vitromodel that adequately mimics the complex architecture and fine interaction between different cell components would give us an important knowledge about development and function of the organs,including skeletal muscle.The difficulty of modeling the skeletal muscle tissuein vitromainly resides in the strict relation existing between architectural complexity and function.Besides the study of molecular and cellular interaction in development or regeneration,3Din vitromodels can also be used for studying disease etiology and for drug testing purposes (Fang and Eglen,2017;Agarwal et al.,2018;Boda et al.,2018;Zhang et al.,2018).As an example,a model of human skeletal muscle was producedin vitroby using fibrin hydrogels and used to test drug disposition and toxicity upon microinjection of several compounds (Gholobova et al.,2018).With this bio-artificial muscle model,in which human myoblasts were able to form striated myofibers expressing MYH1,MYH3,MYH8 and myogenin,it was possible to evaluate different parameters such as compound toxicity,release and biotransformation (Gholobova et al.,2018).
Strategies used for development of 3D skeletal muscle models
The first,more traditional approach to recreate a 3Din vitromodel of functional skeletal muscle involves cell-laden hydrogels,both natural (agarose,alginate,chitosan,collagen,gelatin,fibrin/fibrinogen,hyaluronic acid,silk) and synthetic(polyurethane,polyethylene glycol,polylactic acid,and polyvinyl alcohol) (Boso et al.,2020).The great advantages of using hydrogels in 3Din vitromodeling are the possibility to modify structure,shape,and mechanical stability of the final products,combining different building procedure or materials(Kwee and Mooney,2017).The possibility to modify and adapt elasticity and stiffness to the experimental condition make hydrogels optimal candidates to be used in tissue modelling.Skeletal muscle in particular is a mechanically sensitive tissue,with many aspects (including structure,mass,function) being affected by mechanical forces (Wang et al.,2020).
In a comparative work,Pollot et al.(2018) tested the ability of collagen I,agarose,alginate,fibrin,and collagen chitosan hydrogels to support growing and differentiation of murine primary derived-satellite cells.They demonstrated that fibrin and,to a slightly lesser extent,collagen have the best potential to support the long-term culture of skeletal muscle cellsin vitroin a 3D substrate.Fibrin showed a greater ability to support activation,proliferation,and differentiation of primary murine satellite cells (Pollot et al.,2018).Despite this interesting feature,fibrin gel owns poor mechanical properties,that in addition to its fast biodegradationin vivo,can limit its use as a biomaterial (Moreno-Arotzena et al.,2015).However,these limitations could be overcome combining fibrin hydrogel with synthetic materials,such as poly(ether)urethane (Al Kayal et al.,2020),polyethylene oxide (Gsib et al.,2017) or organosilanes (Wang et al.,2019).Extensive review of the state of the art for hydrogel application in skeletal muscle modelling has been recently reported by Fischer and collegues (Fischer et al.,2020).In the next sections,we will discuss the recent approaches used to improve the modeling of myofibers,which considered the mechanical stimulation required,the microenvironment,the 3D native organization of the tissue,and the simultaneous presence of different cell types.
Dynamic culture of skeletal muscle 3D models
Considering the importance of mechanical stimulus during skeletal muscle development and homeostasis,several groups investigated the effect of the mechanical stimulation on myoblast differentiation and alignment (Liao et al.,2008).Specifically,to mimic mechanical forces during embryonic development,static strain is considered a relevant stimulus(Nakayama et al.,2019).On the contrary,cyclic stimulation recapitulates the functional environment of the adult skeletal muscle (Nakayama et al.,2019).Applying these principlesin vitrois possible with the use of the bioreactors which enable modulation and control of various parameters associated with mechanical stimuli,such as frequency,directionality and degree of strain imposed on the engineered 3D tissue construct (Heher et al.,2015;Chen et al.,2020).Heher and colleagues demonstrated that mechanical stimuli improved the alignment of muscles constructs (Heher et al.,2015).They mounted C2C12 myoblasts-laden fibrin rings in a magnetic bioreactor allowing to applied precise stretching to the samples (in details,10% elongation for 6 hours followed by a rest phase at 3% of stretching).Repetitive stimulation protocol for 6 days led to vastly improved alignment of the cells.As a consequence,myoblasts fused along the axis of strain in a highly ordered manner,resulting in a parallel array of myotubes with increased dimension and more pronounced sarcomeric patterning compared to controls (Heher et al.,2015).Moreover,the promotional effects of uniaxial stretching on the differentiation of C2C12 myoblasts (including the formation of mature myofibers) become more pronounced with increasing strain ratio up to~35% in a gelatinmethacrylate (GelMA) microfibers system (Chen et al.,2020).Aside from cyclic or static strain,tensionper sehave beneficial effects on growth and differentiation of skeletal muscle cells in 3D environment.Mechanical signal required for alignment and differentiation of C2C12 cells could be provided only by attaching type-1 collagen hydrogels to anchor materials that act as pseudo-tendons (Capel et al.,2019).Furthermore,this work demonstrated the scalability of 3D models,enabling the generation of reproducible constructs in the range from 500 to 50 µL of hydrogel volume.
Skeletal muscle 3D models in microscale systems
Skeletal muscle 3D models could be also miniaturized,or even reduced in micrometric scale.A 96-well platform,referred to as MyoTACTIC,enables bulk production of 3D human skeletal muscle micro-tissues (hMMTs) (Afshar et al.,2020).In this custom-designed platform,micro-posts included in each well serve as tendon-like anchor points to establish uniaxial tension in the remodeling hMMT and direct micro-tissue formation and compaction.A suspension of purified primary human myogenic progenitor cells and fibrin hydrogel was pipetted between and around the posts,and cultured for 2 days in myogenic growth media and for additional two weeks in differentiation media.At the end of the culture,hMMTs formed multi-nucleated and aligned myotubes,and exhibited spontaneous contractions and myotube calcium transients(Afshar et al.,2020).
In the last years,3D skeletal muscle models were developed exploiting microfluidic platforms,allowing to combine advantages of 3D culture with microfluidic system,such as more biologically relevant cellular microenvironments and higher throughput analysis platforms of cell behavior than conventional techniques (Shimizu et al.,2015).Agrawal et al.(2017) developed skeletal muscle microtissues using C2C12 myoblasts encapsulated within a GelMA hydrogel surrounding two polyacrylamide pillars and sandwiched between two acellular polyacrylamide hydrogel layers.Within one microfluidic chip is possible to build up to ten of these structures.This organ-on-a-chip model is also characterized by a perfusion system that continuously provides fresh nutrients and removes wastes and metabolic byproducts,while minimizing the usage of reagents and compounds.The engineered tissues were maintained in culture up to 12 days,displaying cylindrical fascicular morphology,multinucleated myotubes,nuclei elongation,and nuclei on myotube periphery (Agrawal et al.,2017).We also recently recapitulated a 3Din vitromodel of human skeletal muscle at the single fiber scale (Urciuolo et al.,2020b).We designed a poly-acrylamide-based hydrogel with topologically organized microchannels and physio-mechanical properties resemblingin vivocharacteristics of skeletal muscle.Both C2C12 cells and human myoblasts (primary and embryonic stem cells (ESC)-derived) formed compact myobundles starting from day 3 of culture,improving maturation (higher in human primary cells)over the days of culture.Moreover,transcriptomic analysis showed a higher similarity of gene expression between myobundles obtained in 3D culture system and skeletal muscle,in respect to myotubes derived in conventional 2D culture systems and skeletal muscle.These results confirm that 3D culture supports better myotube maturation in comparison to conventional 2D myotube culture (Urciuolo et al.,2020b).
In the recent work of Ortega et al.(2019),C2C12-laden GelMA/methacrylate carboxy methyl cellulose hydrogels were custom-made and integrated into a microfluidic platform to measure relevant biomarkers of the 3D artificial muscle.Based on a microfluidic-sensor system,this device allowed to biochemically and/or electrophysiologically stimulate the 3D construct and to detect relevant soluble factors produced after stimulation.
Skeletal muscle 3D models obtained via 3D bioprinting
Recently developed 3D bioprinting technology and improvement in bioink formulation provide high precision in cell and hydrogel deposition,to rapidly fabricate complex structures (Ostrovidov et al.,2019).3D bioprinting relies on the simultaneous deposition of cells and biomaterials in a layer-by-layer fashion to form 3D well-organized,heterogeneous and highly customizable structures.Mechanical stability could be achieved by printing cell-laden hydrogels together with biodegradable polymers in integrated patterns and anchored on sacrificial hydrogels (Kang et al.,2016;Kim et al.,2018).More in detail,Kim and colleague developed a muscle construct consisted of three components:(i) cell-laden hydrogel bioink,(ii) a sacrificing acellular gelatin hydrogel bioink,and (iii) a thermoplastic supporting poly(εcaprol-actone) polymer.In their printing design strategy,the supporting pillar provides the mechanical tension for the cells and drives the cell alignment in longitudinal direction,while the removal of the sacrificing acellular gelatin hydrogel creates a microchannel structure that ameliorates supply of oxygen and nutrients into the inner part of the constructs (Kim et al.,2018).
In 2017,Costantini and colleagues fabricated an artificial skeletal muscle tissue with functional morphologies by using a 3D bioprinting approach (Costantini et al.,2017).They used two different polymers:photocurable PEG-fibrinogen (PF)and sodium alginate.The latter was used only as temporary templating material to allow a precise deposition of C2C12-laden PF-fiber.PF,after ultraviolet irradiation,generated a covalently cross-linked matrix into which embedded myoblast could spread and differentiate.3D bioprinted constructs were cultured up to 21 days,obtaining highly aligned long-range multinucleated myotubes,with abundant and functional expression of MHC and laminin.Moreover,3D fabricated constructs were implanted subcutaneously in the back of immunocompromised mice.Thein vivoexperiments confirmed the formation of muscle-like architectural organization,characterized by tightly packed,highly parallel and completely striated myotube fibers.Interestingly,3D bioprinted hydrogel fibers were partially degraded duringin vivoculture,and they were substituted by newly formed myofibers (Costantini et al.,2017).
Recent work of our group has demonstrated the possibility of using 2-photon sensitive-hydrogel which has proven to sustain myogenic cell viability and facilitate their alignment and differentiation in bothin vitroandin vivosettings (Urciuolo et al.,2020a).The advantage of such system comes from the tunability of hydrogel stiffness coupled with imaging and the submicrometric control of 3D shape of the hydrogel that is guided by computer assisted design (Urciuolo et al.,2020a).The role of stiffness and 3D shape on myogenic behavior of 3D muscularin vitromodels is becoming even more evident when bio-hybrid actuators are introduced in the field of softrobotics.For instance,Mestre and colleagues developed a 3D-bioprinting technique to generate different designs of biological actuators,which could ultimately be integrated into a soft robotic device or be used to study the development and adaptability of muscle tissue (Mestre et al.,2018).
Decellularized tissues for skeletal muscle 3D modeling
Despite the incredible improvements achieved with biomaterial manufacturing,consolidating the peculiar and complex biochemistry,biomechanics and 3D organization proper of a tissue-specific ECM is very hard (Williams,2019).However,recently it was possible to utilize decellularization process to conserve the architecture and biochemical features of the tissue and,thus,better recapitulate the tissue microenvironment (Hillebrandt et al.,2019).Decellularization process removes cellular and nuclear content retaining ECM mechanical integrity,biological activity and 3D architecture of the native tissue.Decellularized muscles were used as scaffold material directly injecting C2C12 myoblasts (Jank et al.,2015)or satellite stem cells (Urciuolo et al.,2018),showing good cells colonization and formation of aligned,multinucleated myotubes.Importantly,decellularized ECM could be reduced to a soluble powder and used as a bioink capable of providing an optimized microenvironment conducive of the growth of 3D structured tissue in a 3D bioprinting approach (Pati et al.,2014).In fact,decellularization preserves the natural composition of the ECM that induces the cell differentiation and tissue development (Pati et al.,2014).In particular case of the skeletal muscle,Choi and colleagues demonstrated that 3D cell-printing of decellularized skeletal muscle ECM(dECM)-based bioink could be used for the fabrication of functional skeletal muscle (Choi et al.,2016).Following the encapsulation of C2C12 myoblasts in the dECM bioink,they generated the 3D cell-printed muscle constructs using polycaprolactone as anchor scaffold.3D bioprinting technology provide the possibility to mix biomaterials and cells and to build the specific necessary microarchitecture,giving structural and mechanical support.On the other side,dECM provided the myogenic environment that supported high viability and contractility as well as myotube formation,differentiation,and maturation.Compared with cell-laden collagen constructs,at day 14 of culture constructs obtained with dECM bioink showed significantly greater myogenic gene expression of Myf5,MyoG,MyoD,and MHC (Choi et al.,2016).In order to enhance mechanical stability and structural integrity of the dECM bioink,a photo-crosslinkable process using the methacrylate reaction has been proposed (Kim et al.,2020).After ultraviolet crosslinking for 1 minute,the dECM-methacrylate conserved the biochemical compounds of the muscle ECM,including several growth factors and cytokines such as VEGF,TGFβ-1,IGF-1,GH-1 and BMP-7.C2C12 myoblasts in the dECM-methacrylate printed structure were aligned and differentiated with a high degree of myotube formation,owing to the synergistic effect of the skeletal muscle-specific biochemical and topographical cues(Kim et al.,2020).
Integration of Myogenic and Neural Cultures for Three-Dimensional Neuromuscular Tissue Modeling
Effective 3Din vitromodels of mammalian tissues must allow correct interactions between different cell types in order to produce culture microenvironments as similar as possible to those that would normally occurin vivo.In case of the skeletal muscle,integrating muscular and nervous cells within a biomimetic scaffold would be of significant benefit for investigations into the development,functional performance,and pathophysiology of the entire tissue (Smith et al.,2016).Standard 2D coculture of myoblasts and motor neurons to study neuromuscular junctions were well established and have yielded numerous discoveries on the nature of motor neuron and skeletal muscle interaction (Ionescu et al.,2016;Steinbeck et al.,2016).However,in light of recent bioengineering advancement in 3D skeletal muscle modelling,researchers integrated motor neurons within such 3D culture system for the development of the NMJ (Moyle et al.,2020).A common way to evaluate the establishment of functional NMJ in 3D environment is to add motor neurons to a previously assessed 3D myoblast-laden fibrin 3D construct (Martin et al.,2015;Dixon et al.,2018).The addition of primary rat motor neurons to engineered skeletal muscle leads to observable neuromuscular interactions after 14 days of coculture,in terms of colocalization of pre-and postsynaptic proteins and inhibition of spontaneous myotube twitch by using chemical specific motor neurons antagonist (Martin et al.,2015).Similar results were obtained with human myoblasts and human induced neural stem cells in 3D hydrogel composed by 3 mg/mL collagen I,1% silk fibroin and 8% Matrigel® (Dixon et al.,2018).In their recent work,Yoshioka and colleagues induced the formation of 3D ring-like neuromuscular tissue construct co-culturing C2C12 myoblasts with murine induced pluripotent stem cell (iPSC)-derived MN.This study confirmed the important role of the ratio between the multiple cell types as well as the media composition in the establishment of a functional neuromuscular construct (Yoshioka et al.,2020).While co-culture of muscular and neural components can indeed provide avenue for NMJ formation,during development this interaction takes place at a specific window of time where motor neuron axons reach the target muscle soon after the primary myoblasts fuse to form first myotubes(Hurren et al.,2015).In addition,the presence of supporting glial cells is detrimental in the establishment and maintenance of the NMJ (Jung et al.,2019).In their recent work,Kaufman and colleagues co-cultured C2C12 myoblast-seeded hydrogel constructs with lumbar section of neonatal rat spinal cord,which preserved different neural cell types,including supportive glial cells.With such multicellular 3D culture,they achieved recapitulation of the timing and complexity of developmental formation of NMJ and modeled central pattern generator circuitry (Kaufman et al.,2020).
The use of human cells for the generation of neuromuscular constructs has a remarkable impact on human biological studies and disease modelling.Indeed,intrinsic differences between animal models and human cell behavior exist and can play detrimental role in the experimental outcome and model applications.For instance,Kamm’s group compared optogenetic 3D motor unit models derived from murine or human cells (Osaki et al.,2018).In particular,as a murine source they used mouse embrionic stem cell-derived MN and C2C12,while human pluripotent stem cells were used to derive human MNs and myoblasts.The transfection of neural stem cells with the construct integrating light-sensitive channelrhodopsin-2 gene prior to MN lineage differentiation also enabled spaciotemporal control of excitatory stimulation.By using a microfluidic device,co-culture experiments showed MN neurites extending towards skeletal muscle bundle and forming functional and optogenetically-controlled NMJ for both the modelled species.However,they observed thinner 3D muscle fiber bundles with significant decrease in overall muscle force production in a murine-cell model compared to human-cell model (Osaki et al.,2018).Bakooshli and colleagues presented a 3D model of NMJ by co-culturing human ESC-derived MNs and a mixture of human primary myoblast (95%) and fibroblast (5%) producing functional,well-characterized NMJs (Bakooshli et al.,2019).Specifically,the cells were seeded into fibrinogen/Geltrex® hydrogel and allowed to mature under uniaxial tension provided by attachment to two tendon-like pillars.In these conditions,the resulting myofibers produced multinucleated aligned myofibers capable of spontaneous contraction after 10 days in culture and demonstrating functional connections with MNs as seen from co-localization of sarcomeric α-actinin,α-Bungarotoxin and neurofilament heavy markers in addition to electrophysiological assessments performed using the current clamp (Bakooshli et al.,2019).Moreover,the 3D coculture induced maturation of acetylcholine receptor (AchR),as revealed from the transition of γ-to ε-subunit (Bakooshli et al.,2019).The switch between the expression of AchR γ-and ε-subunits is necessary during skeletal muscle development and leads to structural modifications that greatly affect the acetylcholine binding kinetics and conductance (Cetin et al.,2020).The ability to mimic AchR maturationin vitroopens new perspectives to investigate molecular processes involved in AchR subunit switch and to model pathologies involving mutations of ε-subunit,including congenital myasthenic syndromes (Bakooshli et al.,2019).
Since human induced pluripotent stem cells (hiPSCs)represent an easily accessible and expandable human cell sources,allowing the generation of multiple isogenic patient-specific cell types,their use can have a valuable impact for high throughput studies and disease modelling(Badiola-Mateos et al.,2018).In the recent years,there have been several different methodologies developed to establish 3Din vitroskeletal muscle constructs derived from hiPSCs extensively reviewed by Chal and Pourquié(2017).Particularly,the development of 3Din vitroskeletal muscle fabrications containing multiple isogenic cell types have been investigated.As demonstrated in recent work of Maffioletti et al.(2018),uniaxial tension applied to cell-laden fibrin hydrogel stimulated efficient and aligned 3D skeletal myogenic differentiation of hiPSCs.In the same work,authors explored the effects of addition of hiPSCs-derived MNs to the cell construct and found that besides inducing formation of NMJs,the MNs had a positive effect on myofibers maturation which continues to be one of the challenges related to hiPSCderived myogenesis (Maffioletti et al.,2018).InTable 1,we summarized the most relevant features of 3D neuromuscular models mentioned above.
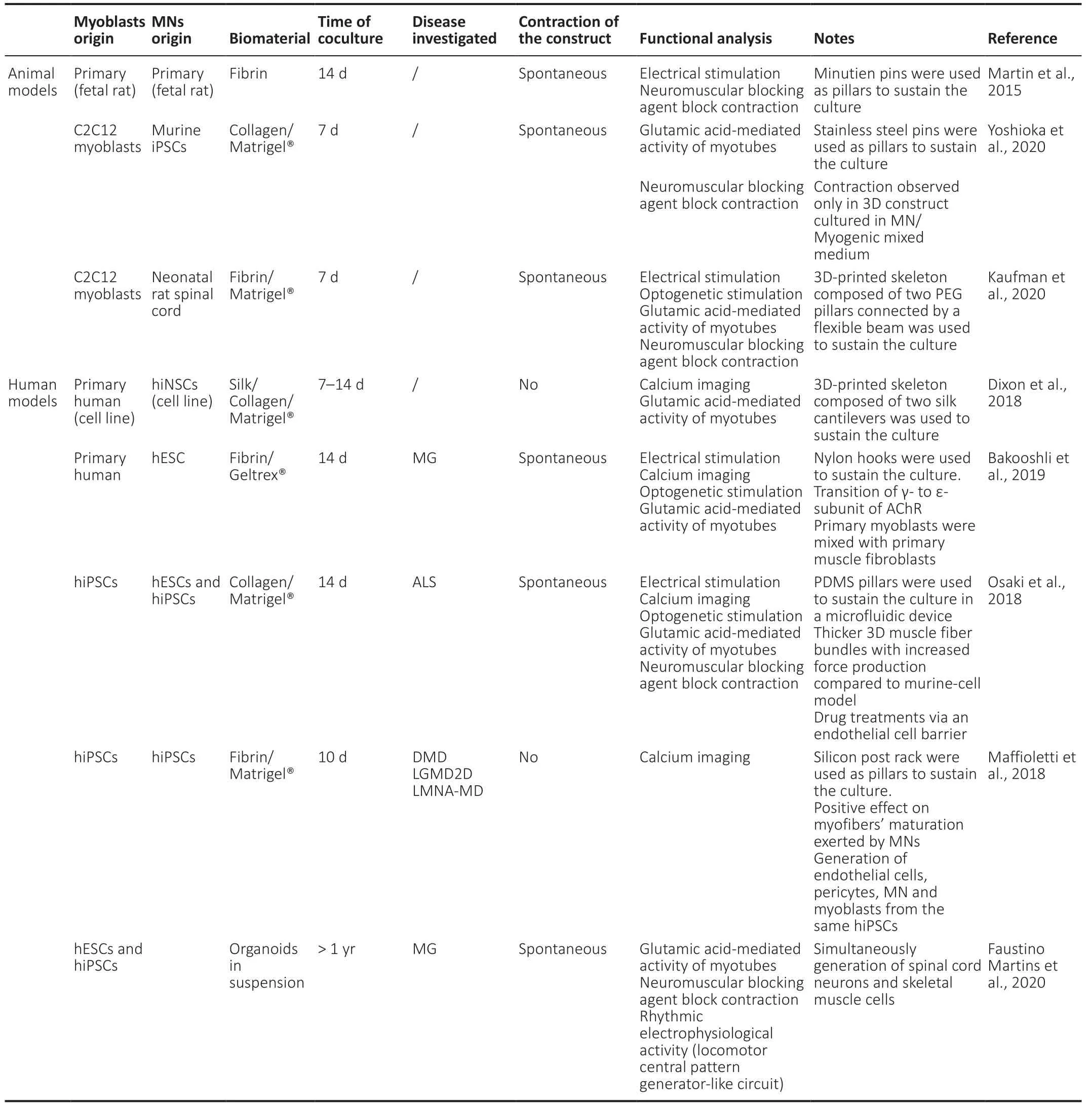
Table 1|Summary of recent established 3D in vitro models of neuromuscular tissue
Limitations and Future Perspectives
Recent years have seen development of emerging cocultures of MNs and myogenic cells coupled with biofabrication techniques for modeling 3D skeletal muscle tissue that have been shown to promote maturation and functionality of both the muscular and neuronal compartment,when compared to conventional 2D culture systems (Kang et al.,2012;Yin et al.,2013;Das et al.,2020).Nevertheless,the studies reported so far,display several limitations that can strongly influence the outcome of physiologically relevant (and thus pathologically relevant) phenotypes revealed by the artificial 3D human skeletal muscle.
One of the limitations is related to the variability of the cellular sources used forin vitro3D modeling.Many authors built their skeletal muscle model systems based on well-studied and characterized C2C12 myoblasts and mouse ESCs (Heher et al.,2015;Jank et al.,2015;Choi et al.,2016;Agrawal et al.,2017;Costantini et al.,2017;Capel et al.,2019;Chen et al.,2020;Urciuolo et al.,2020b).However,several comparative studies involving the use of either murine or human cells,demonstrated divergent physical characteristics as well as variable capacity for fusion and contraction between species(Abdelmoez et al.,2020;Mierzejewski et al.,2020).This and additional variation in methodology and experimental approach creates a challenge for the standardization of the pathophysiological results obtained.This is even more evident when 3D co-cultures are used to mimic human neuromuscular tissue for both basic neuromuscular studies and the investigation of neuromuscular conditions.Indeed,morphometric,compositional and functional analyses have shown that the human NMJ display specific characteristics that cannot be fully recapitulated by animal models (Jones et al.,2017;Slater,2017;Boehm et al.,2020).The development of human skeletal muscle models that include both MNs and myogenic cells represent an extremely powerful tool in which human-specific neuromuscular phenotypes can be investigated,allowing drug screening and testing,with high potential of huge clinical and economic impact.
When considering the use of hiPSCs (Chal and Pourquié,2017),despite the efforts to augment the maturation of hiPSC-derived cells,they display a reduced ability to form functional NMJ and produce contractions compared to maturation level reached with primary cells (Chal and Pourquié,2017;Badiola-Mateos et al.,2018;Moyle et al.,2020).Indeed,the initial source of human myoblasts has to be taken into careful consideration when comparing different studies,since primary and hiPSC-derived myogenic cells possess not only differential gene expression (Chua et al.,2019) but also varied ability to interact with other cells and to migrate (Incitti et al.,2019).Another source of discordance in transcriptional signature of hiPSC-derived myogenic progenitors can be dependent on the hiPSC differentiation protocol used,as demonstrated recently by Xi and colleagues(Xi et al.,2020) comparing transcriptional profile of primary fetal myogenic cells with those produced by three different differentiation protocols.Thus,taking in consideration these intrinsic differences,it is hard to successfully correlate existing models due to the degree of variability of cellular sources and interactions as well as methodology behind myogenic cell derivation.However,there have been recent reports in which authors derived different cells population starting from the same hiPSCs source (Maffioletti et al.,2018;Guo et al.,2020),obtaining multilineage NMJ models.These models are able torecapitulate extremely specific conditions in terms of disease onset and development and that could be the gateway for personalized medicine.
With the same basic idea,Faustino Martins and colleagues reported simultaneous differentiation of both neural and skeletal muscle populations within the same culture conditions producing organotypic tissues containing NMJ by using small molecules (Faustino Martins et al.,2020).They were successful in producing bipartite neuromuscular organoid with functional NMJ from a population of hiPSC-derived axial stem cells.The organoids were electrophysiologically functional,were marked by the presence of terminal Schwann cells and displayed the presence of central-pattern generator-like circuits.In addition,the organoids reacted to the antibodies present in the serum of myasthenia gravis patients,validating the disease modeling applicability of such construct.Overall,the possibility of obtaining multiple populations from the same source could be a convenient approach alongside the long-term capacity of culture of the organoids.
Finally,another major challenge in the development of a 3Din vitromodel of a neuromuscular tissue is its immense complexity relying on coordination of functional and structural properties of the ECM components and interaction with other cell types such as endothelial,smooth muscle,immune and glial cells.The direction of skeletal muscle tissue modelling is clearly moving to solve the above limitations and target the most relevant elements required to recapitulate neuromusculartissue,considering biophysical and biochemical environment,3D architecture of surroundings and mutual interaction of different cell types and extracellular components.
Author contributions:AU supervised and wrote the manuscript.All the authors contributed to manuscript writing‚data search‚collection and analysis‚and approved the final version of the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:This work was supported by IRP Consolidator Grant 2021 (Grant Code:21/05 Irp)‚Fondazione Città della Speranza‚Italy (to AU).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal‚and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License‚which allows others to remix‚tweak‚and build upon the work non-commercially‚as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Towards a comprehensive understanding of p75 neurotrophin receptor functions and interactions in the brain
- Microglia regulation of synaptic plasticity and learning and memory
- Stroke recovery enhancing therapies:lessons from recent clinical trials
- Functional and immunological peculiarities of peripheral nerve allografts
- MicroRNA expression in animal models of amyotrophic lateral sclerosis and potential therapeutic approaches
- Significance of mitochondrial activity in neurogenesis and neurodegenerative diseases

