GDNF to the rescue:GDNF delivery effects on motor neurons and nerves,and muscle re-innervation after peripheral nerve injuries
Alberto F.Cintrón-Colón,Gabriel Almeida-Alves,Juliana M.VanGyseghem,John M.Spitsbergen
Abstract Peripheral nerve injuries commonly occur due to trauma,like a traffic accident.Peripheral nerves get severed,causing motor neuron death and potential muscle atrophy.The current golden standard to treat peripheral nerve lesions,especially lesions with large (≥3 cm) nerve gaps,is the use of a nerve autograft or reimplantation in cases where nerve root avulsions occur.If not tended early,degeneration of motor neurons and loss of axon regeneration can occur,leading to loss of function.Although surgical procedures exist,patients often do not fully recover,and quality of life deteriorates.Peripheral nerves have limited regeneration,and it is usually mediated by Schwann cells and neurotrophic factors,like glial cell line-derived neurotrophic factor,as seen in Wallerian degeneration.Glial cell line-derived neurotrophic factor is a neurotrophic factor known to promote motor neuron survival and neurite outgrowth.Glial cell line-derived neurotrophic factor is upregulated in different forms of nerve injuries like axotomy,sciatic nerve crush,and compression,thus creating great interest to explore this protein as a potential treatment for peripheral nerve injuries.Exogenous glial cell line-derived neurotrophic factor has shown positive effects in regeneration and functional recovery when applied in experimental models of peripheral nerve injuries.In this review,we discuss the mechanism of repair provided by Schwann cells and upregulation of glial cell line-derived neurotrophic factor,the latest findings on the effects of glial cell line-derived neurotrophic factor in different types of peripheral nerve injuries,delivery systems,and complementary treatments (electrical muscle stimulation and exercise).Understanding and overcoming the challenges of proper timing and glial cell line-derived neurotrophic factor delivery is paramount to creating novel treatments to tend to peripheral nerve injuries to improve patients’ quality of life.
Key Words:electrical muscle stimulation;exercise;glial cell line-derived neurotrophic factor;glial cell line-derived neurotrophic factor delivery;motor neuron;nerve gap;neurotrophic factor;peripheral nerve injury;Schwann cells;skeletal muscle atrophy
Introduction
The peripheral nervous system (PNS) is the nervous system branch composed of peripheral nerves branching out the central nervous system’s two main organs,the brain and the spinal cord.These peripheral nerves are in charge of sending and receiving messages from the brain and spinal cord to target tissues and vice versa.Peripheral nerves are made of axons wrapped in endoneurium,perineurium,and epineurium.These nerves are considered the most fragile structure in our body due to the ease of getting damaged by crush,compression,and trauma (Hussain et al.,2020).Peripheral nerve injuries (PNIs) can occur due to trauma,complicated childbirth,and tumor extirpation (Eggers et al.,2020;Fadia et al.,2020;Li et al.,2020).PNIs resulting from trauma are responsible for approximately 5% of patients admitted to a Level I trauma facility in the USA,costing around 150 billion US dollars in health-related costs per year (Taylor et al.,2008).
Neurotrophic factors (NFs) are a group of proteins known for their ability to promote neuronal survival,influence cell proliferation,and differentiation,regulate synaptic plasticity,and modulate both axonal and dendritic elaborations.Additionally,NFs facilitate communication between neurons and their respective target tissues (Henderson et al.,1994;Zhu et al.,2008;Morcuende et al.,2013).NFs include the neurotrophin family where nerve growth factor (NGF),brainderived neurotrophic factor (BDNF),neurotrophin-3 (NT-3),and neurotrophin-4 (NT-4) belong.The glial cell line-derived neurotrophic factor (GDNF) family ligands have four members neurturin,persephin,artemin,and GDNF (Oppenheim et al.,1995;Cobianchi et al.,2017).Although the previously mentioned neurotrophic factors play a role at times of peripheral nerve injuries (Henderson et al.,1994;Glat et al.,2016;Tajdaran et al.,2016;Zheng et al.,2016),this review will be focusing on GDNF due to its formidable role as a regulator for the survival of motor neurons that innervate skeletal muscle,thus having an essential presence in the PNS and making it a target for future treatment developments for PNIs.This review aims to discuss GDNF,Schwann cells’ role and GDNF in nerve repair mechanism,various exogenous GDNF delivery methods to treat PNIs,proper delivery challenges,and the potential role of electrical muscle stimulation and exercise as a complementary treatment following a PNI.
Search Strategy and Selection Criteria
Data retrieval is shown inFigure 1.

Figure 1|Flow chart of article retrieval.
Glial Cell Line-Derived Neurotrophic Factor
GDNF was first isolated from cultured B49 rat glial cells and came to prominence due to the capability of enhancing survival and differentiation of dopaminergic neurons in primary cultures by promoting dopamine uptake.GDNF,similar to the other GDNF family ligand members,functions as a homodimer for the activation of the tyrosine kinase rearranged during transfection (RET) receptor (Lin et al.,1993,1994;Cintrón-Colón et al.,2020).As reviewed by Cintrón-Colón et al.(2020),in order to activate RET,GDNF first binds to a glycosylphosphatidylinositol-linked GDNF (GFRα) receptor,preferably a GFRα1 receptor or a GFRα2,with less affinity,and forms a high-affinity complex.The formed complex attracts two RET molecules causing transphosphorylation of specific tyrosine residues in their domains and initiating intracellular signaling like the mitogen-activated protein kinase,phosphoinositositide-3-kinase,Akt,or Erk pathways.The previously mentioned pathways are distinguished for promoting cell survival (Sariola and Saarma,2003;Kim and Kim,2018;Cintrón-Colón et al.,2020).
GDNF is a protein distributed in both peripheral nervous system and PNS,and its synthesis and secretion occur in various cells,which include a variety of glial cells like astrocytes,oligodendrocytes,and Schwann cells;neurons like motor,enteric,sympathetic,and dopaminergic neurons;and target tissues like a skeletal muscle (Henderson et al.,1994;Springer et al.,1995;Sariola and Saarma,2003).Additional interest for studying GDNF is the ability to promote survival of motor neurons,myelination enhancement post-injury,neurite outgrowth promotion,and play a role as a synaptotrophin to promote both terminal branching and remodeling at the neuromuscular junction (NMJ) (Nguyen et al.,1998;Sariola and Saarma,2003;Li et al.,2007).
Schwann Cell and Glial Cell Line-Derived Neurotrophic Factor Mechanism in Nerve Repair
PNIs have a limited ability of regeneration.Trupp et al.(1995)were the first to report that GDNF expression is induced following a PNI.In the years that followed,several studies have confirmed that after several modalities of nerve injuries(axotomy,sciatic nerve crush,and compression) GDNF expression was upregulated as well (Trupp et al.,1995;Chao et al.,2008).In severe denervation models,GDNF expression is upregulated in the distal stump up to 48 hours post-injury,but as denervation remains for a prolonged period,expression decreases possibly due to Schwann cell senescence and weakened ability to make GDNF (Höke et al.,2002).
As reviewed by Bolívar et al.(2020),Schwann cells are glial cells that originate from the neural crest and have high plasticity capability.These cells differentiate into two mature phenotypes,either myelinating or non-myelinating (Jessen and Mirsky,2008,2016;Bolívar et al.,2020).Schwann cells’maturation starts with migrating neural crest cells forming Schwann cell precursors (multipotent embryonic progenitors).These Schwann cell precursors differentiate into immature Schwann cells,and the association of this immature form with a specific axon determines its course as a mature Schwann cell as reviewed by Jessen and Mirsky (Jessen and Mirsky,2005).
After an injury,the distal nerve stump undergoes Wallerian degeneration,in which the nerve fiber (distal stump)degenerates.Markers related to myelin formation are downregulated,and Schwann cells change to a repair phenotype that bears a resemblance to the immature form as shown inFigure 2A(Jessen and Mirsky,2016;Carr and Johnston,2017;Welleford et al.,2020;Wilcox et al.,2020).Schwann cells with a repair phenotype cast off their myelin,acquire mobility and secrete neurotrophic factors like NGF,BDNF,NT-3,and GDNF to promote axon regeneration (Höke et al.,2006;Xu et al.,2013;Park and Höke,2014;Jessen and Mirsky,2016;Figure 2A).An outstanding study by Welleford et al.(2020) used RNA sequencing to properly analyze the human peripheral nerve’s whole transcriptome profile following injury.The researchers discovered 3641 genes that were significantly differentially expressed,the majority related to growth factor upregulation,most noticeably GDNF(Welleford et al.,2020).
Macrophages,neutrophils,and the reprogrammed Schwann cells breakdown myelin debris and clear the injured axonal membrane and cytoskeleton as seen inFigure 2B(Tomlinson et al.,2018).Additionally,Schwann cells associate with fibroblasts to synthesize adhesion proteins and extracellular matrix,allowing for successful bridging between proximal and distal stumps (review by Jessen and Mirsky,2016;Figure2B).Schwann cells align at the distal stump,forming bands of Büngner to provide trophic and physical support and direct axonal regeneration (Figure 2B).Once axonal regeneration has concluded,Schwann cells differentiate to their mature phenotype and re-myelinate the axons as reviewed by Boerboom et al.(2017;Figure 2C).
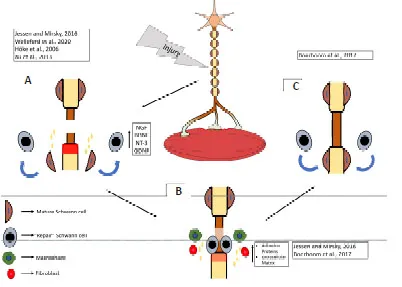
Figure 2|PNI repair mechanism.
At times of injury,skeletal muscle serves as an additional source of GDNF.When skeletal muscles become denervated,GDNF upregulation occurs and remains elevated from weeks to months with the goal of motor neuron survival and reducing atrophy of skeletal muscle (Springer et al.,1995;Zhao et al.,2004).Zahavi et al.(2015,2017) used anin vitromicrofluidic platform that contained the cell body of a motor neuron and muscle cells connected with motor axons extending through microgrooves that allowed NMJ formation.This model’s setup allowed the researchers to study the spatial specificity effects of GDNF.When GDNF is added to skeletal muscle cells,axonal tip growth and muscle innervation were promoted,and retrograde transport of the trophic factor was visualized(Zahavi et al.,2015,2017).This research provides further evidence of the importance of GDNF in the innervation and synapsis of skeletal muscle.
Exogenous Glial Cell Line-Derived Neurotrophic Factor Delivery in Peripheral Nerve Injuries
Nerve allograft and drug delivery system
In cases of a transected peripheral nerve,the current surgical standard consists of using autografts from nerve tissue from the same patient in order to connect the nerve gap.In some cases,these nerve autografts may not be an option where considerable repair is needed due to the limited available length (Tajdaran et al.,2016).An alternate option to using nerve autographs are acellular nerve allografts (ANAs).These allografts maintain the nerve tissue framework and are nonimmunogenic,eliminating the need for immunosuppressants,functioning as a medium for nerve regeneration.ANAs contain low amounts of neurotrophic factors compared to injured/denervated nerve stumps,where upregulation of neurotrophic factors,like GDNF,is increased (Boyd and Gordon,2003;Tajdaran et al.,2016).Previous work from Woods et al.(2013a,b) developed a microsphere-based biodegradable drug delivery system that sustains GDNF release to the injured site in periods of days to weeks.This delivery drug system proved to increase both axon regeneration and functional recovery after a delayed nerve repair (Wood et al.,2013a,b).Tajdaran et al.(2016) combined the previously described drug delivery system with the rat analog of the ANAs and determined how this combination supported nerve repair and regeneration.The experimental groups received the drug delivery system treatment at the suture sites of the allografts for two or four weeks and compared to rats that did not receive the drug delivery treatment.The treated rats had a significantly higher number of regenerated axons from both motor and sensory neurons;and larger fiber diameters than the non-treatment group (Tajdaran et al.,2016).
Glial cell line-derived neurotrophic factor pre-treated sensory graft for motor nerve injuries
Motor nerve grafts are hardly used because of the potential damage to movement functions in the donor area.Instead,sensory nerve grafting has been used to bridge and repair extended segmental motor nerve defects.The problem of doing sensory nerve bridging is that Schwann cells residing in sensory nerves have a subpar ability to support motor neuron axon growth (Chu et al.,2008,2009;Fang et al.,2019).In vitroexperiments using microfluidic devices performed by Marquadt and Sakiyama-Elbert (2015) andin vivoexperiments by Chu et al.(2009) showed that Schwann cells could overcome phenotypic mismatch-induced growth inhibition after exogenous GDNF application and as a result induce motor neuron axonal growth.Taking this previous research into consideration,Fang et al.(2019) designed a brilliant novel surgery starting with a denervated sensory nerve that was pre-treated with a sustained release of GDNF.GDNF was encapsulated into a self-assembling peptide nanofiber scaffold RADA-161 in the donor areain vivo.Following the pre-treatment,the sensory grafts were transplanted to fix motor nerve injury.Following this novel surgery approach,the exogenous GDNF pre-treatment resulted in regeneration and re-myelination of proximal motor axons.Furthermore,muscle function recovery was noted following electrophysiological analysis of the quadriceps femoris muscle (Fang et al.,2019).
Topical addition of glial cell line-derived neurotrophic factor in delayed root reimplantation
A standard model used to study brachial plexus injuries is the root avulsion model.A root avulsion injury is seen in traffic accidents and complicated childbirths.It consists of spinal roots being severed from the spinal cord,and as a consequence,motor neurons die,and peripheral nerves deteriorate (Ruven et al.,2018;Eggers et al.,2020).Ruven and colleagues (2018) used the root avulsion model combined with a 2-week delayed root reimplantation to test the effectiveness of GDNF treatment along with fetal lumbar cell transplantation on motor neuron death and muscle atrophy prevention.A peculiarity of this work is that GDNF treatment was topically added right after the injury.Two weeks after the manipulation,root reimplantation surgery was performed to determine if the motor neurons saved by the trophic factor could regenerate after delayed reimplantation.Results from this research suggest that GDNF treatment significantly prevented motor neuron death and incremented axonal sprouting and regeneration.Due to this regeneration,more neurons managed to make contact with the affected muscles,resulting in some muscle atrophy prevention compared to the delayed surgery-only group (Ruven et al.,2018).
Gene therapy as a glial cell line-derived neurotrophic factor delivery system
Patients that suffer a brachial plexus injury,even after surgical repair,could result in dysfunction and pain for the rest of the patient’s life (Eggers et al.,2020).Gene therapy has been suggested as a form of delivering GDNF to treat PNIs.As reviewed by Eggers et al.(2020),using gene therapy for GDNF can result in a continual supply of biologically active protein limited to the site of injury or where the viral vector was applied.A barrier for this approach is by-passing a potential immune response against the gene being inserted.An additional obstacle that has to be tackled is the time of expression and amount of expression (Tajdaran et al.,2016;Eggers et al.,2020).Eggers et al.(2019) completed a preclinical study testing these challenges.The researchers developed an immune-evasive,doxycycline-inducible,GDNF gene switch,with a time-restricted expression of one month.With this novel GDNF delivery system,the researchers managed to reduce the localized entrapment in avulsed reimplanted ventral spinal roots,promoted long-term motor neuron survival,and augmented long-distance regeneration motor axons.These results provide evidence that controlling the time of expression of GDNF can rectify the harmful effects of uncontrolled GDNF delivery.Also,gene therapy can improve axon regeneration post-surgical repair.
Biodegradable nerve conduit
A constant problem with PNIs is the length of the nerve gap after injury.If the nerve gap is over two to three centimeters is considered a large nerve injury,and the standard remedy is autografting (Fadia et al.,2020).PNIs that surpass three centimeters may encounter challenges with unresponsive growth cones from the proximal stump to NF signals released by the distal stump that lead axonal sprouting causing substandard nerve regrowth (Grinsell and Keating,2014).Autografting could potentially lead to loss of sensory function and neuroma,leading Fadia et al.(2020) to explore a biodegradable poly(caprolactone) (PCL) conduit with an inserted double-walled polymeric microspheres that contain GDNF.This PCL conduit can offer a continuous release of GDNF for more than 50 days in a 5-cm nerve injury in a nonhuman primate (rhesus macaque).The investigators compared the PCL/GDNF conduit with a median nerve autograft and a PCL conduit with empty (no GDNF) microspheres (PCL/empty).Fadia et al.(2020) found that the groups with PCL/GDNF conduit treatment and the autograft-treatment had improved functional recuperation compared to the PCL/empty treated groups.Additionally,the groups treated with the biodegradable nerve conduit with encapsulated GDNF have better nerve conduction velocity 1-year post-surgery.Histological analysis revealed a larger average area inhabited by Schwann cells at the distal nerve when compared to the other two groups (Fadia et al.,2020).This work adds a novel approach on solving PNIs,especially when a large peripheral nerve gap is present in a non-human primate.
Challenges of Glial Cell Line-Derived Neurotrophic Factor Delivery
GDNF poses as a potential NF to treat PNIs in future human trials,but delivery challenges exist.As reviewed by Eggers et al.,understanding the proper relationship between GDNF concentrations and duration of treatment to achieve adequate therapeutic GDNF levels for proper motor neuron survival and axonal outgrowth needs to be further studied.When high or low concentrations of GDNF are prolonged,there is a tendency of axonal coil formation.In low levels of GDNF,proper motor neuron survival does not occur (Eggers et al.,2008,2013,2020;Santos et al.,2016;Wang et al.,2018).
An additional challenge for proper GDNF delivery is maintaining exogenous GDNF in the correct location.Direct injection of the NF in a transplanted nerve tends to leak and causes unorganized axonal regeneration in the leakage site(Fang et al.,2019).Differences between distal and proximal stumps when treated with GDNF exist.When treated at the distal end,it did not improve motor neuron survival,in contrast to the peripheral end (Eggers et al.,2008,2019).
Although a powerful tool,gene therapy can be hindered by the immune system.Thus finding ways to bypass the potential immune response is essential.Hoyng et al.and Eggers et al.have developed a successful way of doing so,as previously discussed,by developing a doxycycline-inducible GDNF system(Hoyng et al.,2014b;Eggers et al.,2019).
Electrical Muscle Stimulation as a Complementary Treatment
A common feature of PNIs is the disconnection of the active nerve from the muscle,eventually causing muscle atrophy.Previous research has demonstrated that when there is a disruption in the NMJ,in other words,a disconnection of axon and muscle,GDNF is upregulated and expressed by muscle in higher quantity to try and re-establish that connection(Springer et al.,1995;Zhao et al.,2004).Electrical stimulation is used as a form of therapy to prevent muscle atrophy and build up strength in patients with injuries.Previous work done in rat models has demonstrated that daily electrical muscle stimulation (EMS) increases reinnervation following a PNI and repair.Willand et al.(2016) provide a possible explanation of why reinnervation is increased following EMS by using a rat model with a transected tibial nerve and immediately repaired post-injury.The rat model had intramuscular electrodes implanted in the gastrocnemius muscle (calf muscle) for electrical stimulation.The researchers used two sets of rats with the same injury.One set was used to measure functional reinnervation using electromyographic recordings,and the other set had the muscle and distal nerve stump removed for molecular analysis.GDNF mRNA levels from muscles that had daily EMS were significantly upregulated compared to the no stimulation groups.However,there was no difference in trophic factor mRNA levels in the distal stump compared to the non-stimulated rats,suggesting that EMS does not regulate Schwann cell-derived GDNF transcription.This work suggests that adding electrical stimulation to a denervated muscle after a PNI upregulates intramuscular levels of GDNF mRNA.This increase in trophic factor diffuses into the distal nerve stump providing the beneficial effects of axon regeneration at the growth cone facilitating nerve regeneration (Willand et al.,2016).
Exercise as a Complementary Treatment
There is increasing evidence of exercise having beneficial regenerative,rehabilitative,and neuro-plasticity-associated effects in central and peripheral nervous systems (Park and Höke,2014;Arbat-Plana et al.,2017;Theisen et al.,2017).Additionally,exercise has been linked with possible neurotrophic factor signaling regulation,as reviewed by Cobianchi et al.(2017) and Cintrón-Colón et al.(2020).A study from Wehrwein et al.(2002) suggests that GDNF is regulated in an activity-dependent manner by using a hindlimb unloading model and walk-training exercise.After following a 4-week walk regimen,the researchers noticed that GDNF protein content in the soleus,gastrocnemius,and pectoralis major muscles increased.In contrast,when hindlimb unloading was performed,GDNF protein content significantly decreased in the soleus and gastrocnemius but increased in the pectoralis major muscle due to recruitment of the muscle.This study indicates that mechanical activity in the form of stretch from increased physical activity might be a sufficient stimulus for neurotrophic factor production(Wehrwein et al.,2002).
Park and Höke (2014) used a peripheral nerve regeneration model to study treadmill exercise’s impact.The animals were divided into a control group,nerve repair without exercise group,and a nerve repair plus exercise group.The animals had the median nerve transected and repaired,while the ulnar nerve was prevented from regeneration after the injury.The researchers noticed that following a daily treadmill exercise regimen;there was improved regeneration after due to a higher number of axons regenerated and increased myofiber size in the target muscles.Additionally,there was an increment in serum,muscle,and nerve of various neurotrophic factors that included GDNF,BDNF,and insulinlike growth factor-1.Also,there was a faster functional recovery as demonstrated by grip power and inverted holding test.This study suggests that muscle derived neurotrophic factors,like GDNF,and an appropriate exercise regimen might offer enhanced regeneration following injury (Park and Höke,2014).
Gyorkos et al.(2014) explored swimming and walk-training exercises.After two weeks of either swimming or walktraining exercises,the researchers noticed that it promoted changes in both GDNF protein content and NMJ structures in slow-twitch and fast-twitch muscles.Muscle-derived GDNF protein content significantly increased,and the total area of the motor end plates significantly increased in slow-twitch muscles but decreased in fast-twitch muscles.These results suggest that increased physical activity is enough to increment GDNF protein content and alter the NMJ components offering insights for potential exercise program developments as an added treatment for patients with PNIs.
Conclusion
The faulty recovery of function following PNIs is attributed to the continuous degeneration and reduction in the motor neurons’ ability to regenerate their axons that lead to chronically denervated muscles (Eggers et al.,2019).Even though surgical repair for PNIs exist,functional recovery in patients remains inefficient and could lead to longlasting or lifelong dysfunction (Eggers et al.,2020).GDNF is a neurotrophic factor that evokes interest because of its ability for axonal outgrowth,role in neuron differentiation,and its role as a potent survival factor for motor neurons(Henderson et al.,1994).Although GDNF shows promise due to its pro-survival effects on motor neurons,it is challenging to deliver this protein.Therefore,further research is needed to understand the optimum amount of the protein and time needed to treat a PNI and better develop methods of keeping exogenous GDNF in the correct location.Previous work has found that uncontrolled GDNF distribution could cause irregular sprouting and axon and nerve entrapment(Höke et al.,2002,2003;Eggers et al.,2008;Su et al.,2009;Hoyng et al.,2014a;Ee et al.,2017).Recently,researchers have advanced in the development of conduits that are biocompatible and possess enough mechanical strength to aid injured neurons and Schwann cells from potential apoptosis(Cebral et al.,2017;Alsmadi et al.,2018;Du et al.,2018;Sarker et al.,2018;Subbiah and Guldberg,2018).EMS and exercise can be considered as complementary treatments following surgery.Previous research has shown that EMS increases levels of trophic factors providing positive effects for axonal regeneration (Willand et al.,2016).Exercise has been suggested to trigger the release of GDNF from skeletal muscle in an activity-dependent manner and be retrogradely shipped to the soma of motor neurons triggering pro-survival genes(Wehrwein et al.,2002;McCullough et al.,2013;Gyorkos et al.,2014;Cintrón-Colón et al.,2020).Finally,figuring out the proper dosage,time,and delivery method for GDNF therapy and combining it with a proper physical activity regimen or EMS during rehabilitation can ameliorate and enhance a patient’s quality of life post-injury.
Author contributions:AFCC wrote the manuscript‚created the figure‚performed the search strategy and edited the manuscript.GAA‚JMV‚JMS critically reviewed and edited the manuscript.All authors approved the final version of the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:This work was funded by the NIH Grant 1R15AG022908-01A2 and the Western Michigan University (to JMS).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal‚and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License‚which allows others to remix‚tweak‚and build upon the work non-commercially‚as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Tomohiro Torii‚Doshisha Daigaku‚Japan;Jian Weng‚Peking University People’s Hospital‚China.
- 中国神经再生研究(英文版)的其它文章
- Towards a comprehensive understanding of p75 neurotrophin receptor functions and interactions in the brain
- Microglia regulation of synaptic plasticity and learning and memory
- Stroke recovery enhancing therapies:lessons from recent clinical trials
- Functional and immunological peculiarities of peripheral nerve allografts
- MicroRNA expression in animal models of amyotrophic lateral sclerosis and potential therapeutic approaches
- Significance of mitochondrial activity in neurogenesis and neurodegenerative diseases

