Viral hepatitis: A narrative review of hepatitis A-E
Zunirah Ahmed, Akshay Shetty, David W Victor, Sudha Kodali
Abstract Viral hepatitis continues to be a major health concern leading to hepatic decompensation ranging from acute hepatitis to cirrhosis and hepatocellular carcinoma. The hepatic and extrahepatic manifestations are not only debilitating but also associated with a significant economic burden. Over the last two decades, the field of virology has made significant breakthroughs leading to a better understanding of the pathophysiology of viral hepatitis, which in turn has led to new therapeutic options. The advent of direct-acting antiviral agents changed the landscape of hepatitis C virus (HCV) therapy, and new drugs are in the pipeline for chronic hepatitis B virus (HBV) treatment. There has also been a significant emphasis on screening and surveillance programs, widespread availability of vaccines, and linkage of care. Despite these efforts, significant gaps persist in care, and there is a pressing need for increased collaboration and teamwork across the globe to achieve a reduction of disease burden and elimination of HBV and HCV.
Key Words: Viral hepatitis; Recent advances; Novel therapies; Barriers to cure; Future direction
lNTRODUCTlON
Hepatitis A
Epidemiology:Hepatitis A virus (HAV) belongs to the Picornaviridae family and is a single-stranded RNA virus that affects around 1.5 million people annually[1]. HAV is a resilient virus and is able to survive in most environments for months despite freezing temperatures, acidic environments, and exposure to chemical agents, thus making it an ideal agent for infection through exposure to contaminated water and food supplies[2]. Since the advent of the HAV vaccine in 1996, there has been a significant decline in HAV incidence rate worldwide[3]. Incidence and prevalence depend on socioeconomic status and geography of the population. Seroprevalence rates are inversely proportional to general hygiene and sanitary conditions and socioeconomic status[4,5].
Prevalence of viral hepatitis in pediatric population:The prevalence in children of HAV varies based on region from where the data are reported, with higher exposure rates in Africa and South East Asia compared to Europe and the USA[6]. Global estimates for HBV prevalence are significantly lower among children, estimated at 1.3% in children under 5 years. There are limited data on hepatitis D prevalence in children[7]. Estimates show that HCV infection in the pediatric population to be approximately 5 million children and adolescents globally. Studies show that the prevalence rates are rising, ranging from 0.05%-0.36% in the USA and Europe to 1.8%-5.8% in developing countries, including Mexico[8-10]. A systematic review of hepatitis E infection in pediatric population projected a worldwide, seroprevalence of 10% with rising prevalence with age[11].
Disease phases:The incubation period of HAV is usually 14-28 d, and patients are contagious for 2 wk prior to, and up to 1-2 wk after symptom onset[6]. Most patients recover spontaneously without chronic consequences[12]. Clinical presentation is variable where children can be completely asymptomatic, while adults can present with jaundice, changes in stool and urine color. Relapsing hepatitis A is characterized by the reappearance of clinical features and laboratory abnormalities consistent with acute hepatitis A after initial resolution of symptoms. Relapse can occur during 6 mo after initial illness. The duration of clinical relapse is generally < 3 wk, however biochemical relapse can last as long as 12 mo. A minority of patients can progress and develop acute liver failure and may need a liver transplant[13,14]. Hepatitis A infection resolves completely in the majority (> 99%) of the cases[15]. HAV infection, unlike some other viruses, does not cause chronic liver disease[16].
Hepatitis А vaccine and future directions:The current recommendations by The Advisory Committee on Immunization Practices are two shots of HAV vaccine, 6 mo apart[17]. There has been a decline in HAV infection from 11.7 to 0.4 cases per 100 000 population, a reduction by 96.6% because of aggressive screening and vaccination protocols[18]. Despite intense public health measures, sporadic hepatitis A cases continue to occur, highlighting the need for ongoing efforts for screening, surveillance, immunization, and education programs.
Hepatitis B
Epidemiology:Hepatitis B has emerged as a global health problem with estimated 350 million cases worldwide of chronic hepatitis B infection[19]. WHO Western Pacific Region and the WHO African Region are estimated to have the highest burden of chronic hepatitis B infection. Countries with high prevalence include Ghana, Gabon, Somalia, China, Cambodia and Mongolia[20]. In the USA, 2.2 million have chronic hepatitis B (CHB) with a higher prevalence (3.45%) among first-generation immigrants[21]. Patients with CHB carry a 15%-40% lifetime risk of developing serious sequelae of infection with an increased risk of death from complications such as cirrhosis and[22] hepatocellular carcinoma (HCC)[23].
Disease transmission and phases:The route of hepatitis B virus spread isviacontact of blood or bodily fluids of an infected person. The route of HBV transmission varies depending on the prevalence and geographic area. Vertical transmission at birth and close household contact among children are among the more common modes in Asia and Sub-Saharan Africa where HBV is endemic[24]. In areas with low prevalence, especially developed nations, transmission of HBV among adults usually occursviasexual transmission, percutaneous inoculation through contaminated needles, blood transfusions, or healthcare-associated risk factors such as hemodialysis[24-29]. In the USA and Europe, prevalence rates are higher in areas with a larger ratio of immigrant population, who likely contracted HBV in their country of origin[30,31].
The natural history of HBV depends on the age of the patient at which infection is acquired. For example, in adults, it usually presents as an acute, self-resolving infection where patients who are immunocompetent develop hepatitis B surface antibody to hepatitis B surface antigen (HBsAg), while only 1%-5% progress to developing chronic infection[32]. In contrast, the majority of patients infected by vertical transmission or horizontal infection during early childhood are likely to develop CHB, with the risk of developing CHB rising to 90% if the infection was acquired at birth and 16%-30% if infected during childhood[33,34].
Chronic hepatitis B can be divided into five phases based on the patient’s viral load, elevation in liver enzymes, and hepatitis B serologies[35]. The early high replicative phase or immune tolerant phase is characterized by positive hepatitis B e antigen (HBeAg), high levels of HBV DNA and normal serum alanine transaminase (ALT). The next stage’s hallmark is immune activation, where HBeAg remains positive along with high levels of HBV DNA and elevated serum ALT with associated hepatic necroinflammation. Based on the immune activation, the disease may progress to loss of HBeAg and development of hepatitis B e antibody (anti-HBe). This stage is characterized by moderate to high levels of DNA with risk of progression to hepatic fibrosis and cirrhosis. In the nonreplicative phase (previously known as inactive carrier phase), in which HBV DNA is usually low or undetectable, HBeAg is absent and patients have normal serum ALT. Lastly, the HBsAg loss/occult phase is defined by loss of HBsAg but detectable HBV DNA in the liver and measurable HBV DNA in serum[36].
Extrahepatic manifestations:Both acute and chronic hepatitis B have extrahepatic manifestations. Polyarteritis nodosa is vasculitis of small and medium-sized vessels and manifests as a serious systemic complication of hepatitis B[37]. HBV-associated glomerulonephritis is commonly seen in children and is self-limited. In adults however HBV glomerulonephritis can slowly progress to renal failure[38]. Approximately one-third of the patients with hepatitis B can have the serum-sickness-like arthritisdermatitis prodrome[39]. Many cutaneous disorders typically related to immune complex deposition are associated with hepatitis B. These include bullous pemphigoid, lichen planus and Gianotti-Crosti syndrome (papular acrodermatitis of childhood). Neurological manifestations include Guillain Barré syndrome, anxiety/depression and psychosis[40].
Definition of cure:Spontaneous seroconversion is the spontaneous loss of HBeAg and development of anti-HBe. This state is associated with low HBV-DNA levels and clinical remission of liver disease in many patients[41,42]. There is improvement in liver fibrosis when patients have HBeAg seroconversion[43]. Overall 0.5% and 0.8% of chronically infected patients will clear HBsAg per year[44]. This clearance of HbsAg is referred to as the recovery phase of hepatitis B.
Resolved CHB is characterized by sustained loss of HBsAg in a patient who was previously HBsAg positive, along with undetectable HBV-DNA levels and no clinical or histological evidence of active viral infection[45].
Functional cure is defined as loss of HbsAg with gain of anti-HbS. True cure is defined as elimination of HBsAg and closed covalent circular DNA (cccDNA)[45].
BARRlERS TO CURE
Clearance of hepatitis B and host immune response
HBV is a DNA virus with a complex structure and categorized into 10 different genotypes (A-J) based on global distribution, and the severity of the disease, risk of HCC, and response to certain treatments[46]. HBV enters the hepatocytes as a consequence of an interaction between the surface antigen and the sodium taurocholate cotransporting polypeptide[47]. After entry into the hepatocyte, the cccDNA develops when the relaxed circular DNA integrates with host cell nucleus, and at the time of HBV replication, cccDNA can generate pregenomic RNA to function as the template for the fully doublestranded DNA[48]. Figure 1 shows the lifecycle of HBV virus.
A few copies of cccDNA can initiate a full-blown infection after active replication, especially when the host is immunosuppressed[49]. Persistent cccDNA has been detected in hepatocytes of patients with resolved HBV infections, and hence the ultimate goal of HBV eradication should aim to clear any remnant cccDNA[42-46]. Another important reason to aim for clearance of cccDNA is the risk of progression to cirrhosis and HCC in patients with low to no HBV DNA in serum, highlighting the important of the role of ccc DNA[50,51].
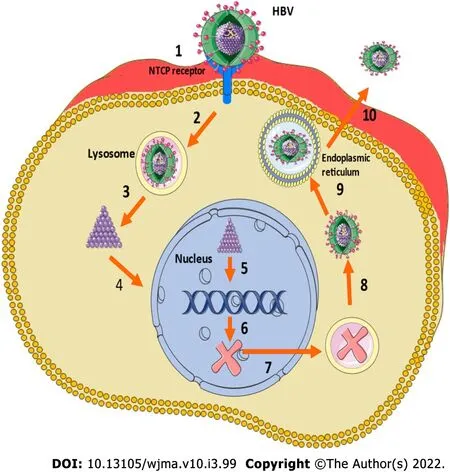
Figure 1 Lifecycle of hepatitis B virus (HBV). 1: Attachment of the virion to the sodium taurocholate cotransporting peptide (NTCP); 2: Endocytosis; 3: Capsid release; 4: relaxed circular DNA entry into the nucleus; 5: closed covalent circular DNA synthesis; 6: Transcription; 7: mRNA transfer to the cytoplasm and encapsidation; 9: DNA synthesis and budding of virions into the endoplasmic reticulum lumen; 10: Virus release through multivesicular body transfer to hepatocyte surface.
HBV is unique when compared to other hepatotropic viruses as there is a lack of innate response during HBV infection[52,53]. Chronic HBV affects the immune system by interfering with the function of T cells and in the synthesis of neutralizing antibodies, which are essential in mounting an appropriate immune response to the virus[54,55]. HBV exposurein uteroinduces a state of trained immunity against HBV[56], and HBV exposed neonates have variable levels of IL-10 and proinflammatory cytokines, and new pharmacotherapeutics exploring this pathway needs further research[56,57]. The goals of therapy have been to control viral replication so that inflammation, development of fibrosis and cirrhosis, and risk of HCC can be reduced, hence lowering the risk of decompensated liver disease and its sequelae and need for a liver transplant.
HBV vaccine and linkage of care
HBV vaccine, although available since 1982, was not widely available because of its high cost. The Global Alliance for Vaccines and Immunization was able to increase vaccine coverage in the early 2000s[58]. There still are major discrepancies in the availability and utilization of the vaccine, especially with regard to universal birth dose administration[59]. Different regions of the world have variable vaccine administration rates. In 2016, the rate of universal HBV single dose vaccine administration was 93% in the Western Pacific followed by 73% in Southeast Asia, 49% in America and Europe and only 19% in the African Region[60]. WHO recommends HBV vaccine at birth, followed by two or three doses at least 4 wk apart. Hepatitis B vaccine within 12 h is recommended for newborns born to mothers whose HBsAg status is unknown[61]. Adults who were not vaccinated as children can also receive HBV vaccine with first dose as soon as possible followed by 2 doses at 1 and 6 mo after the first dose[61]. Currently approved vaccines in the USA include single antigen hepatitis B vaccine and combined hepatitis A and hepatitis B vaccines. In adults aged 19-59 years they can either receive two doses 4 wk apart of single antigen hepatitis B vaccine or a three-dose series for the combination vaccine.
Multiple factors account for variation in vaccination and linkage of care for HBV across the globe. In China, the major limitation to access and care is secondary to the large population, high prevalence and the low coverage of diagnosis and treatment programs[62]. In resource-rich nations like Australia and New Zealand, despite subsidized screening, specialist management, and treatment for HBV, the barriers include lack of awareness about the implications of HBV infection in healthcare workers, and absence of consistent clinical guidelines regarding diagnosis and referral to a specialist[63,64]. In the USA, a recent systematic review highlighted the obstacles to care, which include access to medical care and lack of education and awareness amongst the patients, along with fear of stigma regarding the diagnosis as barriers to testing and care[65]. Implementation of effective vaccination policies worldwide, along with strategies to prevent vertical transmission and widely available testing and treatment, would be necessary to attain a reduction in HBV infections worldwide[66].
Current therapies and limitations:Current treatment options for CHB include interferons (IFNs) and nucleoside analogs but they suppress the viral replication and do not eliminate the virus, and aid in achieving a functional cure[67]. In HBeAg+patients, the loss of serum HBeAg and appearance of HBeAb and loss of circulating HBV DNA is the major goal[22]. Current therapies lead to HBeAg seroconversion in only 20%-30% of treated patients and a mortality reduction by 50% over a 10-year period[22,68,69]. Table 1 summarizes the current antiviral therapies for adults and children.
Goal of new therapies: Eradication of cccDNA is the ultimate goal of ongoing research for novel HBV treatments. Hurdles to measurement of cccDNA include lack of sophisticated assays and challenges to biopsy. There is a need for surrogate markers for loss of cccDNA, HBV DNA, HBeAg[70]. Table 2 summarizes the newer therapies.
Gene editing - future direction: The major hurdle to eradication of HBV is that current antiviral therapies do not eradicate latently integrated or nonreplicating episomal viral genomes. Furthermore, HBV infection disseminates extensively beyond the liver and broad range of cell lines, including neurons, endothelial cells, macrophages, polymorphic nuclear leukocytes, peripheral blood mononuclear cells, and are permissive for HBV replication.
Gene editing provides the ability to alter an organism’s DNA. Targeted endonucleases are highly specific enzymes designed to introduce DNA double-strand breaks into desired target sequences .The major classes of DNA-cleaving enzymes include zinc finger nucleases, Tal-effector nucleases, RNAguided engineered nucleases such as CRISPR/Cas9 and mega nucleases/homing endonucleases[71].
The features that make HBV amenable are small viral genome (3.2 kb) with four proteins (envelope, nucleocapsid, polymerase, and X protein). HBV also has low to intermediate mutability rate and the polymerase mutation rate rages from 1.4 × 105-3.2 × 105mutations/site/year. These factors make it a good target for cleavage enzyme. CRISPR/Cas9 inhibits HBV replication and can be used to target HBV[72,73]. Recently, when Cas9 and guide RNAs were delivered using plasmids into mouse liver, cccDNA could be cleaved, disrupted and cleared[74]. Animal studies after gene editing of CRISPR-Cas9 gene showed improved survival with entacavir with reduced HBV DNA and cccDNA levels[75]. Recent findings of removal of full-length 3175-bp integrated HBV DNA fragment using CRISPR-Cas9 demonstrated that CRISP-Cas9 system may emerge as powerful tool capable of promoting a radical or “sterile” HBV cure[76,77].
HEPATlTlS C
Epidemiology
Globally, hepatitis C virus (HCV) infection prevalence is 1%, and there are about 2.3 million cases in the USA[78]. The highest prevalence is in the Eastern Mediterranean and European Regions, followed by South East Asian and Western Pacific Regions. Countries with high prevalence are Russia, Gabon, Egypt and Syria[78,79]. HCV is an RNA virus, and similar to HBV, it is transmittedviacontacting blood or body fluids of infected individuals, with most common routes of transmission being intravenous drug use, blood product transfusion, solid organ transplantation, or unintentional cross-contamination in hospitals and other medical facilities[80]. Intranasal cocaine use and tattoos administered in unclean parlors are other risk factors[81,82]. Perinatal transmission, though very rare, has been reported in 2%-8% of infected mothers[83].
Hepatitis C in pediatric populations:The estimated prevalence of HCV in 2018 in the pediatric population aged 0-18 years was 0.13% corresponding to 3.26 million children[84]. Direct-acting antivirals (DAAs) are the treatment option for HCV infection in children and adolescents aged ≥ 3 years. Presence of extrahepatic manifestations like rash, advanced fibrosis, cryoglobulinemia, and glomerulonephritis is an indication for early antiviral therapy. Table 3 summarizes the treatment options in pediatric populations.
Extrahepatic manifestations:Chronic HCV, which is untreated can cause chronic inflammation, followed by progressive liver fibrosis leading to the development of cirrhosis and HCC[85,86]. Various extrahepatic manifestations are reported in chronic HCV infection like mixed cryoglobulinemia, vasculitis, glomerulonephritis, and B-cell non-Hodgkin’s lymphoma, along with increased rates of insulin resistance, diabetes, atherosclerosis, and cognitive impairment[87-89]. A meta-analysis of 102 studies looking at prevalence, quality of life, and economic burden of extrahepatic manifestations of HCV showed diabetes (15%) and depression (25%) were the most common extrahepatic manifestations[90].
Barriers to elimination:The goal of WHO has been to develop and work on strategies to reduce new infections while treating patients who are infected with HCV[91].

Table 1 Approved antivirals for adults and children for chronic hepatitis B
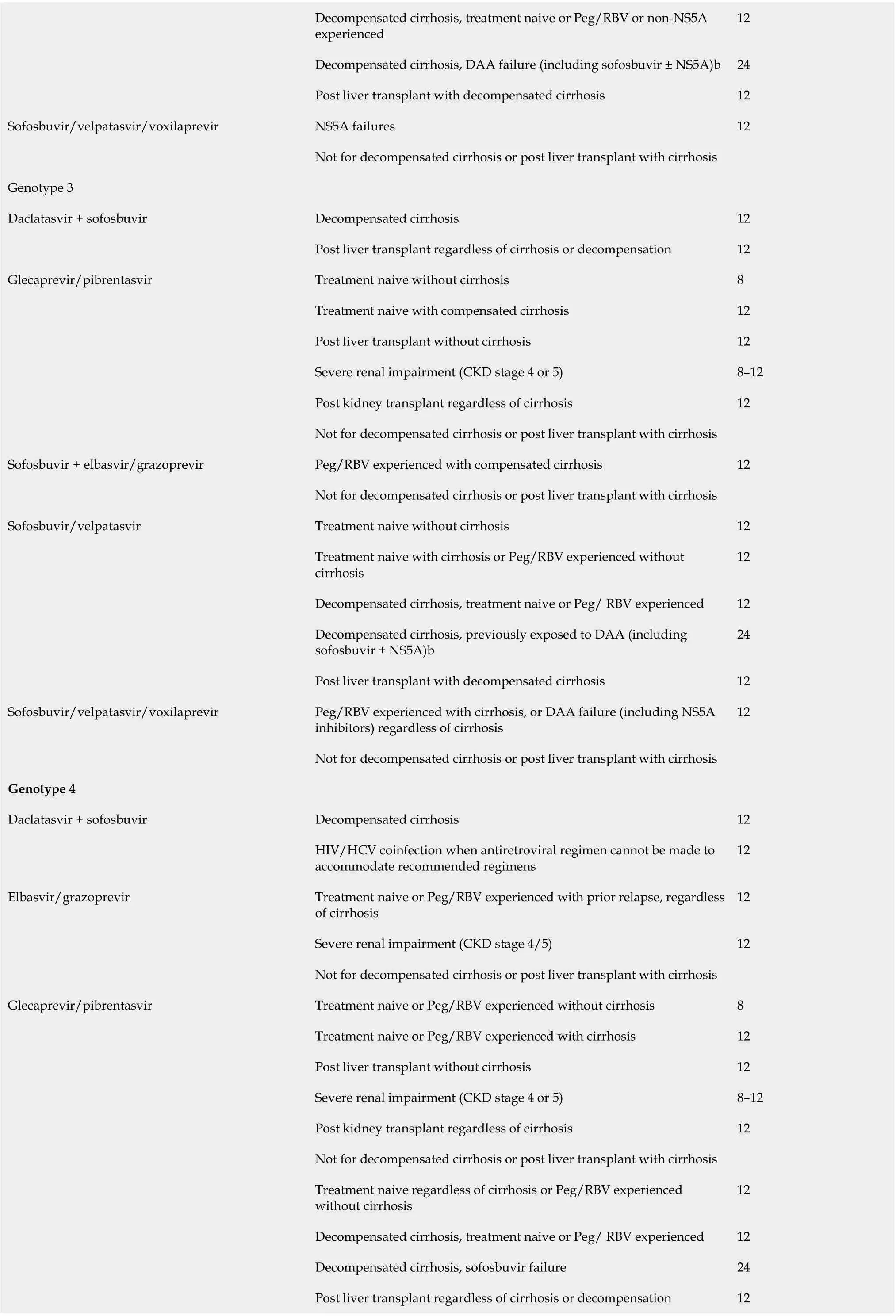
Since the advent of oral DAAs in 2014, there has been a dramatic change in the landscape of HCV therapy[92,93]. DAA therapies are not only well-tolerated and safe but offer cure rates of > 95%[94,95]. In comparison to IFNs, treatment with DAAs is short term[96]. With the new agents and multiple options, treatment can be tailored based on presence and absence of cirrhosis, decompensated disease, coinfection with human immunodeficiency virus (HIV) and renal function and need for dialysis. Table 4 summarizes the available DAAs, their target population, and genotype coverage. DAAs are promising and have changed the landscape of chronic hepatitis C infection, but there are several barriers to care and cure and below mentioned are a few.
Awareness and screening programs
There remains a general misunderstanding and also lack of awareness regarding HCV in the general population worldwide, as evident by a 2017 WHO Global Hepatitis Report, where only 20% of 71 million people with HCV worldwide, were aware of the infection at the time of confirmation[91]. A large population-based study in the USA from 2001 to 2008 showed only 49.7% of patients with HCV infection were aware of their status[97]. Another National Health and Nutrition Examination Survey study showed that cirrhosis was equally common in patients who were unaware of their diagnosis compared to those who knew about their infection[98]. At a patient level, fear of the stigma associated with the diagnosis and at the provider level, lack of time, knowledge, and discomfort in asking about high-risk behaviors are barriers to screening, testing, and cure[99]. The provider perceptions have changed over the years and now most providers believe that they play an active role in their patient’s treatment and their decisions to start treatment are not influenced by high risk behaviors amongst patients[100].
In developing countries, the absence of screening programs and limited resources have resulted in the vast majority of patients being undiagnosed[101-103]. A systematic review and meta-analysis of studies published after 1995 showed that in Africa, only 19% of blood transfusions are screened for HCV due to cost constraints[104].
The screening strategies have to be tailored according to the population and the country to make these cost effective[105]. As shown in a systematic review of 67 screening programs, in low HCVprevalence populations, prescreening can increase efficiency, whereas in high prevalence countries widespread screening programs are cost effective[106-108].
High risk groups:Intravenous drug users (IVDUs) are a well-known high-risk population and the global burden of HCV in injecting drug users is approximately 67%[109]. In Europe the prevalence of HCV is estimated to be 50 times higher in individuals who inject drugs compared with the general population[110]. In the past, this subset of HCV patients was not regarded as eligible for treatment due to concern for poor adherence, reinfection and psychiatric ailments[111]. Current guidelines recommend that people who inject drugs (PWIDs) should not be excluded from HCV treatment[112] and multiple recent studies have shown that there is direct relationship between influences of IV drugs on the efficacy of DAA therapy among adherent patients[113-115], and the SIMPLIFY trial demonstrated that PWIDs should be offered HCV treatment regardless of ongoing drug use[116]. In this high-risk group testing, access to care, prescription of DAA therapy, along with the elimination of stigma associated with the infection have been proposed as effective strategies for this specific population[117-119].
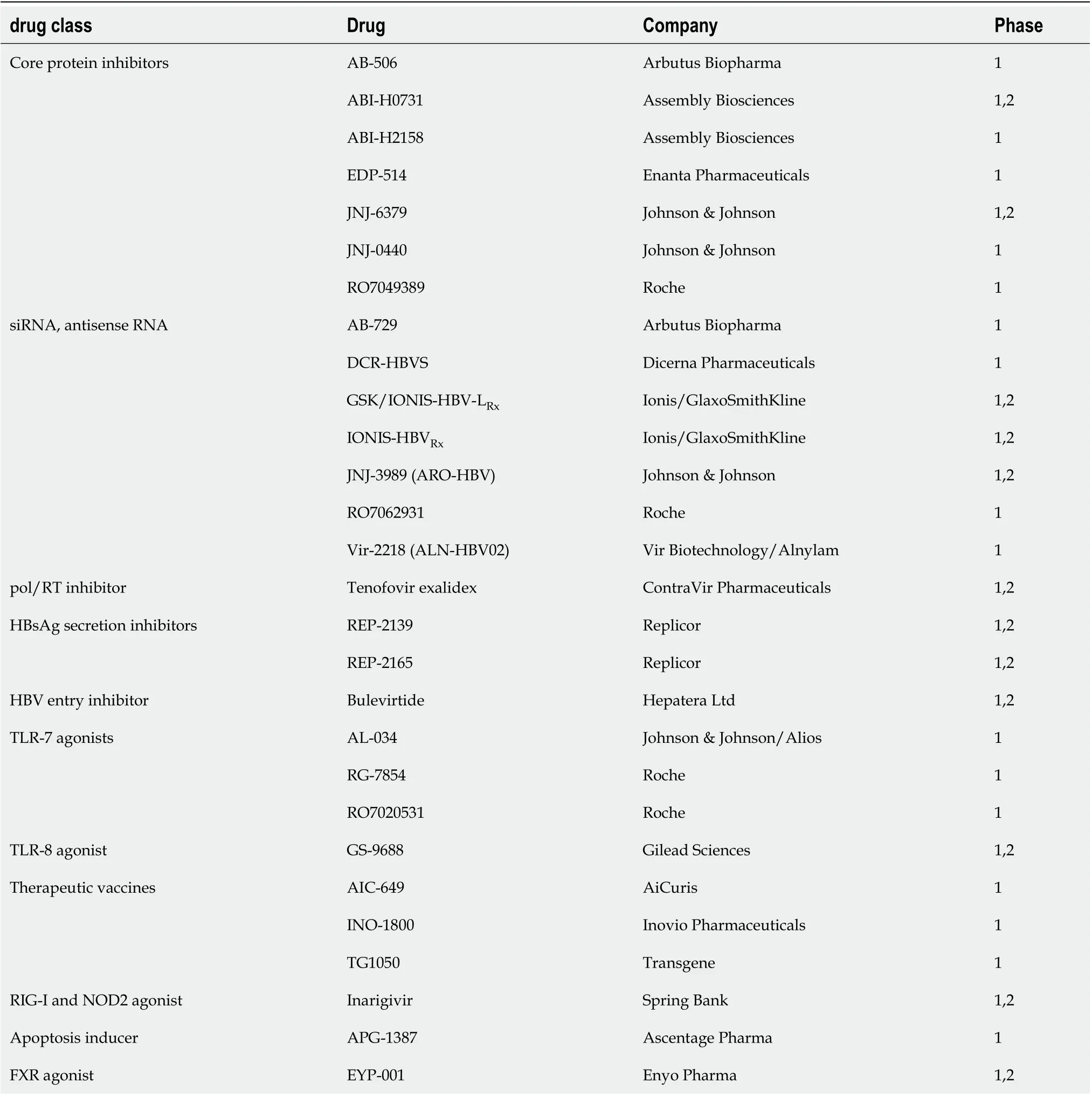
Table 2 Drugs in pipeline for hepatitis B virus
Prevention of HBV/HCV infection:Both HBV and HCV can be transmitted perinatally,vianeedle stick injury andviahousehold contacts. Perinatal transmission for HBV can be prevented by providing hepatitis B immunoglobulins and vaccines within 12 h of birth to infants of HbsAg-positive mothers[120]. Unfortunately for HCV infection, there are no interventions or prophylactic measures that have been proven to prevent perinatal transmission. Management of needle stick injury for HBV depends on the vaccination status of exposed individual and HBV status of patient. For individuals who suffer needle stick injury and are unvaccinated, vaccination series should be initiated. For vaccinated individuals with documented vaccine response no treatment is required. If the vaccination status is unknown, then its recommended to check anti-Hbs titers and if negative, initiating vaccine series is recommended[121]. Recommendations for prevention of HCV after needle stick injury include testing for HCV RNA, HCV antibodies and ALT immediately after the event, repeat laboratory analysis in 2-8 wk, and referral to specialist if infection occurs[122]. Household contacts should be extensively counselled and education includes measures to avoid sharing razors or toothbrushes etc. that predisposes one to contact with body fluids, HCV/HBV positive individuals should refrain from donating blood, organ and tissue[123].
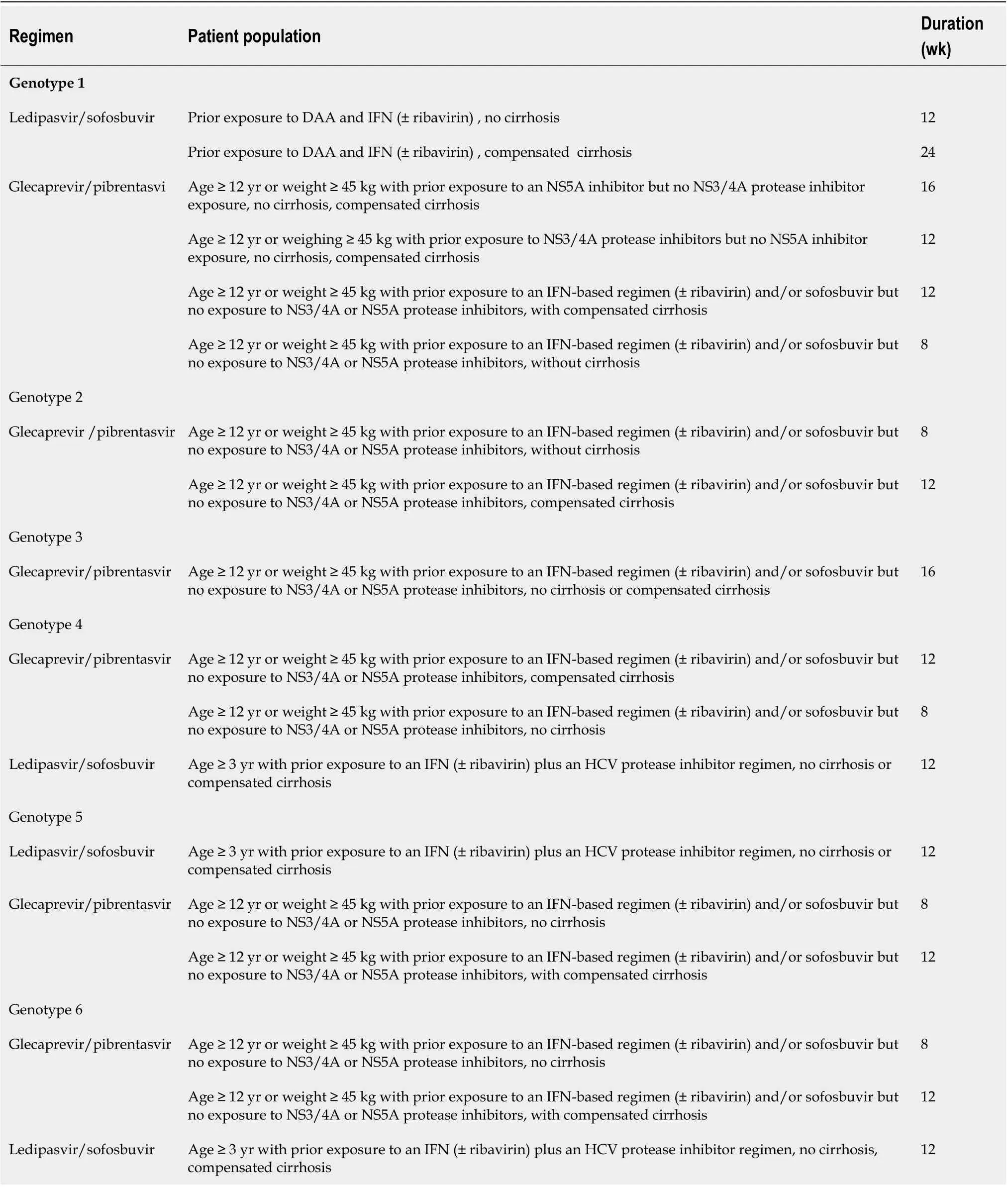
Table 3 DAA therapy in pediatric population
HBV/HCV coinfection:The worldwide incidence of HBV/HCV coinfection is reported to range from 5% to 15%[124,125]. The incidence varies significantly depending on geographic location, with higher incidence in endemic areas[126]. HBV/HCV coinfection leads to higher rate of cirrhosis, HCC and decompensated liver disease compared to monoinfection[124,127]. Four serological profiles are seen in coinfection-codominant, HCV dominant, HBV dominant, and neither replicative, and these can evolve over period of time[128]. The aim in these scenarios would be to identify and eradicate the dominant virus and then monitor for reactivation of the other virus. Close monitoring of HBV DNA and HCV RNA is essential before determining viral dominance[129]. HBV monoinfection is treated with a nucleo(s)tide analog (e.g., entacavir or tenofovir, lamivudine) and/or pegylated IFN (Peg-IFN). Currently, DAAs are the mainstay of treatment for HCV monoinfection although Peg-IFN plus ribavirin is effective, but is rarely used[126].
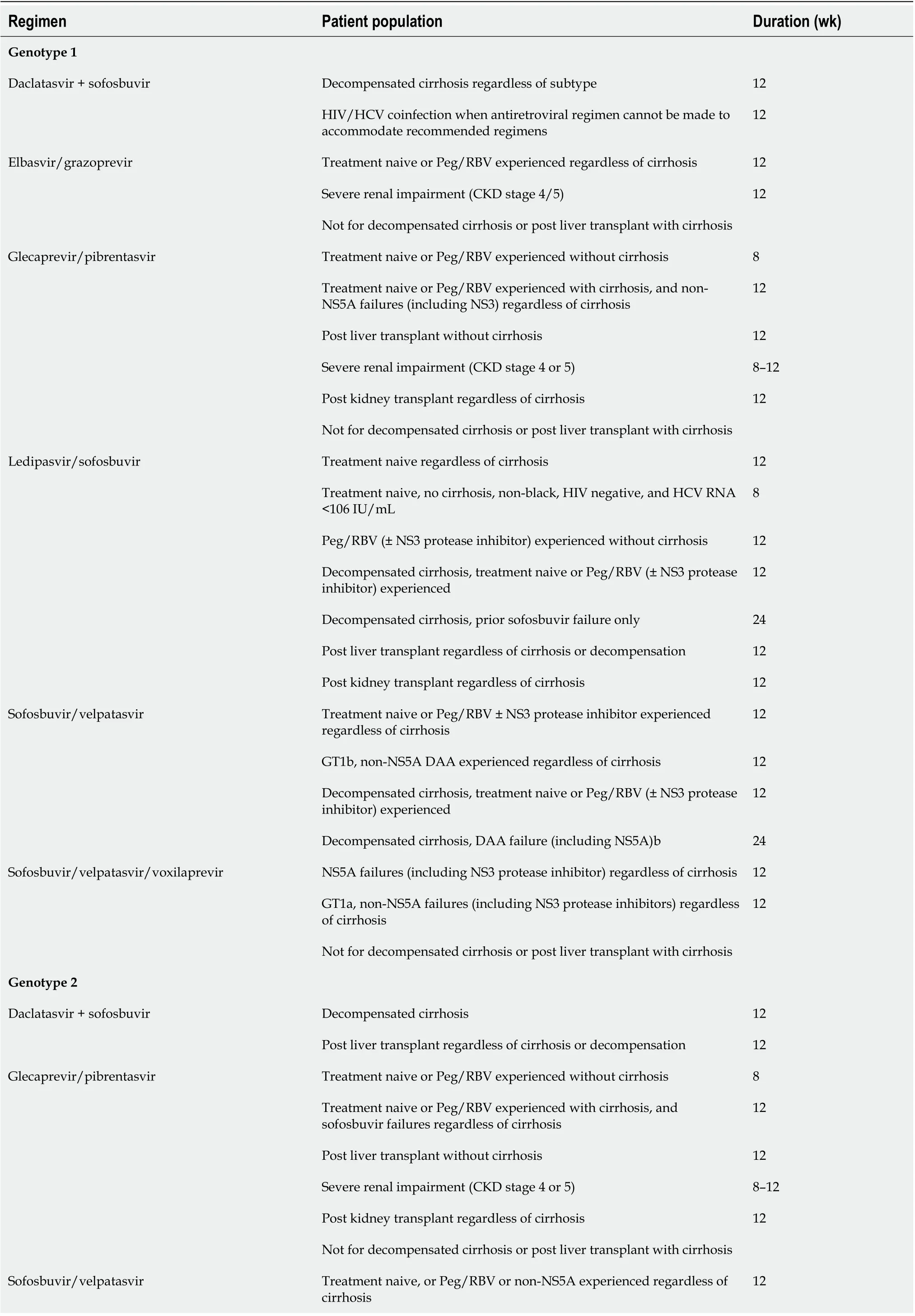
Table 4 DAA therapy for chronic hepatitis C virus
Decompensated cirrhosis, treatment naive or Peg/RBV or non-NS5A experienced 12 Decompensated cirrhosis, DAA failure (including sofosbuvir ± NS5A)b 24 Post liver transplant with decompensated cirrhosis 12 Sofosbuvir/velpatasvir/voxilaprevir NS5A failures 12 Not for decompensated cirrhosis or post liver transplant with cirrhosis Genotype 3 Daclatasvir + sofosbuvir Decompensated cirrhosis 12 Post liver transplant regardless of cirrhosis or decompensation 12 Glecaprevir/pibrentasvir Treatment naive without cirrhosis 8 Treatment naive with compensated cirrhosis 12 Post liver transplant without cirrhosis 12 Severe renal impairment (CKD stage 4 or 5)8-12 Post kidney transplant regardless of cirrhosis 12 Not for decompensated cirrhosis or post liver transplant with cirrhosis Sofosbuvir + elbasvir/grazoprevir Peg/RBV experienced with compensated cirrhosis 12 Not for decompensated cirrhosis or post liver transplant with cirrhosis Sofosbuvir/velpatasvir Treatment naive without cirrhosis 12 Treatment naive with cirrhosis or Peg/RBV experienced without cirrhosis 12 Decompensated cirrhosis, treatment naive or Peg/ RBV experienced 12 Decompensated cirrhosis, previously exposed to DAA (including sofosbuvir ± NS5A)b 24 Post liver transplant with decompensated cirrhosis 12 Sofosbuvir/velpatasvir/voxilaprevir Peg/RBV experienced with cirrhosis, or DAA failure (including NS5A inhibitors) regardless of cirrhosis 12 Not for decompensated cirrhosis or post liver transplant with cirrhosis Genotype 4 Daclatasvir + sofosbuvir Decompensated cirrhosis 12 HIV/HCV coinfection when antiretroviral regimen cannot be made to accommodate recommended regimens 12 Elbasvir/grazoprevir Treatment naive or Peg/RBV experienced with prior relapse, regardless of cirrhosis 12 Severe renal impairment (CKD stage 4/5)12 Not for decompensated cirrhosis or post liver transplant with cirrhosis Glecaprevir/pibrentasvir Treatment naive or Peg/RBV experienced without cirrhosis 8 Treatment naive or Peg/RBV experienced with cirrhosis 12 Post liver transplant without cirrhosis 12 Severe renal impairment (CKD stage 4 or 5)8-12 Post kidney transplant regardless of cirrhosis 12 Not for decompensated cirrhosis or post liver transplant with cirrhosis Treatment naive regardless of cirrhosis or Peg/RBV experienced without cirrhosis 12 Decompensated cirrhosis, treatment naive or Peg/ RBV experienced 12 Decompensated cirrhosis, sofosbuvir failure 24 Post liver transplant regardless of cirrhosis or decompensation 12

Post kidney transplant regardless of cirrhosis 12 Sofosbuvir/velpatasvir Treatment naive or Peg/RBV experienced regardless of cirrhosis 12 Decompensated cirrhosis, treatment naive or Peg/ RBV (± NS3 protease inhibitor) experienced 12 Decompensated cirrhosis, DAA failure (including NS5A)24 Sofosbuvir/velpatasvir/voxilaprevir NS5A failures (including NS3 protease inhibitors) regardless of cirrhosis 12 Not for decompensated cirrhosis or post liver transplant with cirrhosis Sofusbuvir/ledipasvir Treatment naive, compensated cirrhosis - not for decompensated cirrhosis 12 Genotype 5 or 6 Glecaprevir/pibrentasvir Treatment naive or Peg/RBV experienced without cirrhosis 8 Treatment naive or Peg/RBV experienced with cirrhosis 12 Post liver transplant without cirrhosis 12 Severe renal impairment (CKD stage 4 or 5)8-12 Post kidney transplant regardless of cirrhosis 12 Not for decompensated cirrhosis or post liver transplant with cirrhosis Ledipasvir/sofosbuvir Treatment naive or Peg/RBV experienced regardless of cirrhosis 12 Decompensated cirrhosis, treatment naive or Peg/ RBV experienced 12 Decompensated cirrhosis, sofosbuvir failure 24 Post liver transplant regardless of cirrhosis or decompensation 12 Sofosbuvir/velpatasvir Treatment naive or Peg/RBV experienced regardless of cirrhosis 12 Decompensated cirrhosis, treatment naive or Peg/RBV (± NS3 protease inhibitor) experienced 12 Decompensated cirrhosis, DAA failure (including NS5A)24 Sofosbuvir/velpatasvir/voxilaprevir NS5A failures (including NS3 protease inhibitors) regardless of cirrhosis 12 Not for decompensated cirrhosis or post liver transplant with cirrhosis Adapted from American Association for the Study of Liver Diseases/IDSA guidelines (https://www.hcvguidelines.org/). CKD: Chronic kidney disease; DAA: Direct-acting antiviral; HIV: Human immunodeficiency virus; HCV: Hepatitis C virus; RBV: Ribavirin; Peg: Pegylated interferon.
HBV/HCV infection after liver transplantation:HBV recurrence after liver transplantation is a major causes of graft failure, graft cirrhosis and allograft dysfunction. Patients can be categorized into high and low risk for recurrent HBV based on pretransplant viral load, HbeAg positivity and history of antiviral drug resistance[130]. Combination of potent nucleos(t)ide analog and hepatitis B immunoglobulin (HBIG) is recommended after liver transplantation for the prevention of HBV recurrence in patients with CHB. Recent data suggest that patients with low risk of recurrence need to be on continued monoprophylaxis with nucleos(t)ide analogs; however, HBIG can be discontinued[77]. HCV recurrence after liver transplantation is universal in patients with HCV viremia at the time of transplantation. The viral levels are shown to rebound and reach pretransplant level with 72 h and DAA therapy should be started within this timeframe to prevent graft reinfection and loss[131].
HEPATlTlS D
Epidemiology
Hepatitis delta virus (HDV) is a defective virus that encodes its own genome but needs HBsAg and hence HBV for replication, propagation and transmission[132]. The two high-risk groups at risk of infection include IVDUs and patients with high-risk sexual behaviors[133]. In the USA, HDV infection was considered to be a rare, but data over the last decade estimating seroprevalence of HDV have shown higher rates, especially in Asians and immigrants[45].
Transmission:HDV is transmitted parenterally and sexually, while vertical transmission is thought to be rare[134,135]. In low-endemicity regions and developed nations, IVD use is the main route of transmission[133].
Clinical presentation:HDV infection is always associated with HBV infection as HBV is integral to the assembly of the hepatitis D virion and release. Two major patterns of infection can occur: superinfection and coinfection.
Coinfection is concurrent infection with both HDV and HBV. Clinically, the presentation is difficult to differentiate from other causes of hepatitis and especially acute HBV[136]. Patients who are coinfected can present with symptoms that can be mild to severe fulminant hepatitis[137]. Coinfection is usually self-limited, but it is important to highlight the fact that coinfection can cause severe fulminant hepatitis compared to superinfection[137].
Superinfection occurs when HDV infects an individual with CHB, in whom pre-existing HBsAg provides an ideal environment for HDV expression. Patients progress from acute hepatitis to chronic infection in up to 90% of cases, whereas the rest either resolve or progress to fulminant disease[136]. Chronic HDV infection, in comparison to HBV monoinfection, is more severe and, up to 70% percent of patients rapidly progress to cirrhosis within 5-10 years[138].
Diagnosis and management:In patients suspected to have HDV, the first-line screening test is ELISA for anti-HDV. The acute phase of HDV infection is characterized by positive IgM anti-HDV in serum. IgG anti-HDV antibodies are representative of chronic HDV infection or past exposure[139]. HDV screening for all HBV-infected individuals is recommended by the European guidelines whereas in the USA screening is limited to patients with high-risk factors such as HIV or HCV infections and patients with low or undetectable HBV DNA presenting with elevated aminotransferases[45]. Screening of all HBsAg-positive patients should be considered given concerns regarding underestimation of prevalence of HDV[140,141]. This approach would lead to more accurate determination of HDV prevalence and would also lead to earlier intervention and treatment[142].
The current treatment option for chronic HDV infection is Peg-IFN-α for 12 mo based on guidelines from the American Association for the Study of Liver Diseases, Asian Pacific Association for the Study of the Liver, and the European Association for the Study of the Liver[45,77,143]. Overall, the response rate to therapy is low with only a 10%-20% rate of sustained HDV clearance and 10% rate of HbsAg clearance with 1 year of standard IFN-α[144,145]. Studies revealed that 1 year of therapy with Peg-IFN proved to have a better response rate than standard IFN therapy however it hardly exceeded 25% of sustained HDV clearance[146,147].
Combination therapy involving standard IFN-α with ribavirin[148] or lamivudine[149,150] is not more efficacious than monotherapy with IFN for chronic hepatitis D. Similar results are obtained when Peg-IFN-α is used in combination with ribavirin[151] or adefovir[147].
Novel therapeutics:Given the low overall virological response rate and high rate of relapse, there is an increasing need for therapeutic strategies aimed at improving efficacy and offering it to patients for whom IFN is contraindicated due to advanced liver disease. Currently, three new medications that affect HDV life cycle are being studied in clinical trials, with varying mechanism of actions: hepatocyte entry inhibitors, farnesyltransferase inhibitors, and nucleic acid polymers. Table 5 summarizes these novel therapies with the associated adverse effects. Figure 2 highlights the different targeted approaches for treatment of chronic hepatitis D.
Аdditional approaches:siRNAs have shown early promise in this field. In a phase IIa clinical trial that showed that a single injection of siRNA ARC-520d decreased HbsAg levels in HbeAg negative CHB patients in a dose-dependent fashion[152]. A multi-dose extension study of up to 12 doses, with once monthly dosing, demonstrated an increased decline of HbAg level, especially in HbeAg-positive rather than HbeAg-negative patients[153]. The study was stopped because of adverse effects of the carrier molecule but demonstrated the effect and highlighted the scope of siRNA as a potential treatment option.
Currently, for the management of HDV, new approaches such as DNA vaccines[154], anti-HB immune complexes[155]https://www-ncbi-nlm-nih-gov.ezproxy3.lhl.uab.edu/pmc/articles/PMC5580405/-ref-75, and immunologically active adjuvants such as β-glucosylceramide are being explored. Targeting the HBV and immune system interaction is another area that has garnered significant interest. Preclinical studies have shown that the Toll-like receptors (TLRs) play a key role in sensing pathogen-associated molecule patterns and activating intracellular antiviral pathways as well as the production of proinflammatory cytokines and antiviral effectors like IFN[156]. In a study assessing the safety, pharmacokinetics, and pharmacodynamics of oral TLR-7 agonist, GS-920 led to induction of peripheral mRNA expression of IFN-stimulated gene 15 production in CHB patients; however, there was no effect on HBV DNA[157]. Immune checkpoint inhibitors have also been studies in chronic viral hepatitis, and a phase Ib clinical study of nivolumab in CHB patients highlighted its tolerance and association with significant decline in HbsAg after a single dose over a 24-wk period[158].
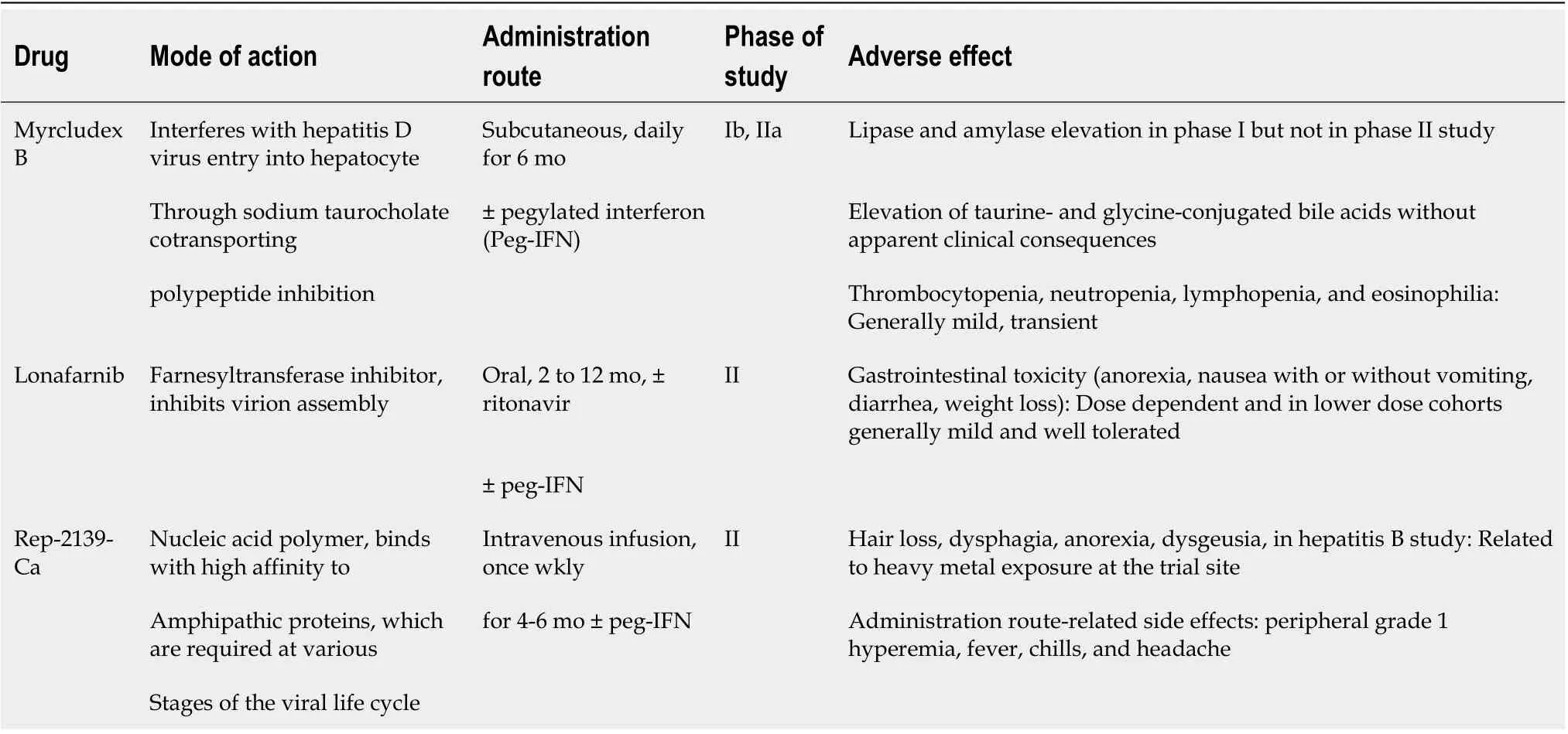
Table 5 Novel drug treatments for chronic hepatitis D virus
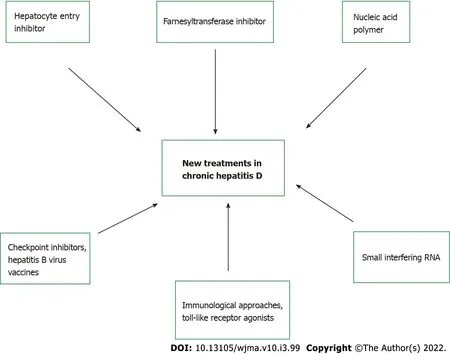
Figure 2 New treatments in chronic hepatitis D, with specific targets.
HEPATlTlS E
Epidemiology
HEV is a small, nonenveloped virus and belongs to the Hepeviridae family and is further classified into genotypes 1-4 and 7[159]. Globally an estimated 2.2 billion people are infected by HEV with 70 000 deaths attributed to HEV annually[160,161].
HEV is mainly transmittedviacontaminated water and consumption of undercooked pork or wild boar and other foods, while reports of blood transfusion related transmission has been recently recognized[162,163]. Outbreaks of HEV1 and HEV2 genotypes are documented in areas with inadequate sanitary conditions and lack of access to clean water[164]. The prevalence of anti-HEV IgG in Africa ranges from 4.6% to 10.7%, and 34% to 94% in Asia[165-169]. HEV prevalence is probably underestimated as seen in a large German cohort study, as the majority of practitioners do not regularly test for HEV in the presence of acute hepatitis symptoms; in part, due to lack of high clinical suspicion but also absence of standardized testing, leading to increased morbidity and mortality among susceptible individuals[170].
The diagnosis of HEV infection is often challenging given lack of standardized testing and need for HEV PCR for definitive diagnosis[171-173]. Paradoxically, in immunocompromised hosts who do not mount an adequate antibody response, PCR testing should be the cornerstone of diagnosis[174,175]. There is no US FDA-approved diagnostic test and available serological assays have variable sensitivities and specificities making accurate diagnosis often challenging[176-178].
Clinical presentation:Clinical presentation in HEV is variable, ranging from asymptomatic carriers to fulminant hepatitis. In acute HEV, the incubation period is typically 3-8 wk. followed by a short prodrome leading to a symptomatic phase that can last for several days to weeks (mean 4-6 wk)[179]. HEV can also infect patients with chronic liver disease and can cause decompensation, and lead to high mortality[180-182]. Extrahepatic manifestations of HEV include rash, arthralgias, Guillain-Barré syndrome palsies, and pseudotumor cerebri[183].
Based on the patients’ immune response to acute HEV, some may progress to chronic HEV infection, which is defined by persistent elevated aminotransferase levels for at least 3 mo combined with positive serum HEV RNA and consistent histological findings on liver biopsy[182]. Chronic HEV primarily occurs in immunosuppressed patients such as organ transplantation recipients or those with HIV infection, and hemodialysis[184-189].
Infection with HEV, specifically genotype 1, during pregnancy leads to increased risk of adverse outcomes to the fetus such as spontaneous abortion,in uterofetal demise, and premature delivery, while placing the mother at risk of severe hepatitis and complications[190]. HEV in pregnancy is associated with eclampsia, hemorrhage, and acute liver failure, and carries a high mortality rate of 15%-25%, especially in the third trimester[182,191].
Treatment:Acute HEV in immunocompetent hosts is self-limiting illness followed by spontaneous clearance and usually does not require treatment[192]. Monotherapy with ribavirin is the current treatment of choice for patients with chronic HEV infection[193]. Three months of ribavirin monotherapy for chronic HEV has been associated with around 78% sustained virological response[194]. No established treatment for HEV is available for pregnant women as ribavirin is contraindicated in pregnancy, hence supportive care is recommended[195,196]. Peg-IFN, as an alternative to ribavirin has shown limited success[197,198]. There is a need for direct-acting novel therapies as HEV remains a serious public health concern particularly among pregnant women and immunocompromised patients. The current efforts for these drug developments are focusing either on the inhibition and manipulation of host components or developing DAA therapies that can target viral enzymes without affecting host components[199].
Hepatitis E vaccine and surveillance programs:The need for hepatitis E vaccine was recognized secondary to its worldwide prevalence and severe complications in high risk populations. Early studies of recombinant vaccines in healthy adults have shown promising results, but study populations have unfortunately not included the high-risk groups who are most susceptible to severe and chronic HEV. A Nepalese randomized, placebo controlled, double blinded , phase 2 clinical trial of a recombinant HEV vaccine given at 0, 1 and 6 mo to 898 patients (vs896 placebo) revealed vaccine efficacy of 88.5% in intention to treat analysis[200]. In a different phase 3 clinical trial, Hecolin (Xiamen Innovax Biotech, China) had 112 604 participants, of which 56302 in the study arm and 56 302 in the placebo arm, received three doses of rHEV and hepatitis B vaccine at 0, 1 and 6 mo respectively. The vaccine efficacy of 95.5%was reported in an intention to treat analysis[201]. This vaccine is only approved and commercially available in China[202].
An HEV vaccine that is available worldwide would reduce the incidence of the infection in endemic areas and also confer protection to travelers[203]. Areas and countries with high prevalence should also focus on improved sanitation, access to clean water with a specific focus on high-risk groups, especially pregnant women and patients with chronic medical conditions[167,204].
CONCLUSlON
With the recent advancements in the area of molecular virology, the landscape for the management of viral hepatitis has evolved dramatically. We have a better understanding of the molecular structure of these pathogens and their interplay with our immune system, which has paved the way for novel drugs and therapeutics. While the success of the decade is focused on DAAs as the cure for HCV, the burden of chronic HBV and HDV infections persists as research is ongoing for both a cure for HBV and treatment options for HDV. Drugs that hold promise regarding complete eradication of HBV cccDNA from hepatocytes are under investigation and may be pivotal in complete eradication of the infection in the future. Despite the advancement in the field of serological and PCR testing for HBV, HCV, and HDV, there is a continued need for improvements in screening protocols for these infections. Standardized testing along with options for treatment and vaccination remain areas of interest for HEV. Work continues on implementation of universal vaccination for HAV and HBV, while clinical trials are ongoing for HEV vaccination. There remains a pressing need for increased collaborative efforts to help combat these illnesses, as we continue to learn about the viral hepatitis to fill the gaps in our knowledge.
FOOTNOTES
Author contributions:Ahmed Z, Shetty A, and Kodali S contributed equally to this work; Ahmed Z, Shetty A, Victor DW and Kodali S designed the research study; Ahmed Z Kodali S performed the research; Ahmed Z, Shetty A, Victor DW and Kodali S wrote the manuscript; all authors have read and approve the final manuscript.
Conflict-of-interest statement:All the authors declare no conflict of interest.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:United States
ORClD number:Zunirah Ahmed 0000-0002-5205-7805; David W Victor 0000-0003-1414-3128; Sudha Kodali 0000-0003-0352-6019.
S-Editor:Liu JH
L-Editor:Kerr C
P-Editor:Liu JH
 World Journal of Meta-Analysis2022年3期
World Journal of Meta-Analysis2022年3期
- World Journal of Meta-Analysis的其它文章
- Difference in incidence of developing hepatocellular carcinoma between hepatitis B virus-and hepatitis C virus-infected patients
- Clinical outcomes of the omicron variant compared with previous SARS-CoV-2 variants; meta-analysis of current reports
- ls cellular therapy beneficial in management of rotator cuff tears? Meta-analysis of comparative clinical studies
- Evidence analysis on the utilization of platelet-rich plasma as an adjuvant in the repair of rotator cuff tears
- Review with meta-analysis relating North American, European and Japanese snus or smokeless tobacco use to major smoking-related diseases
- Rare post-endoscopic retrograde cholangiopancreatography complications: Can we avoid them?
