Diagenetic mineralogy and its effect on the reservoir properties of the sandstones of the Permian of S120 block (Sulige gas field), Ordos Basin, NW China
Qi Wn , Ai-Ping Fn ,*, Ren-Cho Yng ,b, Nils Lenhrdt
a Shandong Provincial Key Laboratory of Depositional Mineralization & Sedimentary Minerals, Shandong University of Science and Technology, Qingdao, 266590, China
b Laboratory for Marine Mineral Resources, Qingdao National Laboratory for Marine Science and Technology,Qingdao, 266071, China
c Department of Geology, University of Pretoria, Private Bag X20, 0028, Pretoria, South Africa
Abstract The characteristics of diagenetic minerals and their effects on reservoir quality of the tight sandstones of the Permian in Sulige gas field of the Ordos Basin were studied through observations on thin sections, scanning electron microscopy, energy dispersive spectroscopy analysis and electron microprobe analysis. Diagenetic minerals in the Permian sandstones consist of illite, kaolinite, chlorite, siliceous and calcite cements. Large amounts of intercrystalline pores between kaolinite and illite provide channels for acidic fluids flow and thereby were conducive to the formation of clastic solution pores,intergranular solution pores and composite pores. Authigenic chlorite occurs in the form of three morphotypes as grain-coating,pore-lining and pore-filling. Grain-coating and pore-lining chlorite with different crystal shapes occur as coatings on the framework grains. Pore-filling chlorite precipitated as discrete flaky plates in pore spaces.Quartz microcrystals developed but quartz overgrowth did not develop because of the occurrence of porelining chlorite. This, in turn, led to the preservation of primary pores by occupying potential nucleation sites for quartz overgrowth and thereby preventing quartz microcrystals from merging into quartz overgrowth.This process is regarded as the most important for influencing the quality of the lithophysical properties.Calcite cement was mainly precipitated during a late diagenetic stage and has a negative effect on the reservoir quality. This study provides important insights into analyzing the relationship between diagenetic minerals and reservoir quality and the results are directly applicable to the exploration and development of tight sandstone reservoirs all over the world.
Keywords Ordos basin, Sulige gas field, Permian, Diagenetic processes, Siliceous cement, Chlorite
1.Introduction
The formation of diagenetic minerals is closely related to chemical and mineralogical changes in sediments and the formation and destruction of pore spaces, making them as important factors affecting the physical properties of rock and reservoir types.The study of diagenetic minerals, such as, siliceous cement, clays and carbonates, is therefore of special significance for evaluation of a (hydrocarbon)reservoir.
Siliceous cements in the form of quartz overgrowth or authigenic quartz have long been recognized(Aagaard et al., 2000; Worden et al., 2012; Zhong et al., 2012; French and Worden, 2013; Li et al.,2013). However, their effects on the physical properties of sandstone are still under debate (Zhong et al.,2012; Zhang et al., 2012; Worden et al., 2012; Li et al., 2013). Quartz overgrowth may fill primary pores to a large extent,which may lead to a significant reduction in the reservoir porosity (Aagaard et al.,2000). Furthermore, it may be closely embedded at a later stage, and thereby occupying pore center and blocking pore throats.This may eventually lead to the destruction of the reservoir porosity (Zhong et al.,2012; Li et al., 2013). Nevertheless, some studies have shown that the quartz overgrowth of different stages may have different effects on reservoirs, for instance, the initial quartz overgrowth can resist mechanical compaction and may therefore even protect the grains and primary intergranular pores (Worden et al., 2012; Zhong et al., 2012; French and Worden,2013; Li et al., 2013). Authigenic quartz is mainly composed of microcrystalline quartz (1-10 μm) and mesoquartz (10-20 μm). It is generally believed that authigenic quartz can inhibit quartz overgrowth,which is helpful to maintain the porosity of oil and gas reservoirs (Vagle et al., 1994; Weibel et al., 2010;Zhang et al., 2012).
Clay minerals are also widely distributed in sandstones, but their effects on porosity and permeability are still relatively unclear, which made them a ‘hot spot’ for current research on reservoir geology and form one focus for the present study.Kaolinite mainly precipitates in an acidic medium due to the dissolution of feldspar and unstable rock fragments (Cao et al.,2005; Shaw and Conybeare, 2009; Guo et al., 2013).Commonly, the formation of kaolinite leads to the blockage of pores and has a destructive effect on the physical properties of the reservoir (Cao et al., 2005;Gong et al., 2020). However, the appearance of kaolinite implies the occurrence of dissolution and the development of intercrystalline pores, which will be beneficial to increasing the reservoir micropores (Hu and Zhu, 2002; Chen et al., 2014b; Ma et al., 2017).The formation of illite in sandstone is mainly caused by the transformation of montmorillonite and kaolinite into illite during diagenesis (Bjørlykke and Aagaard,1992; Chuhan et al., 2000; Uysal and Golding, 2003;Huang et al., 2012). The formation of illite leads to severe damage to reservoirs by reducing the size and connectivity of pore-throats (Sun et al., 2019; Weibel et al., 2020). Authigenic chlorite is mainly formed in an environment that is rich in Fe2+and Mg2+. Graincoating and pore-lining chlorite mainly develop during the early to middle stages of diagenesis, because they occupy the crystalline basement and hinder the reaction between pore fluid and clastic particles (Sun et al., 2008; Chen et al., 2014a; Zhu et al., 2017),which can effectively restrain the quartz overgrowth,resist compaction and retain primary pores (Chen et al., 2009; Li et al., 2012; Nguyen et al., 2013; Zhu et al., 2017). Thin grain-coating and pore-lining chlorite commonly show excellent reservoir quality.On the other hand, thick grain-coating and pore-lining chlorite may block pore throats and drastically reduce permeability.Pore-filling chlorite is highly detrimental to reservoir quality(Worden et al., 2020; Zhou et al.,2020; Hansen et al., 2021; Griffiths et al., 2021).Studies on carbonate cementation show that calcite,formed during an early stage of diagenesis, can resist compaction of sandstone,while calcite,formed during a late stage, may fill pores and reduce porosity (Lv et al., 2009; Wang et al., 2014). All studies described above show that the content, crystal morphology,occurrence state of aggregates,and formation process of diagenetic minerals in the reservoir have an important and complex influence on the physical properties of the reservoir.
Previous researches on the Sulige gas field mainly focus on the important influence of different diagenetic processes on the reservoir densification or reservoir pores(Yang et al.,2012a;Chen et al.,2013;Carvalho and De Ros, 2015; Lai et al., 2016; Zhou et al., 2016). Compaction and cementation are the main factors for reducing porosity, while dissolution has a constructive effect on pores(Fan et al.,2016;Su et al., 2016; Cui et al., 2019). In contrast, so far, few researches were conducted regarding understanding the sources, controlling factors and symbiosis sequences of various diagenetic minerals and their influence on the evolution of pores and the physical reservoir properties in the Sulige gas field.Therefore,the aims of this contribution are:(1)to investigate the types of diagenetic minerals, their origin and the environmental conditions under which they originally formed; (2) to ascertain pore types and physical reservoir properties;and(3)to better understand the effects of diagenetic minerals on pore types, physical properties and the evolution of a reservoir.This work,integrating new information on diagenetic minerals and reservoir quality of sediments of the Sulige gas field, attempts to understand the diagenetic characteristics and sequence of these sandstones and provide insights into reservoir quality prediction for tight sandstone reservoirs.
2.Geological setting
The Ordos Basin(located between the coordinates 106.20°- 110.30°E and 34.1°- 41.3°N) is located in northwestern China and covers an area of approximately 250,000 km2(Fig. 1). The Helan, Liupan, Qinling, Lvliang, and Yinshan mountain ranges form the basin boundaries respectively in four directions. The Ordos Basin was uplifted by the Caledonian orogeny from Late Ordovician to Early Carboniferous (Dai et al., 2006; Yang et al., 2019). As a result, the basin underwent 130 million years of erosion, forming an ancient peneplain surface (Yang et al., 2012b; Xu et al., 2018). From Late Carboniferous to Late Permian, the Hercynian orogeny caused the subsidence of the Ordos Basin in Late Paleozoic which resulted in the basin's tectonic evolution and marine and continental changes.In addition,from Mesozoic to Cenozoic, the basin subsequently experienced the Indosinian, Yanshanian and Himalayan orogenies and eventually formed the present structural forms. The Ordos Basin can be subdivided into six first-order tectonic units(Fig.1):The Yimeng High in the north,the Weibei High in the south, the Jinxi Fold Belt in the East,the Yishan Slope in the center,and the Tianhuan Depression and the Western Thrust Belt in the west(Yang et al., 2005).

Fig. 1 A) Location and tectonic units of the Ordos Basin. The map of China is modified after the Standard Map Service of the National Administration of Surveying, Mapping and Geoinformation of China (http://bzdt.ch.mnr.gov.cn/) (No. GS(2019)1823); B) Composite stratigraphic column of the Permian in the Ordos Basin (modified from Fan et al., 2019). M−mountain.
The S120 Block is located in the west of the Sulige gas field which is located in the north-central part of the Yishan Slope, a structural unit that is inclined towards the west.During the Late Carboniferous until the Early Permian, the sedimentation was represented by alternating deposition of terrestrial and epeiric sea sediments. From Middle to Late Permian,sedimentation took place in a fluvial-deltaic to lacustrine environment adjacent to the ancient sea(Yang et al.,2014).Deposition within the Ordos Basin was dominated by continental fluvial, deltaic and lacustrine facies during Mesozoic times (Yang et al.,2014a,b, 2017; Fan et al., 2017,2018). The Permian Shanxi Formation and Lower Shihezi Formation are the main gas-bearing strata in the Sulige area. The Shanxi Formation is characterized by thickness of 90-110 m and can be divided from base to top into two members,P1s2and P1s1.P1s2consists of a group of quartz sandstones with thinly intercalated layers of siltstones, mudstones, and coal seams. P1s1is mainly composed of gray-white, medium to fine-grained sandstone that is sandwiched between carbonaceous and dark gray mudstones. The Lower Shihezi Formation is bounded by the Luotuobozi sandstone at its base and the Taohua mudstone at its top. The formation as a whole, however, is mainly composed of gray coarse sandstones, gray-white medium-grained sandstones, gray-green lithic quartz sandstones,and variegated mudstones. According to the sedimentary cycle, the Lower Shihezi Formation can be subdivided into four gas reservoirs from base to top:P2h8, P2h7, P2h6, and P2h5.
3.Materials and methods
All samples used in this study were collected from cores of 7 wells drilled in the Permian of S120 Block of the Sulige gas field (Fig. 2). 120 drill core samples were used for macroscopic description and measurements of their physical properties. The porosity and permeability of these samples were measured by a YRD-FKS-2 instrument under overburden pressure(500-8000 pounds per square inch) via nitrogen gas.Altogether, 127 thin sections with a standard thickness of 30 μm were produced from the collected samples. The thin sections were half-stained with Alizarin Red S and K-ferricyanide to distinguish calcite.As a result of chemical reaction,pure calcite turns red, which may be a purplish red in case the calcite contains iron.If iron-rich dolomite reacts with the solution,its color turns bright blue.Blue resin was used for thin sections to make the statistical analysis more convenient and allow an easier description of pore types, their occurrence, distribution characteristics, and contact relationships between the matrix particles. A Leica microscope was used under transmitted light using both plain polarized and crosspolarized light to examine the detrital compositions,matrix, and cement by counting at least 300 points per thin section.
A scanning electron microscope(SEM)was used at a temperature of 25°C and humidity of 50% to observe the pore structure, differentiate clay mineral types,and determine the mode of clay occurrence within the pore spaces.A total of 50 representative samples were examined with a Phenom Pro X scanning electron microscope. The performance indices of the instrument are the following: acceleration voltage (5 Kv-15 Kv),magnification(150,000×),resolution better than 8 nm;test conditions: voltage (15 Kv), vacuum degree(0.1 mbar).The surfaces of the trims were coated with platinum and gold to avoid electron charging effects.
Quantitative analysis of the elemental compositions for cement was obtained with a JXA-8230(JEOL)electron microprobe analyzer (EMPA), operating at an acceleration voltage of 15 Kv,1×10−8A beam current,and a beam diameter <5 μm. Backscattered electron(BSE) images were recorded from carbon-coated thin sections for the definition of the textural relationships between diagenetic phases.
4.Results
4.1. Petrological characteristics
The results from drill core observations show that the reservoir lithology of the Shanxi Formation in the S120 Block of the Sulige gas field is mainly represented by gravelly sandstones, gray coarse-grained sandstones, gray-green medium-grained sandstones, and light gray fine-grained sandstones, while lithology of the Lower Shihezi Formation reservoir is mainly characterized by gray gravelly sandstones, light gray coarse-grained sandstones, and gray-green mediumgrained and fine-grained sandstones (Fig. 3).
Grain size analysis and observations on castings and conventional thin sections show that the sandstones of the study area are predominantly medium-grained.The main particle size distribution ranges from 0.18 to 0.62 mm. The maturity of the clastic deposits is high. The degree of rounding of the particles ranges from subangular to subrounded.
The main type of sandstone in the study area is sublitharenite,followed by quartz arenite.In addition,there is a small amount of litharenite (Fig. 4A). The clastic composition of the reservoir (Table 1) is predominantly characterized by quartz. The content of feldspar is very small with most crystals being completely kaolinized. The lithic fragments mostly originate from metamorphic and igneous rocks.Only a few lithic fragments are derived from sedimentary rocks(Fig.4B).The sedimentary fragments are usually characterized as mudstone,argillaceous siltstone,and siltstone.

Fig. 2 Logged lithologies and sampling locations (red arrows) in the Permian of S120 Block of the Sulige gas field.
The pore space between the larger sand grains is occupied by matrix and cement (Fig. 4C). The matrix can generally be divided into two types:(1)terrigenous matrix and (2) tuffaceous matrix (Fig. 5). The content of the terrigenous matrix in the samples is relatively high.It appears brown under plane-polarized light and as scaly aggregates under cross-polarized light. A tuffaceous matrix can be seen locally.The cement is mainly composed of clay minerals (kaolinite, illite, chlorite),followed by siliceous and calcareous cements(Table 1,Fig. 4C). In the following sections, the cement will be described in details.
4.2. Diagenetic minerals
4.2.1. Kaolinite
Kaolinite is the most widely distributed diagenetic mineral within the analyzed samples,with an average content of 4.74%, mostly filling intergranular pores.The crystal shape of kaolinite is well developed,with a crystal diameter of ca. 10 μm and a thickness of<1 μm. The kaolinite exhibits a pseudo-hexagonal morphology. Aggregates of kaolinite are sheet-like or worm-like. In the study area, kaolinite can be subdivided into kaolinite formed by feldspar dissolution(Fig.6A),kaolinite formed by partial or complete alteration of the tuffaceous matrix (Fig. 6B), and authigenic kaolinite in pores (Fig. 6C).

Fig. 3 Cores of the sandstone reservoir in the Permian of S120 Block, Sulige gas field. A) Gravelly sandstone, Well S39, 3842.6 m, P1s1; B)Coarse-grained sandstone with unclear parallel bedding, Well S390, 3914 m, P1s1; C) Greenish-gray medium-grained sandstone, Well S176,3718.1 m, P1s1; D) Low-angle cross-bedding, fine-grained sandstone, Well S396, 3835.8 m, P1s2; E) Gravelly sandstone, Well S37, 3610.4 m,P2h6;F)Wedge cross-bedding,coarse-grained sandstone,Well S390,3816.0 m,P2h8;G)Greenish-gray medium-grained sandstone,Well S367,3579.0 m, P2h7; H) Parallel bedding, fine-grained sandstone, Well S396, 3782.6 m, P2h8.

Fig.4 A)Lithologic triangular plot of the Permian sandstones from S120 Block,Sulige gas field(modified from Folk,1980);B)Rock fragments component histogram of the Permian samples; C) Filler component histogram of the Permian samples.
4.2.2. Illite
The content of illite in the samples is relatively high.The mineral is mostly developed in sublitharenite and litharenite,but is rare in quartz arenite.It mainly fills the pores of the sandstones but did not grow in dissolution detritus and feldspar. Generally, there are three types of occurrence: (1) Most of the illite crystals are small and curved. Aggregates form semihoneycomb textures around clastic particles. Some of them are wrapped around the rock particles together with other diagenetic minerals that fill theperiphery of the illite crystals(Figs.6C and 7);(2)The curved lamellar illite between the particles is often mixed with clastic particles and other substances(Fig. 6D). This kind of illite is common in the study area;(3)The fibrous illite in the intergranular pores is partly connected to the nearby particles. Aggregates are reticulate fills, filling the pores together with kaolinite(Fig.6E)as the result of kaolinite illitization(Fig. 6F).

Table 1 Clastic and filler components of the Permian sandstone reservoir in S120 Block of Sulige gas field.

Fig. 5 A) Terrigenous matrix, Well S176, 3718.1 m, P1s1; B) Tuffaceous matrix, Well S37, 3780.6 m, P1s1.

Fig.6 Microfeatures of illite and kaolinite in the Permian sandstones in S120 Block,Sulige gas field.A)Dissolution of feldspar and formation of kaolinite, Well S37, 3719 m, P2h8, SEM; B) Alteration of tuffaceous matrix and formation of kaolinite, Well S37, 3782.2 m, P1s1, plane polarized light;C)Illite and kaolinite,Well S396,3838 m,P1s1,cross polarized light;D)Illitization of matrix,Well S390,3816 m,P2h8,SEM;E)Filamentous and reticulated illite, Well S390, 3822.3 m, P2h8, SEM; F) Illitization of kaolinite, Well S119, 3651.7 m, P2h8, SEM, Q: Quartz.
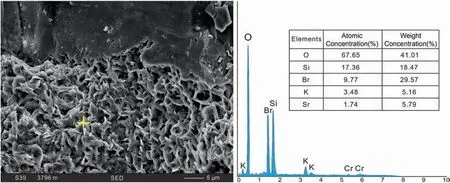
Fig. 7 SEM-EDS for semi-cellular illite of the Permian sandstones in S120 Block, Sulige gas field.
4.2.3. Chlorite
In the sandstones of the study area, chlorite cement appears to be relatively rare and is mainly distributed in the Shihezi Formation. Three types of chlorite cement were observed: (1) Grain-coating chlorite that grew around the sedimentary particles and wrapped around the particles in form of a thin film. The grain-coating chlorite is poorly crystallized with very small thicknesses of 1-5 μm.A shallow thin line can be seen at the contact of the particles under plane-polarized light(Fig.8A);(2)Pore-lining chlorite grew in places where there is no particle contact,often above the early grain-coating chlorite (Fig. 8A,B, D, E). Compared with grain-coating chlorite, the crystal shape of pore-lining chlorite is acicular or‘bamboo-leaf-like’. Its crystals are generally larger than 5 μm and are almost parallel arranged. The aggregates generally grow perpendicular from the surface of the particles towards the pore space;(3)Porefilling chlorite is relatively rare within the samples and often fills the pores together with kaolinite. This kind of chlorite exhibits large crystal sizes,well-developed crystal shapes, and crystal directions that are not related to the particle surface (Fig. 8C). In addition,the crystals may form flakes or rose-shaped aggregates; (4) The chlorite formed due to an alteration of the existing biotite in the Shanxi Formation (Fig. 8F).
4.2.4. Siliceous cement
Siliceous cement is one of the most common cements in the analyzed samples.Two forms of siliceous cements were observed in the samples: (1) Quartz overgrowth is the most common form of siliceous cementation, which is usually distributed around the quartz grains and grew coaxially with quartz particles.A clear “dirty line” (Fig. 9A) can usually be observed between the original clastic quartz grains and quartz overgrowth. The width of the quartz overgrowth is between 20 μm and 70 μm, with straight edges, indicating a large amount of growth space during the quartz overgrowth.All quartz overgrowth occurrences in the study area developed almost at the same diagenetic stage; (2) In comparison, the content in authigenic quartz is less and can only be observed in a few samples. The authigenic quartz can generally be found on top of the grain-coating chlorite. There are two stages of authigenic quartz crystals in the samples(I and II).The authigenic quartz crystals I are generally smaller, i.e. less than 10 μm. In contrast, the authigenic quartz crystals II are larger with sizes greater than 20 μm(Fig. 9B, C).
4.2.5. Carbonate cement
Most of the carbonate cement in the samples is stained purplish red after Alizarin Red S staining, indicating that it is iron-rich calcite.Three types of calcite cement were observed within the study area: (1)Calcite fills intergranular pores and thereby occupies the central part of the pores. In these cases, calcite,siliceous cement,and clay films are grown sequentially from the pores to the edge of the particles(Fig.9D).(2)Calcite fills almost the whole pore in the form of pore cementation (Fig. 9E). (3) Calcite replaces kaolinite,illite,and siliceous cements(Fig.9E,F).

Fig. 8 Chlorite and siliceous cement in the Permian sandstones from S120 Block, Sulige gas field. A) Grain-coating chlorite and pore-lining chlorite, Well S37, 3640.3 m, P2h6, plane polarized light; B) Pore-lining chlorite and authigenic quartz, Well S37, 3640.3 m, P2h6, plane polarized light;C)Pore-filling chlorite,Well S176,3658 m,P2h8,plane polarized light;D)Chlorite and authigenic quartz,Well S37,3640.3 m,P2h6, SEM; E) Detail of grain-coating chlorite and pore-lining chlorite from the yellow boxed area in D, Well S37, 3640.3 m, P2h6, SEM; F)Chloritization of biotite, Well S396, 3835.8 m, P1s1, plane polarized light. Q: quartz, Rd: rock debris.

Fig. 9 Siliceous cement and calcite cement in the Permian sandstones from S120 Block, Sulige gas field. A) Quartz overgrowth, Well S39,3784 m,P2h8,cross polarized light;B)Formation of authigenic quartz outside grain coating chlorite,Well S37,3640.3 m,P2h6,cross polarized light; C) Chlorite and authigenic quartz (I and II), Well S364, 3664.8 m, P2h8, SEM; D) Quartz overgrowth and calcite cement, Well S119,3651.7 m,P2h8,cross polarized light;E)Calcite cement and replacement of quartz(yellow arrow),Well S39,3801.7 m,P2h8,cross polarized light; F) Calcite cement and replacement of kaolinite, Well S39, 3801.7 m, P2h8, plane polarized light, Q: Quartz.
4.3. Physical reservoir properties

Fig. 10 Pore types of the Permian sandstone reservoir in S120 Block, Sulige gas field. A) Residual intergranular pore, Well S37, 3640.3 m,P2h6,plane polarized light;B)Kaolinite intercrystalline pore,Well S364,3664.8 m,P2h8,plane polarized light;C)Illite intercrystalline pore,Well S39,3801.2 m,P2h8,BSE;D)Intragranular dissolved pore,Well S396,3784 m,P2h8,plane polarized light;E)Matrix dissolved pores,Well S37, 3782 m, P1s1, plane polarized light; F) Microfracture, Well S37, 3640.3 m, P2h6, plane polarized light, Q: Quartz, Rd: rock debris
The pores within the samples include primary and secondary pores. The primary pores are mainly intergranular (residual intergranular pores). Primary intergranular pores are filled by well-developed minerals during diagenesis, while some primary intergranular pores are still preserved, mostly irregular,triangular,or polygonal(Fig.10A).Secondary pores are widely distributed in the study area, including intergranular pores, dissolved pores, microfractures,etc. The intercrystalline pores (mainly the pores between diagenetic minerals) predominantly include kaolinite (Fig. 10B) and illite intercrystalline pores(Fig. 10C). Dissolved pores are formed by the dissolution of clastic particles,diagenetic minerals,and parts of the matrix in the process of diagenesis. There are mainly lithic-dissolved pores (Fig. 10D), feldspardissolved pores, matrix-dissolved pores (Fig. 10E) and calcite solution pores in the study area. Lithicdissolved pores were observed predominantly within the studied sublitharenites. The contents of feldspar dissolved pores and calcite dissolved pores are very small and were only observed in individual thin sections. The matrix-dissolved pores are more developed and widely distributed in the samples containing volcanic material and a tuffaceous matrix.Microfractures are not widely developed in the study area(Fig.10F).Statistical analysis of the pore types and the plane porosity in the study area shows that the plane porosity of the Shanxi Formation is lower than that of the Lower Shihezi Formation. This is particularly true for the content of residual intergranular pores, lithic dissolved pores and matrix dissolved pores (Fig. 11A).
Porosity and permeability are important index to indicate whether the physical properties of the reservoir are good or bad. Logging data within the study area show that the porosities of the analyzed reservoir rocks range from 2.01% to 13.34% (Table 2)with the main distribution range as 3%-7% (Fig. 11b).The permeabilities range between 0.01 and 1.76 mD(Table 2), and the main distribution ranges in 0-0.5 mD(Fig.11C).According to the porosity-permeability relationship diagram (Fig. 11D) of the samples within the study area,there is a positive correlation between porosity and permeability (correlation coefficient R2= 0.5), indicating that the reservoir is a porous reservoir with high pore connectivity.
4.4. Electron microprobe analyzer (EMPA)data
4.4.1. Matrix
The EMPA data of the matrix in the study area(Fig. 12) shows that the content of FeO in the tuffaceous matrix is slightly higher than that of the terrigenous matrix, while the content of K2O is slightly lower. The content of K2O, FeO, SiO2, and Al2O3are relatively high in both the terrigenous and tuffaceous matrices.
4.4.2. Diagenetic minerals
The EMPA results of kaolinite (Table 3) show that,the average SiO2and Al2O3contents are 45.87 wt%and 38.30 wt%,respectively.Other impurities are less than 0.6 wt%, which is similar to the chemical composition of standard kaolinite (Teng et al., 2009), indicating that the kaolinite is pure.
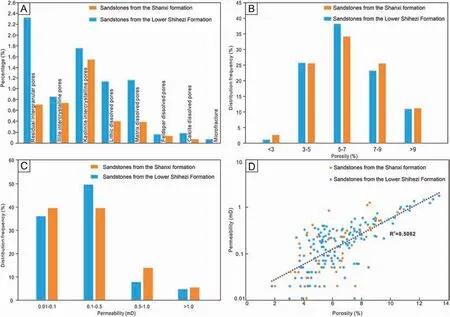
Fig.11 A)Types and ratios of pores in the Permian sandstone reservoirs from S120 Block,Sulige gas field;B)Histogram showing the porosity distribution in the Permian sandstone reservoir in S120 Block, Sulige gas field; C) Histogram showing the permeability distribution in the Permian sandstone reservoir in S120 Block,Sulige gas field;D)Cross plot of the relationship between porosity and permeability of the Permian sandstone reservoir in S120 Block, Sulige gas field.

Table 2 Physical properties of the Permian sandstone reservoir in S120 Block, Sulige gas field.
The EPMA data of measured chlorite show that it primarily consists of Al2O3, FeO, SiO2, and MgO, with small amounts of Na2O,K2O,CaO,and MnO,and some impurities (e.g. TiO2) (Table 4). The chemical compositions of the different forms of chlorite are slightly different, from the grain-coating chlorite with poor crystal shape near the particle surface to the porelining chlorite or pore-filling chlorite with better crystal shapes near the center of the pores.There are no obvious differences in Al2O3between chlorites with poor crystal shape that formed at the edge of a sedimentary particle and the chlorites with better crystal shapes that formed in the center of a pore.Content of FeOTand MnO increased gradually from the former to the latter, while MgO only slightly increased. Content of SiO2decreases gradually from the inside towards the outside, and K2O can be observed to decrease from chlorites with poor crystal shapes to chlorites with well-developed crystal shapes (Fig. 13).

Fig. 12 Major elements for matrix of the Permian sandstones in S120 Block, Sulige gas field.

Table 3 Major elements for kaolinite of the Permian sandstone reservoir in S120 Block, Sulige gas field.
4.5. Paragenetic sequence
Based on textural and contact relationships between primary and secondary minerals,the sequence of diagenetic mineral formation within the analyzed sandstones or paragenetic sequence were reconstructed (Yang et al., 2014a,b; Shao et al., 2019).However, the exact time and duration of diagenesis was not completely inferred. Grain-coating chlorite can be observed at the contact of some clastic particles, indicating that the formation of grain-coating chlorite was earlier than that of mechanical compaction. Pore-lining chlorite can be found on the periphery of grain-coating chlorite (Fig. 8A, B, D, E),indicating that the pore-lining chlorite was formed after the compaction and formation of the graincoating chlorite.
Authigenic quartz grew on pore-lining chlorite(Fig. 14A), demonstrating that authigenic quartz formed later than pore-lining. Chlorite was found on some authigenic quartz (Fig. 14B, C), which implies that the chlorite grew for a relatively long time until finally the authigenic quartz started to develop. The kaolinite primarily formed in the periphery of porelining chlorite (Fig. 14D) and is commonly partly surrounded by quartz overgrowth (Fig. 14E). This shows that the formation of kaolinite was later than that of the pore-lining chlorite and earlier than the quartz overgrowth.Illite is commonly seen overlying kaolinite(Fig. 14F), and part of the kaolinite underwent illitization (Fig. 6F), which shows that illite was formed later than kaolinite.Furthermore,pore-filling chlorite is interspersed in the intercrystalline pores of kaolinite(Fig. 14G), also indicating that the formation of porefilling chlorite was later than that of kaolinite. Clay minerals in pores are often symbiotic with authigenic quartz (Fig. 14H, I), showing that siliceous cementation experienced a long time.Iron-rich calcite fills the remaining pores in the form of mosaic cementation and even replaces a part of the earlier quartz cement and kaolinite(Fig.9D,E,F).This is evidence that this mineral formed last.
4.6. Diagenetic stages
Based on the diagenetic sequence described above and existing data on the burial environment (Zhang et al., 2009; Wang and Fan, 2010) of the study area,it can be assumed that the study area is currently in a late diagenetic stage.In the following paragraphs,the different diagenetic stages (Fig. 15) will be discussed in detail.
4.6.1. Early diagenetic (eodiagenesis) stage A
During the deposition of the Shihezi and Shanxi formations,the sedimentary environment of the study area was a river delta environment, and correspondingly a large amount of the dissolved Fe and Mg that was carried by the river started to flocculate and precipitate at the estuary, promoting the formation of grain-coating chlorite.This early diagenesis took place during a stage characterized by the existence of immature organic matter. Due to the mixing of pyroclastic material (originating from volcanic eruptions)and mud, the sandstones in the study area are rich in fragments of igneous rocks and contain a tuffaceous matrix that underwent devitrification and hydration to form montmorillonite.In addition, at this early diagenetic stage,feldspar began to dissolve,forming a small amount of kaolinite. In general, at this stage, the sedimentary particles can be assumed to have formed line contacts, which characterizes the beginning mechanical compaction of the still loose sediment,resulting in the formation of an unconsolidated to semiconsolidated sedimentary rock. The pore types that developed at this stage were mainly primary pores with cements that mainly comprised of grain-coating chlorite,montmorillonite,and a small amount of kaolinite.
4.6.2. Early diagenetic (eodiagenesis) stage B
During this period, the evolution of organic matter was in the semi-mature stage, and the level ofcarboxylic acid, produced by humus, increased. The carboxylic acid mixed with freshwater (groundwater)to form acidic pore waters, which promoted the dissolution of unstable aluminosilicate minerals(feldspar, mudstone fragments, etc.) in sandstone,eventually leading to the formation of kaolinite and early siliceous cements. Meanwhile, a large amount of Fe,Mg,and Si was formed due to the illitization of montmorillonite. In addition, the organic acids also caused the dissolution of volcanic material (igneous rock fragments, tuffaceous matrix, etc.), which provided a basis for the formation of pore-lining chlorite. In conclusion, during this stage, the sediment particles were mainly in point and line contact with each other. Furthermore, the compaction gradually increased, leading to the formation of semiconsolidated rocks. In addition, a small number of secondary pores started to develop. The cement that formed at this stage was mainly kaolinite with small amounts of illite, pore-lining chlorite, and siliceous cement (authigenic quartz and quartz overgrowth).

Table 4 Major elements for chlorite of the Permian sandstone reservoir in S120 Block,Sulige gas field.

Fig.13 BSE images and EMPA data showing the chemical composition of pore-lining chlorite from near-grain boundaries(poorly crystallized)to center of pores(euhedral-crystallized).A)Well S37,3640.3 m,P2h6;B)Well S390,3822.3 m,P2h8;C)Well S39,3801.7 m,P2h8,Q:Quartz.
4.6.3. Middle diagenetic (mesodiagenesis) stage A to stage B
At this stage, organic matter attained a range between low and high maturity. Commonly, during mesodiagenesis,large amounts of CO2are released during the transformation of organic matter into hydrocarbons,making the pore fluid strongly acidic.Carboxylic acid ions can enhance the complexation of Al,compaction, clay transformation, and dissolution,resulting in the dissolution of aluminosilicate skeleton particles,matrix,and volcanic components,which,in turn, results in the formation of large amounts of kaolinite and siliceous cements.A part of the siliceous cement that was produced by dissolution may form the quartz overgrowth,which occasionally wraps kaolinite cement within the study area. Alternatively, the siliceous cement may form authigenic quartz.

Fig.14 A)Chlorite and authigenic quartz,Well S37,3640.3 m,P2h6,SEM;B)Chlorite grows on authigenic quartz,Well S364,3664.8 m,P2h8,SEM;C)The detail of the yellow boxed area in B,Well S364,3664.8 m,P2h8,SEM;D)Kaolinite developed on the outside of chlorite,Well S364,3664.8 m,P2h8,SEM;E)Quartz overgrowth wrapped the kaolinite crystal,Well S37,3725.9 m,P2h8,cross-polarized light;F)Kaolinite covered with illite,Well S37,3782.2 m,P2s1,SEM;G)Chlorite is interspersed in the intercrystalline pores of kaolinite,Well S390,3822.3 m,P2h8,BSE;H)Kaolinite,illite,chlorite and authigenic quartz,Well S39,3802.3 m,P2h8,SEM;I)Detail of the yellow boxed area shown in H,showing illite and chlorite growing on authigenic quartz, Well S39, 3802.3 m, P2h8, SEM, Q: Quartz.
When the burial temperature exceeds 120°C,large amounts of K-feldspar starts to dissolve and release K+,which promotes the illitization of kaolinite. At the same time,the illitization of montmorillonite stops.To sum up, at this stage, sedimentary particles are commonly in line contact or concave-convex contact with each other. Primary intergranular pores are formed and a large number of secondary pores appear,which are mostly formed by the dissolution of feldspar,lithic fragments, and tuffaceous and terrigenous matrix.During this period,the cement is mainly composed of authigenic kaolinite,illite,and silica(quartz).4.6.4. Late diagenetic (telodiagenesis) stage
At this stage,organic matter is characterized by a high maturity to over-maturity. Commonly, large amounts of organic acids are already consumed and the ability of the source rocks to produce organic acids reduced. This results in the decrease of the organic acid concentration and the gradual transformation of the diagenetic fluids to alkaline conditions. Fe and Mg that were previously released by the dissolution of volcanic materials, illitization of kaolinite, and illitization of montmorillonite (and could not be transferred to shallower depths with lower P-T conditions)may become locally enriched to form pore-filling chlorite. At the same time, chloritization of kaolinite may begin to occur in this fluid medium.With the alkaline fluid in the pores gradually becoming enriched in Ca, Mg, and Fe, iron-bearing calcite may start to precipitate and fill the residual intergranular pores at this stage, as can be seen in some of the analyzed samples,and which corresponds to the final products of the diagenetic evolution within the study area.
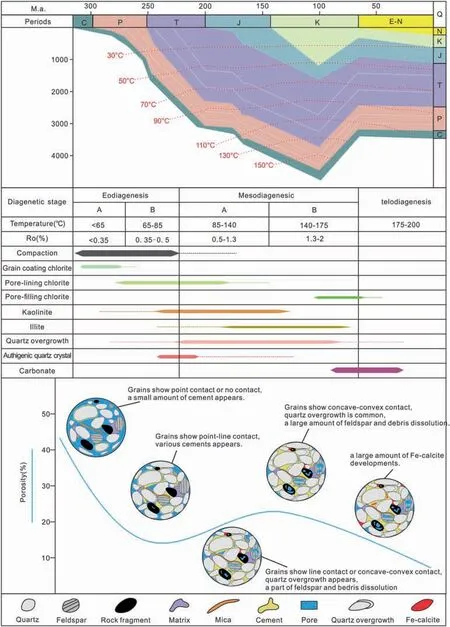
Fig.15 Burial history and paragenetic sequence of the Permian sandstones in S120 Block,Sulige gas field(modified from Han et al.,2020).
5.Discussion
5.1. Cement-forming processes
5.1.1. The formation of kaolinite
The formation of authigenic kaolinite in sandstone is generally considered to be the product of an acidic medium, and its formation is often related to the dissolution of aluminosilicates such as feldspar (Ding et al.,2014; Bjørlykke and Jahren, 2015).
The strata underlying the sandstone within the study area are large-scale coal seams. As a result of burial diagenesis, the organic matter of this coal started to mature, leading to the production of large amounts of organic acids.Eventually,these acidic fluids reacted with the aluminosilicates in the overlying sandstone to form kaolinite.Although the sandstone in the study area underwent structural uplift after deposition during the Cretaceous,it was not exposed.
According to the thermodynamic characteristics,the standard Gibbs free energy of feldspar dissolution in an acidic medium is less than zero.Anorthite exhibits the lowest standard Gibbs free energy,which shows an increase with increasing temperatures. This indicates that anorthite dissolves at lower temperatures.During the early diagenetic stage,the diagenetic environment is open to semi-open.The sandstone in the study area can predominantly be characterized as continental sandstone without an additional source of K+ions(Huang et al., 2012). The K+ions that were released from volcanic material were distributed together with the pore water.Therefore,it was difficult to reach the critical concentration of potassium ions needed for illite precipitation.In consequence, anorthite reacted with the acid fluids to form kaolinite. The corresponding reaction equation is as follows:
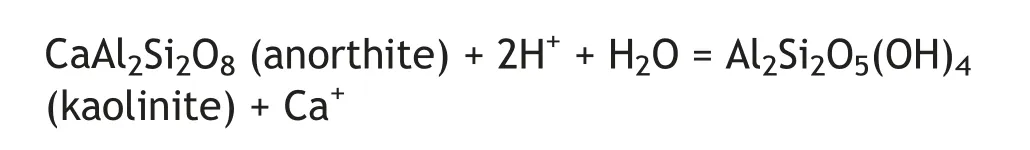
In contrast, the standard Gibbs free energy of Kfeldspar decreases with increasing temperatures.This indicates that K-feldspar is more likely to dissolve at higher temperatures. In sedimentary basins, the dissolution of K-feldspar often occurs in the temperature range of 100-120°C (Wilkinson et al., 2001). A large amount of organic acid was released from organic matter, and K-feldspar dissolved and released K+ions into the pore water. If an illitization of montmorillonite would have occurred, a large amount of these K+ions would have been consumed during this process. Therefore, between 100 and 120°C, K-feldspar dissolved to form kaolinite rather than illite.The reaction equation is written as follows:

This is consistent with the precipitation of kaolinite adjacent to dissolved feldspar,which can be observed under SEM (Fig. 6A). Additional sources for kaolinite include dissolution and transformation of the tuffaceous matrix in acid pore fluids (Fig. 6B) and the precipitation of authigenic kaolinite (Fig. 6C) from pore water at burial temperatures above 100°C. The existence of dickite (Fig. 14D) in the study area can be explained by the increase in burial depth and temperature that led to a change in the structure of some of the kaolinite and a gradual coarsening of the crystals from flake-shaped to blocky particles, and finally resulted in the formation of dickite (Beaufort et al.,1998; Cuadros et al., 2014).
5.1.2. The formation of illite
Illite is a common clay mineral in clastic rocks and usually forms at the mesogenetic stage. The different occurrences of illite in the study area indicate the different controlling factors in the process of illite formation. The semi-honeycomb illite, which wraps some of the clastic particles, may be related to the transformation from montmorillonite. During the temperature range of 70-120°C,the montmorillonite within the studied sandstones simultaneously reacted with the K+ions to form illite (Cuadros, 2006). This chemical reaction can be expressed as follows:

Commonly, the illitization of montmorillonite preserves some of the original characteristics of the montmorillonite aggregates, for instance, a semihoneycomb shape (Fig. 7). The curved flake illite,which is often mixed with clastic particles, may have been formed by the illitization of the matrix. During the deposition in the study area, a large number of volcanic fragments (lithics, tuff, etc.) formed part of the matrix of the sandstones. These particles started to release K+ions during diagenesis, which interacted with pore fluids, and finally led to the formation of illite.As the matrix is not completely dissolved,illite is often mixed with other particles,which were the main source of illite in the study area.The observed illite in the analyzed samples (usually fibrous and reticulated in pores) is mostly interpreted to originate from the transformation of kaolinite.As the burial temperature continued to increase within the analyzed strata, the illitization of montmorillonite (>120°C) stopped,changing to a pervasive illitization of kaolinite at temperatures above 130°C (Bjørlykke,2014). In addition, the pore space collapsed and the organic acid in the pore fluid gradually decreased, which also promoted the beginning of illitization of kaolinite. The reaction equation is as follows:

Under plane-polarized light, it can be observed that the outline edge of the sheet-like kaolinite is blurred.Furthermore,many fibrous edges of kaolinite can be seen under SEM, clearly providing additional evidence for this hypothesis(Fig.6D,F).Therefore,it can be assumed that at the stage of illite formation,after the relatively shallow burial in the study area,the content of K-feldspar was less than that of kaolinite. The burial was not enough to cause all kaolinite to transform to illite, which led to the coexistence of kaolinite and illite in the study area.
5.1.3. The formation of chlorite
The EMPA data show that the content of FeOTin chlorite with a good crystal shape (Chl-a) is significantly higher than that in chlorite with a poor crystal shape (Chl-b). Therefore, the content of FeOTcan be used as a marker to distinguish between Chl-a (more than 27 wt%) to Chl-b (less than 27 wt%). In consequence, the correlation between FeOTand other oxides in chlorite needs to be analyzed and interpreted.The results of this analysis show that SiO2, TiO2, CaO,Na2O, and K2O are negatively correlated with FeOT(Fig.16).Chl-a and Chl-b form distinct clusters in SiO2,CaO, and K2O vs. FeOT. Although the content of CaO,K2O and TiO2are low,the correlation coefficient(R2)is high,i.e.0.70,0.77 and 0.51 respectively.The results show that the pore water contains a lot of Ca2+and K+that were released by feldspar and volcanic material,during the formation of Chl-b (Bahlis and De Ros,2013). MnO vs. FeOTand MgO vs. FeOTexhibit relatively low correlation coefficient with R2(Mn)=0.0236 and R2(Mg)=0.0353,respectively.Distinct clusters are missing in these major element oxides.The contents of SiO2and Al2O3(the main components of chlorite) in chlorite are high. SiO2shows a strong negative correlation with FeOT, while the correlation of Al2O3with FeOTis less apparent. This is related to the different positions of Si and Al in the chlorite crystal lattice.
As the Al ions in chlorite can be divided into AlIV(in a tetrahedron) and AlVI(in an octahedron), it is necessary to analyze the correlation of various ions in chlorite (Fig. 17). Therefore, based on the 28 oxygen equivalents,the molecular formulas of different forms of chlorite are calculated from our EMPA data(Table 4).The molecular formula of Chl-b is (Fe4.25Al4.55Mg1.06-Ca0.04K0.36Na0.04)Σ=10.3(Si6.95Al1.05)8O20(OH)16.The molecular formula of Chl-a is (Fe6.52Al3.53Mg1.28Ca0.01-K0.02Na0.02)Σ=11.38(Si5.45Al2.546)8O20(OH)16.The analysis shows that the octahedral occupancy is deficient within the crystal lattice of the samples,i.e.there is less than the ideal total of 12 cations for a fully trioctahedral chlorite and metamorphic chlorite. In fact, the measured cations for Chl-b are 10.3,and for Chl-a are 11.38. Therefore, it can be assumed that the total number of chlorite cations with a good crystal shape is closer to the ideal state. The Si/Al ratio decreases,accompanied by an increase of AlIV,from Chl-b to Chl-a.In almost all cases, the octahedral Al (AlVI) is higher than the tetrahedral Al(AlIV),i.e.n(AlVI)/n(AlIV)=4.32 in Chl-b and n(AIVI)/n(AIIV)=1.39 in Chl-a.This shows that the octahedral AlVIin chlorite with a good crystal shape is replaced by other cations more frequently.By analyzing the correlation between octahedral AlVIand Fe and Mg,it can be seen that from Chl-b to Chl-a,AlVIis negatively correlated with Fe and Mg, with correlation coefficient of R2(Mg)=0.26 and R2(Fe)=0.75.This indicates that octahedral AlVIin good crystalline chlorite is mainly replaced by Fe.
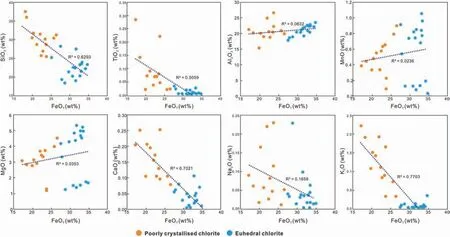
Fig.16 Correlation of major elements and FeOT for chlorite with different crystal shape in sandstone reservoirs,from the Permian of S 120 Block, Sulige gas field.
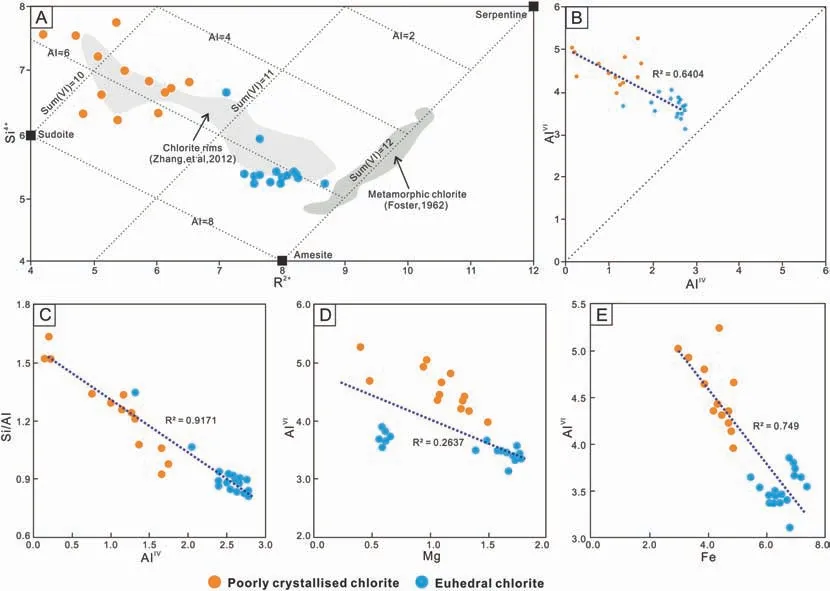
Fig. 17 Correlation of main cations for chlorite with different crystal shape. A) The structural formula of chlorite is plotted in the trioctahedral region of the vector representation of chlorite compositions (Wiewi′ora and Weiss, 1990) with Si and R2+ as orthogonal axes and octahedral occupancy and total Al shown by contours; B) The grey dotted line has a slope of 1. AlIV:aluminium in tetrahedral coordination;AlVI:aluminium in octahedral coordination;R2+:the total content of Fe,Mg and Mn;Sum(VI):octahedral occupancy;C)Correlation of Si/Al and AlIV; D) Correlation of AlVI and Mg; E) Correlation of AlVI and Fe.
The differences in crystal morphology and element changes of chlorite described above may be caused by the influence of the burial environment during diagenesis (Jahren and Aagaard, 1989; Hillier and Velde,1991;Ehrenberg,1993; Zhang et al.,2012b).It has been noted that there is a systematic decrease in Si, AlVI, and Si/Al, and an increase in octahedral occupancy, (Fe + Mg) and AlIVwith increasing depth or temperature in a diagenetic system(Hillier and Velde,1991; Jahren, 1991; Caritat and Walshe, 1993).Furthermore the increase of (Fe + Mg) mainly appears as an increase in Fe content, as Fe ions with a larger radius can occupy the octahedral sites more easily than Mg ions with increasing temperature (Caritat and Walshe, 1993). The chlorite in the study area also shows this trend.Grain-coating chlorite often develops in sedimentary environments and particularly forms during an early phase when particles are not or only slightly in contact with each other. Given a sufficient growth space and the addition of Fe and Mg,provided by the river, chlorite crystals can start developing within the pore fluid. Due to the strong pore fluid activity, chlorite crystals do not grow completely perpendicular to the particle surface. At the same time,chlorite crystals occupy a large number of clastic particle surfaces within a short time. If the growth rates are too high and too many crystals are growing at the same time, this leads to a restriction in growth,which results in poor crystal shapes and a relatively disorderly arrangement of grain-coating chlorite.Furthermore,due to low temperatures and pressures in the diagenetic environment, the hydration of Fe and Mg ions is so strong that it is difficult to enter the crystal lattice(Wu et al.,2020).Therefore,the content of Fe and Mg,the occupation rate of the octahedron,and the degree of crystallization are poor in grain-coating chlorite. The Fe and Mg of pore-lining chlorite mainly originate from mafic volcanic fragments, which are commonly rich in these elements. During the stage of pore-lining chlorite formation, the porosity and permeability of sandstone,as well as the temperature and pressure, are relatively high and the source material is abundant.If there is sufficient space and time for crystal growth, more Fe and Mg can enter the crystal lattice, and the occupation rate of AlVIincreases, forming better crystal shapes and an orderly arrangement of the crystals. The material source and formation mechanism of the pore-filling chlorite is similar to that of the pore-lining chlorite.Furthermore,the Fe and Mg ions that are released by the illitization of kaolinite and montmorillonite provide an additional source for pore-filling chlorite.
In consequence, after chlorite formation, the temperature and pressure conditions of the diagenetic environment are relatively high, the growth rate of pore-filling chlorite is lower, and the number of crystals is greatly reduced. In addition, Fe ions have sufficient time to replace cations in the chlorite lattice.Therefore,the content of Fe is higher,the octahedron occupancy is close to ideal, and the crystal form of chlorite is intact. However, at this stage, the porosity and permeability of sandstone are relatively low, the material transport is slow,and the sources of Fe and Mg are reduced. As a result, the development of filled chlorite is relatively low in the study area.
5.1.4. The formation of siliceous cement
There are many studies on the siliceous sources of quartz cement in sandstone,such as the transformation of biological silica and clay minerals, the pressure solution of clastic quartz particles,and the dissolution of unstable igneous rock fragments and feldspars(Hendry and Trewin,1995;Kampvan de.,2008;Peltonen et al.,2009; Thyberg and Jahren, 2011; White et al., 2011;Chen et al., 2019). However, for different sandstones,there are relatively unique sources of silica,which may be affected by many factors, such as clastic composition, sedimentary structures, reservoir structure, and so on (Molenaar et al., 2007). No siliceous sponge needles or other evidence for biogenic SiO2were found within the study area. Therefore, biological silica may be disregarded as a possible source for quartz cement.However,according to the optical observations and SEM analysis of the samples in the study area, the quartz cement may come from dissolved feldspar. Feldspar dissolves and releases a large amount of SiO2under acidic conditions, which is one of the sources of siliceous cement. The fragments in the sandstone of the study area dissolved and released not only Fe and Mg ions but also large amounts of silica, which formed another source of siliceous cement in the study area.An alternative silica source for the siliceous cement may have been the SiO2that was released by clay mineral transformation as illitization of montmorillonite and kaolinite continuously released SiO2(Metwally and Chesnokov, 2012). Therefore, the montmorillonite in the studied sandbodies and adjacent mudstones may be one of the sources for the siliceous cement.
5.1.5. The relationship between chlorite and siliceous cement
There are different views on the mechanism of chlorite affecting quartz cement (Bahlis and De Ros,2013; Lai et al., 2016). In the present study, it can be seen that the formation of quartz cement is closely related to chlorite formation.According to the growth relationship between chlorite and siliceous cement in the study area, the effect of chlorite on the siliceous cement can be divided into four cases (Fig. 18): (A)Grain-coating chlorite wrapped the surface of clastic particles and thereby inhibited the formation of authigenic quartz by occupying the nucleation points on the surface of the particles.In places where graincoating chlorite is missing, silica concentrates and readily forms authigenic quartz.The authigenic quartz grows along the grain surface in a consistent crystal direction, which may easily merge into large crystal units or form quartz overgrowth. Then, due to the continuous growth of chlorite, the quartz overgrowth may be relatively narrow (Fig. 18A). (B) Where both grain-coating chlorite and pore-lining chlorite are developed, pore-lining chlorite is thicker and exhibits better developed crystal shapes. Furthermore, it can often be found growing nearly vertically along the edge of the particles.Authigenic quartz developed on the chlorite with random crystal directions, which made it more difficult for the quartz to grow into large crystal units than authigenic quartz with the same crystal orientation. Simultaneously, the chlorite,growing on the authigenic quartz crystals, also hindered the formation of quartz overgrowth.In this case.Siliceous cement occurs in the form of authigenic quartz crystals (Fig. 18B). (C) When chlorite and authigenic quartz grow together along the particle surface, and a part of the chlorite is interspersed between authigenic quartz crystals, it may block their mutual contact with each other and limit their growth.Nevertheless, the authigenic quartz that was not blocked by chlorite could merge into larger crystals that eventually continue to grow (Fig. 18C). (D) If chlorite is not developed, authigenic quartz crystals with the same crystal orientation grow along the particle surface and are not blocked by chlorite. Therefore, it is easier to combine into larger crystal units,and then merge to grow to form a wider quartz overgrowth(Fig. 18D).

Fig. 18 Mechanism of chlorite affecting siliceous cementation (modified from Weibel et al., 2010) A) Authigenic quartz with the same direction grows on where chlorite is absent (yellow arrow),and chlorite grows on authigenic quartz(red arrow),Well S364, 3682.9 m, P2h8,SEM;B)Authigenic quartz with random direction(red arrow)and chlorite,which continues to grow on authigenic quartz(yellow arrow),Well S39, 3651.7 m, P2h8, SEM; C) Chlorite blocks the binding of authigenic quartz, authigenic quartz (yellow arrow), chlorite (red arrow), Well S39, 3804.8 m, P2h8, SEM; D) Quartz overgrowth, Well S396, 3837.7 m, P1s1, plane polarized light, Q: Quartz.
5.1.6. The formation of calcite cement
In the study area, calcite is mostly purplish red after alizarin red staining.Most of the calcite replaces quartz cement and kaolinite, which indicates that calcite formed during the late diagenetic stage and is often related to organic acid decarboxylation. With the increase in burial depth, organic acids began to remove carboxyl groups under thermal catalysis and released large amounts of CO2. The dissolution of volcanic fragments and tuffaceous matrix in the study area, as well as the discharge of Fe, Mg, and Ca ions from silicate minerals and clay minerals during diagenesis, buffered the pH of the pore water and thereby provided a material basis for the precipitation of the iron-bearing calcite. The research data of formation water also show that there are Ca ions (Tian et al., 2020) in the water of the study area, which eventually led to the formation of iron calcite.
5.2. The diagenetic difference between the Shanxi Formation and the Lower Shihezi Formation
The results of this contribution show that the Shanxi and the Lower Shihezi formations exhibit similar contents in clastic particles and types of diagenetic minerals. Nevertheless, the Shanxi Formation contains smaller particle sizes, a higher matrix content and fewer residual intergranular pores in its sandstones (Table 1, Fig. 11). This indicates that the compaction resistance of the Shanxi Formation is weak, and correspondingly, its porosity is lower than that of the Lower Shihezi Formation (Table 2). When comparing the diagenetic mineral content of the two formations, the Shanxi Formation exhibits higher contents in kaolinite, illite and siliceous cement than the Lower Shihezi Formation(Table 1, Fig. 4A).
The Lower Shihezi Formation predominantly contains grain-coating chlorite, pore-lining chlorite and pore-filling chlorite,whereas the chlorite of the Shanxi Formation primarily formed due to diagenetic alteration of the preexisting biotite. In the Lower Shihezi Formation, a lower matrix content and more residual intergranular pores provided nucleation locations and growth space for chlorite.In contrast,the high matrix content in the Shanxi Formation covered the surface of the sand grains and filled the pore space in between the grains, which strongly inhibited the growth of chlorite. Correspondingly, the absence of graincoating chlorite and pore-lining chlorite within the Shanxi Formation resulted in the formation of large amounts of quartz overgrowth.
The lower part of Shanxi Formation was characterized by a large number of mudstones and coal seams.Both rock types exhibit high contents in organic acid and complex anion and complex aluminum elements within these organic acids (Zhang et al., 2021)that led to an upwards migration of Al-rich pore waters to the sandstone of the Upper Shanxi Formation.However,due to the high matrix content,a weak anticompaction degree, and few residual intergranular pores,the pore water of the Shanxi Formation did not flow smoothly. During flowing, the organic acid was consumed and the ability of Al complexation was weakened in the process of migration,which led to the enrichment of kaolinite in the sandstones in the middle and upper part of the Shanxi Formation. The high matrix content of the Shanxi Formation provided additional K+ions for the formation of illite,which may be the reason for the higher illite contents within the Shanxi Formation as compared to the Lower Shihezi Formation.
5.3. Effect of diagenetic minerals on reservoir physical properties
The physical properties of the Permian sandstone reservoirs from the Sulige gas field are characterized by low porosity and low permeability. Nevertheless,the results of this contribution show that some intergranular pores and secondary dissolution pores are well preserved in the samples of the study area.It can be assumed that these pores are closely related to the development of diagenetic minerals during the diagenetic evolution.
Grain-coating chlorite and pore-lining chlorite formed a “protective film” on the surface of the particles, which occupied a part of the pore space but largely prevented the combination of authigenic quartz crystals and the formation of quartz overgrowth.Most of the primary intergranular pores are preserved,which plays a constructive role in the physical properties of the reservoir.Pore-filling chlorite occupies a large area of the pore space or is interspersed in some kaolinite intercrystalline pores, and therefore reduces the porosity and permeability of the reservoir.
The siliceous cementation in the study area includes authigenic quartz and quartz overgrowth. The quartz overgrowth generally has a larger thickness,and sometimes multiple quartz overgrowths are connected, even completely occupying the intergranular pores, and thereby seriously destroying the pores of the reservoir. Although authigenic quartz occupies part of the pore space,its crystal shape is smaller than that of quartz overgrowth, which can enhance the degree of reservoir compaction resistance to some extent. Consequently, its destructive capacity is less than that of quartz overgrowth,even,to some extent,it contributes to the preservation of primary pores.
Most of the kaolinite that primarily fills intergranular pores in the study area is from the dissolved feldspar and matrix(tuffaceous matrix,etc.)or authigenic kaolinite. Nevertheless, the volume reduced after dissolution and transformation of feldspar,matrix,and clay minerals (Hurst and Nadeau, 1995; Fan et al.,2016), and a large number of intercrystalline pores developed by kaolinite,have made a great contribution to the formation of secondary pores and played a constructive role in improving reservoir porosity.
The illitization of montmorillonite and kaolinite improves the reservoir porosity to a certain extent.However,the illite content in the study area is relatively low and therefore can be assumed to have little effect on increasing the reservoir porosity. On the contrary, the permeability of the reservoir is reduced because illite fills the throat or the position of small pores.
The iron calcite cement fills the primary intergranular pore almost completely and thereby destroys the kaolinite intergranular pores during the replacement of kaolinite. This significantly reduced the reservoir porosities.
6.Conclusions
1) The main diagenetic minerals of the sandstone reservoirs in the Permian of S120 Block, Sulige gas field,Ordos Basin are kaolinite, illite, chlorite, siliceous cement (quartz overgrowth and authigenic quartz),and calcite.The pore types are mainly intercrystalline pores,matrixsolution poresandresidual intergranular pores,accompanied by a few fragments,calcite,and feldspar solution pores and microfractures.
2) The occurrence of diagenetic minerals has a great influence on the porosity and permeability of the reservoir. Grain coating chlorite and pore-lining chlorite can inhibit the quartz overgrowth so that the primary pores are relatively well preserved,which provides a good channel for the fluid medium,and the overall physical properties of the reservoir are very good. In contrast, pore-filling chlorite occupies a large area of pore space,which makes the physical properties of pores worse. The kaolinite formed by feldspar dissolution improves the reservoir porosity due to the decrease in volume.On the other hand, the authigenic kaolinite in the pores fills the space and therefore occupies parts of the pores,which results in a decrease in porosity.
3) In the Lower Shihezi Formation, with its high content in grain coating and pore-lining chlorite,authigenic quartz is developed, while quartz overgrowth is rare. Some primary intergranular pores are preserved well. However, in the Shanxi Formation without chlorite,the primary pores are mostly filled with quartz overgrowth and calcite.
4) The formation succession of diagenetic minerals also affects the porosity and permeability of the reservoir. The pore types are mainly kaolinite and illite intergranular pores, matrix, and fragments solution pores, and the reservoir physical properties are relatively poor. In addition, because the formation of kaolinite is earlier than siliceous cementation,siliceous cement does not completely fill the pores in the sandstone with many kaolinites,but only wraps part of kaolinite and retains partly intergranular pores of kaolinite. In the sandstone with a little kaolinite, siliceous cementation completely fills the pores and is in close contact with each other.
Therefore, the occurrence, content, and formation succession of diagenetic minerals in the Permian sandstone reservoir of S120 Block, Sulige gas field,are the important reasons that explain its low porosity,low permeability,and severe heterogeneity.Through this study, it can be inferred that the reservoir physical properties are not only affected by diagenesis,the characteristics of diagenetic minerals have a great influence as well. Therefore, it is of great significance to identify and judge the reservoir physical properties of oil and gas reservoirs by studying the characteristics of diagenetic minerals in the reservoir.
Authors’ contributions
Qi Wan,designed experiments and measurements of sandstone samples, drew figures and made tables; Ai-Ping Fan,finished diagenesis research of the sandstone reservoirs; Ren-Chao Yang, finished core examination,geological setting analysis; Nils Lenhardt, polished the English, rewrote many sentences, reviewed and commented critically from scientific aspects.
Funding
This study was supported by the National Natural Science Foundation of China(grant No.42172167)and China- ASEAN (Association of Southeast Asian Nations)Maritime Cooperation Fund Project(grant No.1212010 0500017001).
Availability of data and materials
All data generated or analyzed during this study are included in this published paper.
Conflicts of interest
The authors declare that they have no competing interests.
List of abbreviations
SEM scanning electron microscope
EMPA electron microprobe analyzer
BSE Backscattered electron
Acknowledgements
This study was supported by the National Natural Science Foundation of China (grant No.42172167) and China-ASEAN(Association of Southeast Asian Nations)MaritimeCooperationFundProject(grant No.12120100500017001). The authors would like to thank anonymous reviewers and the Associate-Editorin-Chief Yuan Wang for their constructive comments and corrections and Dr.Lei Shu in Shandong Institute of Geological Sciences for instruction and help in the electron probe microanalysis experiment.
 Journal of Palaeogeography2022年3期
Journal of Palaeogeography2022年3期
- Journal of Palaeogeography的其它文章
- Research status of lacustrine mudrock deposition constrained from astronomical forcing
- Origin of soft-sediment deformation structures in Nihewan Basin
- Hydroclimate changes related to thermal state of the tropical Pacific in the northern coast of the South China Sea since ~8000 cal yr B.P.
- Petrography and tectonic provenance of the Permian Tunas Formation: Implications on the paleotectonic setting during the Claromec′o Foreland Basin evolution, southwestern Gondwana margin, Argentina
- Intensive peatland wildfires during the Aptian-Albian oceanic anoxic event 1b:Evidence from borehole SK-2 in the Songliao Basin, NE China
- Clay mineral formation and transformation in non-marine environments and implications for Early Cretaceous palaeoclimatic evolution:The Weald Basin, Southeast England
