N/S co-doped interconnected porous carbon nanosheets as high-performance supercapacitor electrode materials
WEI Yu-chen, ZHOU Jian, YANG Lei, GU Jing, CHEN Zhi-peng, HE Xiao-jun
(School of Chemistry and Chemical Engineering, Anhui Key Laboratory of Coal Clean Conversion and High Valued Utilization, Key Lab of Metallurgical Emission Reduction and Resources Recycling, Ministry of Education, Anhui University of Technology, Maanshan 243002, China)
Abstract:The synthesis of porous carbon nanosheets without acid treatment for high-performance supercapacitors (SCs) is difficult. We report the construction of N/S co-doped porous carbon nanosheets (NS-PCNs) from coal tar pitch (CTP), using Na2S2O3·5H2O as the sulfur source and K2CO3 as an activator, under flowing ammonia at high temperature. NS-IPCN800 prepared at 800 °C is composed of two-dimensional (2D) nanosheets with abundant pores and an interconnected 3D carbon skeleton. The abundant microspores increase the number of active sites for electrolyte ion adsorption and small mesopores act as channels for fast ion transmission. The 3D carbon skeleton provides paths for electron conduction. Heteroatom doping provides an additional pseudocapacitance for the NS-IPCN electrodes. As a result the NS-IPCN800 electrode has a high capacitance of 302 F g−1 at 0.05 A g−1 in a 6 mol L−1 of KOH electrolyte, and has a high energy density of 9.71 Wh kg−1 at a power density of 25.98 W kg−1. It also has excellent cycling stability with a capacitance retention of over 94.2% after 10 000 charge-discharge cycles. This work suggests an environmentally friendly way to produce NS-IPCNs from CTP for use as high-performance SC electrode materials.
Key words: Coal tar pitch;N/S co-doped interconnected porous carbon nanosheets;Hierarchical pores;Supercapacitor
1 Introduction
Energy crisis and environmental pollution prompt the exploitation and utilization of clean energy sources such as solar energy and wind energy.However, these energy sources are unstable due to geographical and weather effects. Thus, it is urgent to develop efficient and green energy[1-4]. As clean energy storage devices, supercapacitors (SCs) have been widely concerned due to their rapid charge and discharge capability, long lifespan and high-power density[5-7]. However, the performance of SCs is mainly affected by electrode materials. The traditional electrode materials of SCs include porous carbons, metal oxides and organic conductive polymers[8]. Among them, porous carbons have drawn much attention because of their abundant resources and low cost[9-12].Heteroatom doping is an effective method to increase capacitance of carbon materials by improving surface wettability and providing additional pseudocapacitance[13-16]. For example, N/S co-doped carbon material presented a specific capacitance of 169 F g−1(10 A g−1)[17]. Frustratingly, the preparation processes of porous carbons usually require acid or alkali for post-treatment, which inevitably increases environment pollution. In short, it is urgent to develop a no pickling method for synthesizing of carbon materials for high-performance SCs.
Coal tar pitch (CTP) is a by-product in the process of coal chemical industry. CTP can be used as precursors of porous carbon materials because of its cheapness and abundance[7]. In addition, CTP contains rich polycycle aromatic hydrocarbons, which are easy to be converted into graphene films at high temperatures[14]. Therefore, we report a no pickling method to synthesize N/S co-doped interconnected porous carbon nanosheets (NS-IPCNs) from CTP by using Na2S2O3·5H2O as template and ammonia as dopant coupled within-situK2CO3activation. The as-obtained NS-IPCN has three-dimensional (3D) structure composed of two-dimensional (2D) flakes with abundanthierarchical pores. Moreover, the N and S elements in IPCN provide additional pseudocapacitance[17]. As a result, NS-IPCN800electrode presents excellent electrochemical performance due to its ultrathin sheet-like structure, reasonable pore size distribution and heteroatom doping. This study reports a no pickling method to construct NS-IPCNs from CTP for high-performance energy storage devices.
2 Experimental
2.1 Preparation of IPCNs
The CTP was reserved from Maanshan Iron &Steel Co. Ltd. Polytetrafluoroethylene (PTFE, purity,50%) was purchased from DuPont Co. Ltd. of USA.Ammonia (purity, 99.99%) and Argon (purity,99.99%) were purchased from Nanjing Specialty Gas Co. Ltd. of China. The purity of Na2S2O3·5H2O and K2CO3is 99.5% and 99%, respectively.
Firstly, 4 g of Na2S2O3·5H2O, 3 g of CTP and 12 g of K2CO3were ground and mixed in the solid state. Secondly, the mixture was heated to 130 °C and kept for 30 min in flowing Ammonia (99.99%, 30 mL min−1) and then heated to 800 °C for 60 min, followed by being cooled down to room temperature naturally at last. The obtained sample was washed several times with deionized water to ensure that the final filtrate was neutral, and dried at 110 °C for 24 h to obtain a product named as NS-IPCN800,where 800 represents final activation temperature. The samples synthesized at 750 and 850 °C were named as NSIPCN750and NS-IPCN850, respectively. Subsequently,the N-IPCN800was synthesized from 3 g of CTP and 12 g of K2CO3in the absence of Na2S2O3·5H2O at 800 °C. The S-IPCN800was prepared from 4 g of Na2S2O3·5H2O, 3 g of CTP and 12 g of K2CO3in Argon atmosphere at 800 °C.
2.2 Characterization
The crystal powder of Na2S2O3·5H2O was investigated by Thermogravimetric (TGA). The morphology of IPCNs was investigated by field emission scanning electron microcopy (FESEM, Nanosem430)and transmission electron microscopy (TEM, JEOL-2100). The pore structure parameters of IPCNs were obtained using N2adsorption/desorption at 77 K (Autosorb-IQ, Quantachrome, USA). The chemical bonding states of elements in IPCNs were analyzed by Xray photo-electron spectroscopy (XPS, Thermo ESCALAB250, USA). The Raman spectra of IPCNs were recorded on Raman spectroscopy (JYLab-Ram HR800, excited by a 532 nm laser).
2.3 Electrochemical evaluation
The preparation process of the electrode is as below: (1) 90% IPCNs and 10% PTFE were mixed in deionized water; (2) the obtained mixture was dried into a paste substance; (3) the paste substance was rolled into thin carbon film and cut it into 6 mm of radius; (4) the carbon films were heated at 110 °C oven for 24 h under vacuum. The as-obtained carbon film was pressed onto foam nickel to fabricate electrode.The mass loading of active material for each electrode was about 2.0 mg cm−2. Finally, the symmetrical button-type SCs were assembled with two similar electrodes separated by a polypropylene membrane in 6 mol L−1of KOH electrolyte.
The cyclic voltammetry (CV) curves of SCs were obtained using an electrochemical workstation (CHI 760E, Shanghai Chenhua Instrument Co., Ltd.). The galvanostatic charge/discharge test (GCD) was investigated by SC test system on the Arbin Instruments(SCTS). The electrochemical impedance spectroscopy(EIS) was obtained on Power Transmission Impedance Analyzer (SI1260, Solartron Analytical, UK)with a frequency range of 10−3-105Hz. The specific capacitance (Cg, F g−1) of the single IPCN electrode was calculated by the formula (1)[18].
WhereI(A) represents the discharge current, Δt(s) is the discharge time,m(g) represents total mass of the active material in the two electrodes, ΔV(V) stands for the discharge voltage after IR drop.
The energy density (E, Wh kg−1) and average power density (P, W kg−1) of SCs were calculated according to equations (2) and (3)[19].
WhereV(V) is the discharge voltage after IR drop and Δtd(h) is the discharge time.
3 Results and discussion
Fig. 1 shows the direct fabrication of NS-IPCNs from CTP and the mechanism involved in the process.CTP, K2CO3and Na2S2O3·5H2O were ground and mixed homogeneously at first. The weight loss of Na2S2O3·5H2O template was occurred at 20 to 140 °C due to the loss of crystalline water (Fig. S1(a), Supplementary Materials). In the subsequent heating step,CTP was liquefied at 150 °C, and then the small aromatic molecules in CTP were decomposed and reorganized to form an interconnected spherical film on the surface of the Na2S2O3template and K2CO3. Subsequently, Na2S2O3was decomposed to produce Na2SO4and sodium polysulfide at 300 °C[20]. In addition, Na2SO4and K2CO3were involved the following chemical reactions with carbon in Eqs. (4-6)[21]. Simultaneously, the N and S elements in the raw materials were incorporated into carbon skeleton. The spherical film was broken to form an interconnected 3D sheet-like structure composed of ultrathin 2D nanosheets with rich hierarchical pores as the further increase of temperature. Finally, NS-IPCN was obtained after washing with deionized water.
The FESEM images of IPCNs in Fig. 2 (a-e)demonstrate the interconnected 3D structure. It is noted from Fig. 2b that NS-IPCN800with 3D interconnected structure was composed of ultrathin 2D carbon nanosheets with abundant hierarchical pores. The 3D interconnected structure not only improves the stability of carbon skeleton but also provides the highways for electrons transmission. More importantly, the pores on the flakes are expected to provide channels for electrolyte ion[22]. As shown in the TEM images(Fig. 2(d-e)), the thickness of NS-IPCN800carbon nanosheet is approximately 5 nm, which is thinner than that of NS-IPCN750and NS-IPCN850. Short mesopores are easily formed in the thin nanosheets and are anticipated to shorten the transport distance of ion.Therefore, NS-IPCN800is one of the candidate electrode materials of SCs.
The N2adsorption/desorption isotherms of IPCNs in Fig. 3a are typical IV type with strong N2adsorption at the low relative pressure (p/p0< 0.01) and obvious hysteresis loops at 0.4 <p/p0< 0.95, indicating the presence of abundant micropores and few mesopores in IPCNs. The micropores offer rich active sites for electrolyte ion adsorption, while mesopores are served as channels for ion transportation[22,23]. Fig. 3b and Fig. S1(b) show the detailed pore size distribution of IPCNs. The micropores of IPCNs center are at 0.6-1.2 nm, whereas the mesopores of IPCNs are mainly center at 2-5 nm. As the activation temperature enhances from 750 to 850 °C, theSBETof IPCNs increases from 1 019 to 2 000 m2g−1and then decreases to 1 977 m2g−1. TheDapof IPCNs increases from 2.52 to 2.84 nm(Table 1). Additionally, theVtof NS-IPCNs increases from 0.85 to 1.45 cm3g−1. These results indicate that the etching effect of K2CO3on carbon skeleton increases with the increase of temperature, causing the micropores to collapse into mesopores.
Fig. 4a presents the Raman spectra of IPCNs,showing the typicalD-band at 1 340 cm−1andG-band at 1 590 cm−1. TheDpeak is related to the defects and disorder structure of the samples, while theGpeak is assigned to the well-ordered graphitic structure[24].The peak intensity ratios ofID/IGare 1.01 for NS-IPCN750, 0.99 for NS-IPCN800and 0.94 for NSIPCN850, which are lower than that of graphene oxide of 1.02[7], indicating that NS-IPCNs possess high degree of graphitization and therefore good conductivity.
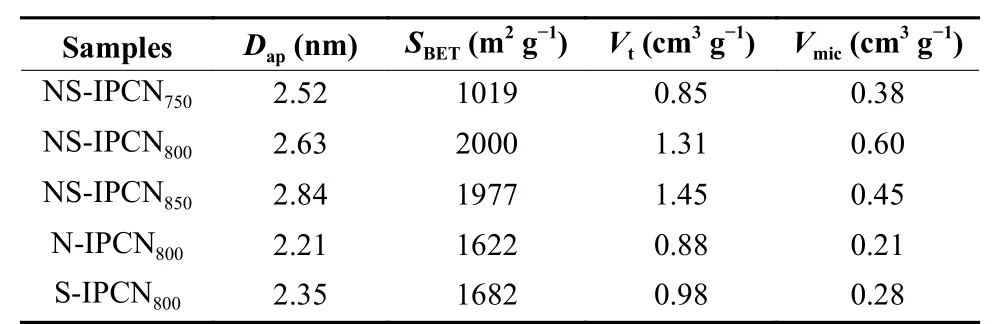
Table 1 Pore structure parameters of IPCNs.
The survey XPS spectra (Fig. 4b) of NS-IPCNs present two strong peaks at C 1s (285.1 eV), O 1s(532.5 eV) and three weak peaks at S 2s (228.4 eV), S 2p (164.3 eV) and N 1s (400 eV), showing that NSIPCNs possess C, N, O and S elements. The O 1s spectra (Fig. S2) of IPNCs are deconvoluted into C=O (531.2 eV), C―O (532.6 eV) and ―OH (536.1 eV) of oxygen-containing functional groups (Table 2).Oxygen-containing functional groups can improve the wettability of electrodes and reduce the diffusion resistance of electrolytes[15]. N 1s and S 2p spectra of IPCNs are exhibited in Fig. S3 and S4. The N 1s spectra of IPCNs can be fitted into four peaks (Table S1), including pyridinic nitrogen (N―6, 398.2 eV), pyrrolicnitrogen (N―5, 399.99 eV), quaternary nitrogen(N―Q, 401.7 eV) and nitrogen oxide (NOx, 403.5 eV).Among them, N―6 and N―5 can provide additional pseudocapacitanceviathe redox reaction on the surface of materials[25]. Moreover, N―5 and N ―6 have favorable electron-donating properties, enhancing the charge transfer capability and the activities of NS-IPCNs[26,27]. In addition, N―Q and NOxprovide extra electrons for IPCNs, which can reduce the electron transfer barrier and improve the conductivity[28,29]. The S 2p spectra of IPCNs are resolved into three peaks,corresponding to SOx(168.5 eV), S 2p1/2(165.0 eV)and S 2p3/2(163.5 eV) (Fig. S4 and Table S1). The introduced S elements are expected to increase structural defects of carbon skeleton and enhance the polarizability of atoms, further improving electrochemical activity[30].
Fig. 5a shows the CV curves of IPCNs at 10 mV s−1in 6 mol L−1of KOH electrolyte. The CV curves of all electrodes are quasi-rectangular shape without obvious redox peaks, indicating that IPCN electrodes have ideal electric double-layer capacitance (EDLC) behavior[13,31]. Fig. 5b and Fig. S5(a-d)present the CV curves of IPCN electrode at different scan rates. The curves exhibit approximately rectangular at a low scan rate. Moreover, the CV curve of NS-IPCN800still retains quasi-rectangular shape at 500 mV s−1, proving the excellent rate performance of NS-IPCN800electrode. These results also indicate that the micropores in carbon material play a buffering role in ion migration by providing active sites for electrolyte adsorption and desorption[32,33].
The GCD curves of IPCN electrodes (Fig. 6a)present isosceles triangle, indicating the ideal EDLC behavior[19]. Obviously, the GCD time of NS-IPCN800electrode is the longest, suggesting the highest specific capacitance. Fig. 6b shows the GCD curves of NSIPCN800electrode. Fig. 6c demonstrates the specific capacitance curves of IPCN electrodes at various current density. The specific capacitance of NS-IPCN800electrode is 302 F g−1at 0.05 A g−1, which is higher than that of NS-IPCN750of 198 F g−1, NS-IPCN850of 223 F g−1, N-IPCN800of 210 F g−1and S-IPCN800of 224 F g−1. Similarly, the specific capacitance of NSIPCN800electrode of 231 F g−1is also higher than that of NS-IPCN750of 150 F g−1, NS-IPCN850of 170 F g−1,N-IPCN800of 166 F g−1and S-IPCN800of 165 F g−1at 40 A g−1. The capacitance retention of NS-IPCN800capacitor reaches 76.5% with the increase of current density from 0.05 to 40 A g−1. The specific capacitance of NS-IPCN800is higher than that of other electrodes reported in the literature (Table 3)[34-42].
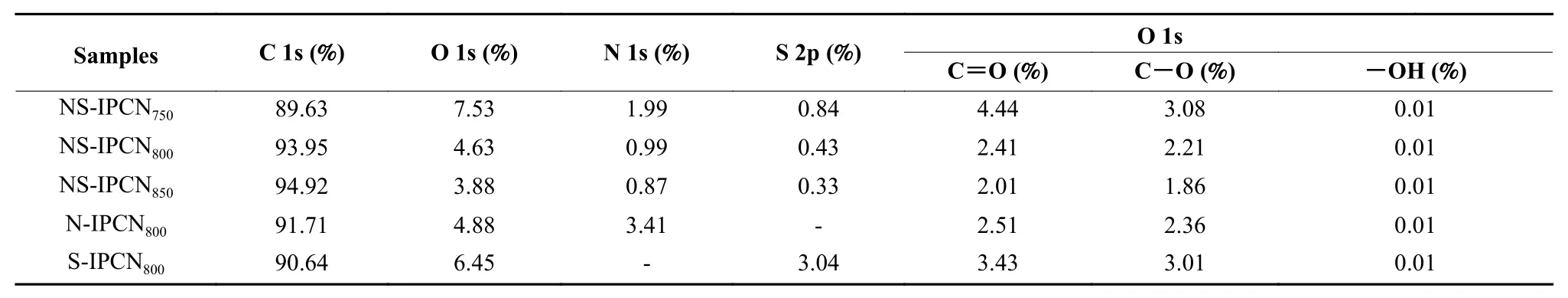
Table 2 Contents of C, O, N and S elements in IPCNs.
Energy density is an important indicator of SCs[43].Fig. 6d reflects the energy density of IPCN capacitors at different power densities. The energy density of NS-IPCN800capacitor of 9.71 Wh kg−1at a power density of 25.98 W kg−1is significantly higher than that of NS-IPCN750(7.27 Wh kg−1) and NSIPCN850(7.73 Wh kg−1). The ideal Nyquist diagram is a straight line perpendicular to theZ' axis[44,45]. In the low-frequency region, the Nyquist diagram of IPCN capacitor is almost perpendicular to theZ' axis, indicating the ideal EDLC characteristics. The x-intercept of theZ' axis corresponds to the intrinsic ohmic resistance (Rs) of IPCN capacitors, while the diameter of semicircle represents the charge transfer resistance(Rct)[46]. TheRsandRctof NS-IPCN800capacitor of 0.19 and 0.42 Ω are the smallest among the five IPCN capacitors (Fig. 6e), demonstrating that NSIPCN800electrode possesses better electronic conductivity and lower resistance. Cycle stability is another important indicator for the practical application of SCs. Fig. 6f shows that the capacitance retention of NS-IPCN800capacitor maintains 94.12% at 5 A g−1after 10 000 cycles. The excellent cycle stability confirms that NS-IPCN800is very suitable as electrode material for long-lifespans SCs.
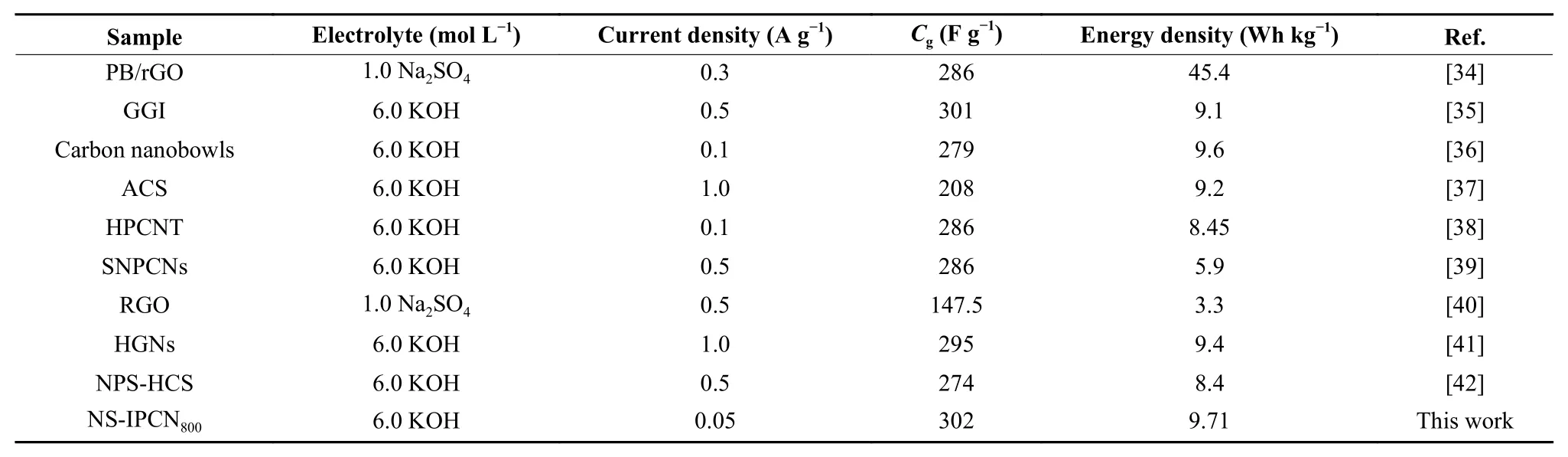
Table 3 Comparison of the specific capacitance of NS-IPCN800 electrode.
4 Conclusion
In summary, N/S co-doped IPCNs are prepared from CTP with Na2S2O3·5H2O as template and ammonia as dopant coupled within-situK2CO3activation.The as-prepared NS-IPCN800features interconnected 3D structure is composed of 2D ultrathin nanosheets with rich hierarchical pores. In addition, NS-IPCN800possesses a high degree of graphitization and a good conductivity. Besides, the moderate heteroatom doping provides additional pseudocapacitance for NS-IPCN800electrodes. Benefitting from these merits, NSIPCN800electrode exhibits excellent electrochemical performance such as high specific capacitance of 302 F g−1at 0.05 A g−1and excellent rate performance of 230 F g−1at 40 A g−1. Additionally, NS-IPCN800capacitor presents high cycle stability with only 5.88%decay after 10 000 cycles at 5 A g−1. This work provides a simple method without pickling to construct high-performance electrode materials from CTP for energy storage devices, realizing the high added utilization of chemical by-products.
Acknowledgement
The authors acknowledge the financial support from the National Natural Science Foundation of China (52072002, 51872005, U1710116 and U1508201) and the WanJiang Scholar Program.
- 新型炭材料的其它文章
- 选择透过性石墨烯基薄膜在海水淡化领域中的应用
- Advanced design strategies for multi-dimensional structured carbon materials for high-performance Zn-air batteries
- 生物质炭材料在金属锂负极中的应用
- Review of H2S selective oxidation over carbon-based materials at low temperature: from pollutant to energy storage materials
- 碳包覆磁性纳米粒子吸波机制及研究进展
- Hybridization of activated carbon fiber cloth with electrospun nanofibers for particle filtration

