Application of intravoxel incoherent motion diffusion-weighted imaging in hepatocellular carcinoma
Yi Zhou, Jing Zheng, Cui Yang, Juan Peng, Ning Liu, Lin Yang, Xiao-Ming Zhang
Abstract The morbidity and mortality of hepatocellular carcinoma (HCC) rank 6th and 4th,respectively, among malignant tumors worldwide. Traditional diffusion-weighted imaging (DWI) uses the apparent diffusion coefficient (ADC) obtained by applying the monoexponential model to reflect water molecule diffusion in active tissue; however, the value of ADC is affected by microcirculation perfusion. Using a biexponential model, intravoxel incoherent motion (IVIM)-DWI quantitatively measures information related to pure water molecule diffusion and microcirculation perfusion, thus compensating for the shortcomings of DWI. The number of studies examining the application of IVIM-DWI in patients with HCC has gradually increased over the last few years, and many results show that IVIMDWI has vital value for HCC differentiation, pathological grading, and predicting and evaluating the treatment response. The present study principally reviews the principle of IVIM-DWI and its research progress in HCC differentiation, pathological grading, predicting and evaluating the treatment response, predicting postoperative recurrence and predicting gene expression prediction.
Key Words: Hepatocellular carcinoma; Intravoxel incoherent motion; Differentiation;Pathological grading; Treatment response; Gene expression
INTRODUCTION
The morbidity and mortality of hepatocellular carcinoma (HCC) rank 6thand 4th, respectively, among malignant tumors worldwide[1,2]. The apparent diffusion coefficient (ADC) obtained using traditional diffusion-weighted imaging (DWI) reflects water molecule diffusion in tissue[3]. However, many studies have shown that a voxel contains other diffusion information in addition to the diffusion movement of pure water molecules, namely, the microcirculation perfusion of the capillary network in tissue[3-6]. One limitation of traditional DWI is that it is unable to distinguish water molecule diffusion and microcirculation perfusion information. Le Bihanet al[6,7] proposed that intravoxel incoherent motion (IVIM) quantitatively obtains information on both pure water molecule diffusion and diffusion related to microcirculation perfusion, thus compensating for the shortcoming of traditional DWI.Therefore, IVIM has become a research hotspot in recent years. The liver is suitable for IVIM research because of its relatively large blood supply. Since the first application of IVIM-DWI for the study of liver disease by Yamadaet al[8], the number of studies assessing IVIM-DWI in patients with HCC has progressively increased, and significant progress has been achieved. This study mainly reviews IVIMDWI research progress in the diagnosis and treatment of HCC.
THE BASIC PRINCIPLES OF IVIM-DWI
DWI is currently the only functional magnetic resonance imaging (MRI) technology that noninvasively measures water molecule diffusion in living tissue[3]. According to the theory of traditional DWI, the movement of free water molecules is considered to conform to a Gaussian distribution. However, due to the presence of complex cell structures and multiple diffusion barriers, such as cell membranes, in biological tissues, the diffusion behavior of water in biological tissues is more complicated. Therefore,the displacement of water in tissue may substantially deviate from a Gaussian distribution, challenging the validity of the monoexponential model to varying degrees. Additionally, the ADC, which reflects the water molecule diffusion, is affected simultaneously by microcirculation perfusion[3,5,7]. IVIM-DWI uses sufficient b values and biexponential models for the acquisition and analysis of images and distinguishes information regarding the pure tissue diffusion of water molecules and diffusion related to microcirculation perfusion. Among IVIM-DWI parameters, the simple diffusion coefficient, which is also referred to as the slow apparent diffusion coefficient (D), reflects the diffusion movement of pure water molecules in the tissue in a region of interest (ROI). The false diffusion coefficient, which is also called the fast apparent diffusion coefficient (D*), reflects diffusion movement related to microcirculation perfusion in the capillary network in an ROI. The fraction of the fast apparent diffusion coefficient(f) represents the volume ratio of the local microcirculation perfusion-related effect to the total diffusion effect, which may reflect the blood volume in an ROI. The formula for determining the relationship between IVIM-DWI signal attenuation and b values in tissue is as follows:
Sb/S0= (1 - f) × exp (-bD) + f × exp [-b (D + D*)]
Where b is the diffusion sensitivity coefficient with units of s/mm2, while the unit for both D and D*is mm2/s, and S represents intravoxel signal intensity, with S0and Sbrepresenting the signal intensities when b = 0 and b has other values, respectively. The b value determines the effect of microcirculation perfusion on DWI signal attenuation[6]. When the b value is low (b < 200 s/mm2), the effect of microcirculation perfusion accounts for a large proportion of the total diffusion effect, whereas when the b value is high (b ≥ 200 s/mm2), signal attenuation basically reflects only the pure water molecule diffusion in a voxel.
HCC DIFFERENTIATION
To date, many studies have shown that IVIM-DWI has important value in differentiating HCC from other liver lesions[8-22]. In theory, malignant tumors restrict the movement of water molecules to a significantly greater extent than benign tumors; thus, the ADC and D values of malignant tumors are lower than those of benign tumors[23]. Studies have confirmed that the ADC and D values of malignant liver lesions are remarkably lower than those of benign liver lesions and are useful to distinguish benign from malignant liver lesions, with the D value showing higher differentiation efficiency[8,10-15,17].Yoonet al[14] conducted an IVIM study on 169 lesions in 142 patients. The values of ADC and D in malignant liver lesions [1.14 ± 0.24, 0.95 ± 0.21, (× 10-3mm2/s), respectively] were significantly lower than those in benign liver lesions (1.72 ± 0.37, 1.61 ± 0.34, (× 10-3mm2/s), respectively). The area under the receiver operating characteristic (ROC) curve (AUC) values for the ADC value and D value in differentiating liver malignancies and hypervascular malignancies were 0.933 and 0.971, respectively, and 0.919 and 0.961, respectively (allP< 0.0005).
However, research results for D* values and f values in differentiating benign and malignant liver nodules are inconsistent. A study by Ichikawaet al[16] showed that the D* value of malignant liver lesions was significantly lower than that of benign lesions, but no difference in f values was observed between benign and malignant lesions. A study by Luoet al[10] also revealed that the D* values of HCC were obviously lower than those of focal nodular hyperplasia, with no significant difference in the f value between the two groups. However, another study[11] by the same group showed that the f value in the malignant group was significantly lower than that in the benign group, and the D* value did not differ significantly between the two groups. The studies by Klausset al[12] and Doblaset al[18] both showed no significant differences in the D* and f values between benign and malignant liver lesions and no significance in the values of D* and f in differentiating those two types of liver lesions. Recently,Podgórskaet al[24] reported that the perfusion-diffusion ratio was more effective than IVIM-DWI parameters in differentiating solid benign and malignant primary liver lesions.
The inconsistencies in the results described above may be related to the following factors: (1) D* and f values not only reflect blood perfusion information but may also reflect other information, such as particle or gland excretion[10,15,25]; (2) the D* values of hepatic hemangioma with different blood supply types exhibit a range of fluctuation range[13]; and (3) interference from other factors, such as the b value distribution, ROI settings, and differences in tumor composition, may occur[16,23,26] (Figures 1 and 2).
Relatively few studies assessing the differentiation of malignant nodules with different pathological characteristics in the liver have been published. The few available studies have involved differentiating HCC from intrahepatic cholangiocarcinoma (ICC) and/or metastasis[9,27-29]. Our previous study[27]showed significantly lower ADC and D values for HCC than for ICC, a significantly higher D* value for HCC than for ICC, higher diagnostic efficiency for D* [AUC = 0.896], and no significant difference in f values between the two groups. According to other studies, ADC and D for HCC are obviously lower than those for ICC. However, the results for the IVIM-DWI parameters D* and f are not consistent. Choiet al[9] and Shaoet al[28] reported that the f values of HCC were higher than those of ICC, while the D*values did not differ significantly between the two studied groups. Weiet al[29] did not document significant differences in either D* or f values between HCC and ICC groups.
The results of studies analyzing the differentiation between HCC and liver metastases differ substantially. Choiet al[9] found that the f value of HCC was significantly higher than that of liver metastases,but no significant differences were detected in other IVIM-DWI parameters. The study by Wuet al[30]indicated no obvious differences in the values of ADC, D, D*, and f for HCC from those for hepatic metastases, which may be due to changes in microcirculation and cell density caused by metastatic tumors of different origins. Dividing liver metastases into different subgroups according to different origins may be a more reasonable approach.
PREDICTION OF PATHOLOGICAL GRADING
Preoperative prediction of the pathological grade of HCC has important value in formulating guidelines for individualized treatments. Studies have shown that IVIM helps predict the pathological grade of HCC before surgery[23,26,31-44]. A meta-analysis by Yanget al[32] included 16 studies (1428 cases of HCC) using IVIM-DWI for pathological grading and found that ADC and D values showed high accuracy for the pathological grading of noninvasive HCC and better differentiation efficiency for D values than for ADC values. The explanation for this result may be that a higher tumor pathologicalgrade indicates greater cellularity, a higher nuclear-to-cytoplasmic ratio, and a smaller extracellular space, resulting in more restricted water diffusion, which ultimately leads to lower ADC and D values[23,33,35]. A potential explanation for the superiority of D values compared to ADC values may be that D values theoretically avoid the effect of microcirculation perfusion information. The results for the correlation between D* and f values and pathological grading are inconsistent. In addition to reporting results for ADC and D values and tumor tissue grading consistent with those of previous studies, Liet al[36] used rat models and showed that the D* and f values of higher-grade lesions were higher than those of lower-grade lesions and that D* and f values were positively correlated with the tumor tissue grade. Sokmenet al[26] and Granataet al[38] found that f values were significantly correlated with the HCC pathological grade, while a significant correlation was not identified between D* values and the pathological grade. On the other hand, multiple studies by Zhuet al[23,33,35] showed that neither D*values nor f values were significantly correlated with the HCC pathological grade.


Figure 2 Hepatic hemangiomas in a 35-year-old male. A 3.0-cm-sized mass in the right hepatic section shows hypointensity on the unenhanced T1-weighted image, hyperintensity on the unenhanced T2-weighted image, peripheral globular enhancement in the image of the arterial phase and centripetal fill-in in the image of the portal venous phase. A: Unenhanced T1-weighted image; B: Unenhanced T2-weighted image; C: Image of the arterial phase; D: Image of the portal venous phase; E: Image showing the apparent diffusion coefficient; F: Image showing the D; G: Image showing the D*; H: Image showing the f.
PREDICTION AND EVALUATION OF TREATMENT RESPONSES
Locoregional therapy
IVIM-DWI accurately reflects microstructures and blood perfusion changes before and after tumor treatment and is particularly suitable for the prediction and evaluation of the responses to and efficacy of tumor locoregional therapy (LRT)[45]. Many researchers have investigated the value of IVIM-DWI in the prediction and evaluation of transarterial chemoembolization (TACE) efficacy in patients with HCC[46-52]. Parket al[50] reported that the preoperative D* value of HCC with better lipiodol deposition was markedly higher than that of HCC with poor lipiodol deposition, suggesting that the D* value may predict lipiodol deposition after TACE for patients with HCC. As shown in our previous study[46], the ADC and D values of HCC were obviously elevated four weeks after TACE treatment, the D* value was significantly reduced, and the f value did not change significantly.
Hectorset al[53] investigated the effect of yttrium 90 radioembolization on diffusion and perfusion in HCC. The authors performed multiparametric MRI including IVIM-DWI on 24 patients with HCC before (n= 24) and six weeks (n= 21) after radioembolization. The ADC and D values of HCC were significantly increased at six weeks after radioembolization (P< 0.0004), while the D* values were significantly reduced (P= 0.014), and no significant change was detected in f values (P= 0.765).
Serveret al[54] administered LRT to 15 patients with HCC (11 patients underwent transarterial radioembolization, and four patients underwent TACE, and IVIM-DWI was performed before and eight weeks after LRT). Their results indicated that the ADC and D values obtained after LRT were significantly higher than those recorded before treatment, but the f values were significantly lower than those recorded before LRT. In the responder group, the ADC and D values were significantly increased,and the f values were significantly reduced after LRT. No significant differences were detected in the nonresponder group. Based on these findings, IVIM effectively predicts and evaluates the response to and efficacy of LRT. In these studies, the potential explanation for the inconsistency in the blood perfusion parameters D* and f may be that they represent different types of perfusion in various aspects, with the former usually reflecting the blood flow rate in capillaries and the latter mainly reflecting blood volume (Table 1).
Systemic therapy
Changes in volume are often not obvious in the early stage of targeted therapy for HCC. Compared to traditional imaging methods, IVIM-DWI has more advantages in the early evaluation of the HCC response to targeted therapy[55-59]. Wagneret al[56] revealed that the D value of viable tumor regions in malignant liver tumors was significantly lower than that of necrotic tumor tissue but higher than that of fibrotic tumor regions. Jooet al[55] used IVIM-DWI to evaluate the efficacy of the vascular blocker CKD-516 against rabbit VX2 liver tumors and found that the D* and f values of the treatment group were noticeably reduced at four hours. Based on these studies, IVIM-DWI is useful to assess early responses to tumor vascular blockers[56]. Yanget al[57] investigated the values of IVIM-DWI in evaluating the response of 35 nude mice with HCC to sorafenib. Compared to the values recorded at baseline and in the control group, the ADC and D values of the treatment group at each time point were significantly higher, while the f value was significantly decreased at 7 d and increased at 21 d. The values of ADC, D, and f were significantly correlated with the necrotic fraction. Lewinet al[58] used IVIM parameters to evaluate the efficacy of sorafenib treatment in patients with advanced HCC. The f values of responder patients were obviously increased at two weeks and two months after treatment,while these values decreased in nonresponder patients. Therefore, the authors propose that the f value is useful as a reliable marker to evaluate sorafenib treatment efficacy in patients with advanced HCC.Shirotaet al[59] used IVIM-DWI to evaluate the responses of patients with HCC undergoing sorafenib treatment and found that the D value at baseline was significantly higher in the responder group than in the nonresponder group. The sensitivity and specificity of the D value for evaluating the treatment response were 100% and 67%, respectively (Table 2).
Immunotherapy for HCC is an emerging method with promising results; it exerts an antitumor effect without affecting tumor size. Functional imaging methods will play an important role in evaluating the response to immunotherapy. Anderssonet al[60] evaluated the response of patients with HCC to intratumoral injections of the immune primer ilixadencel using IVIM-DWI and a histogram analysis.Seventeen patients with HCC were enrolled in this study. Their results showed that the 10thpercentile of D* decreased significantly after treatment to baseline, and significant correlations were identified between the 10thpercentile and median D value. Chenet al[61] reported that both pretreatment IVIMDWI and DCE-MRI parameters, especially ADC and slope, may predict progression-free survival and overall survival in patients with HCC receiving lenalidomide (a dual antiangiogenic and immunomodulatory agent) as second-line therapy. Recently, the application of immune checkpoint inhibitors in tumors has become a new research hotspot. Subsequently, IVIM-DWI will inevitably play a valuable role in predicting the response to and evaluating the efficacy of tumor immunotherapy.
PREDICTION OF POSTOPERATIVE RECURRENCE
Some researchers have reported the value of IVIM-DWI in predicting recurrence and prognosis after the surgical resection of HCC. Microvascular invasion (MVI) is one of the main factors affecting postoperative recurrence or survival among patients with HCC. IVIM-DWI predicts HCC MVI prior to surgery[62]. Weiet al[63] prospectively studied the predictive value of preoperative IVIM-DWI and conventional imaging characteristics for MVI. The results of the univariate analysis showed that the characteristics that were significantly correlated with HCC MVI were decreased ADC values, decreased D values, and irregular circumferential enhancement. In the multivariate analysis, only the D value was an independent risk factor for HCC MVI (the AUC was 0.815). Zhaoet al[64] and Liet al[65] reported similar results. Zhanget al[66] studied 157 patients undergoing HCC resection and found that D values might serve as a marker for predicting HCC recurrence after hepatectomy and that the predictive capability would be improved if it was combined with age and the alpha-fetoprotein level.
PREDICTING GENE EXPRESSION
Recently, some studies have indicated that IVIM-DWI predicts gene expression in patients with HCC[67-69]. We recently investigated the correlations of HCC IVIM-DWI parameters with angiopoietin-2(Ang-2) and transketolase (TKT) expression[67]. We observed significantly higher D* and f values in the high Ang-2 expression group than in the low Ang-2 expression group, and the values of D* and f were positively correlated with the Ang-2 expression levels . On the other hand, the ADC and D values of the TKT high expression group were significantly lower than those of the TKT low expression group, and the ADC and D values were negatively correlated with TKT expression. Based on this result, IVIM-DWI noninvasively predicts Ang-2 and TKT expression in HCC before surgery. Shiet al[68] performed IVIMDWI of 52 patients with HCC and identified four IVIM-derived histogram metrics with the capability for differentiating Ki67 expression, withPvalues less than 0.05. By establishing a diagnostic model based on a logistic regression model, the AUC value for diagnosing high Ki67 expression was 0.861. The results of this study suggest that the histogram indicators obtained from IVIM-DWI scanning can accurately predict the Ki67 expression status.

Table 1 Summary of locoregional therapy studies
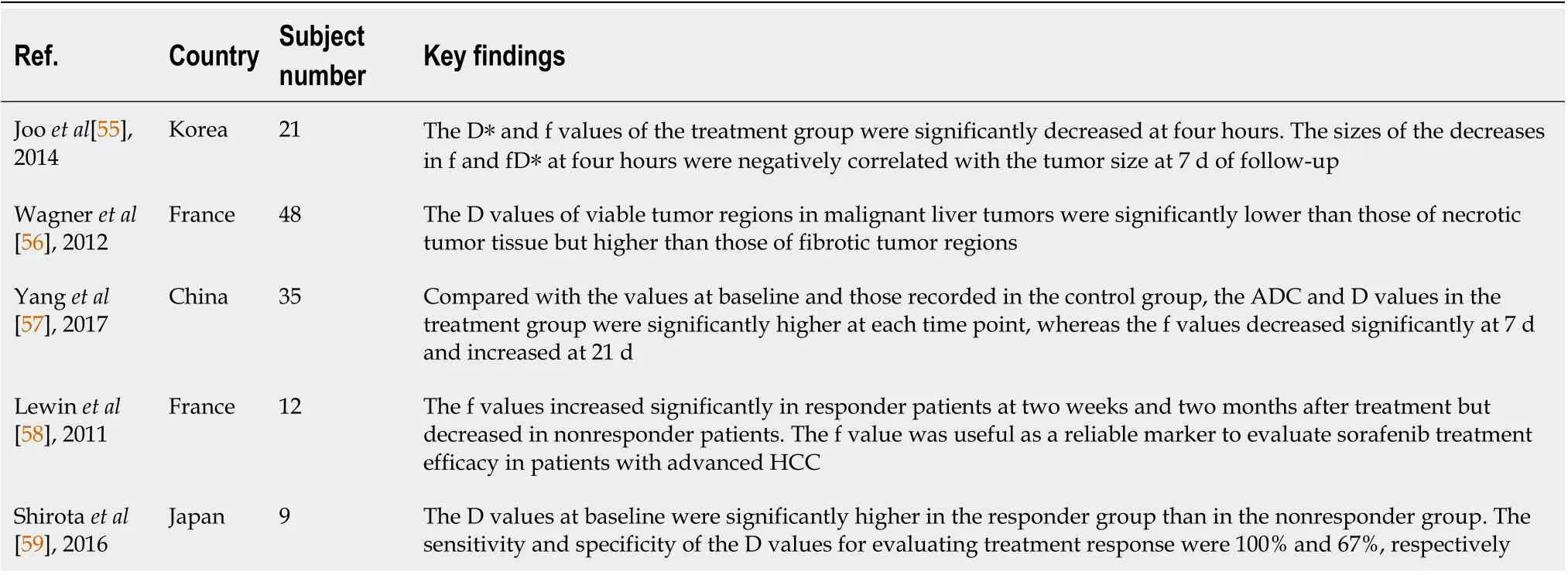
Table 2 Summary of systemic therapy studies
CONCLUSION
IVIM-DWI accurately and precisely reflects tissue structures and pathophysiological changes and has important application value for HCC differentiation, pathological grading, and predicting and evaluating the treatment response. However, the factors affecting IVIM-DWI measurement results are complicated and diverse[8,20,25,70-76]. Factors including the number, distribution, and fitting methods of b values, other physiological activities (such as gland secretion and flow), ROI settings, scanning devices and field strength, imaging acquisition methods, and motion artifacts all affect data quality[23,25,34,41]. Increased standardization of data acquisition and analysis is imperative to facilitate the generation of reliable IVIM-DWI biomarker measures that are broadly applicable[74,77,78]. In addition,recent studies have shown that IVIM-DWI modeling of the perfusion component is constrained by the diffusion component, and a reduced D slow measure leads to artificially higher PF and D fast measures[75,76]. These results should also be considered in the design of future IVIM-DWI studies. We postulate that with advances in imaging technology and further in-depth research, IVIM-DWI will certainly play a more prominent role in the diagnosis and treatment of HCC.
FOOTNOTES
Author contributions:Zhou Y wrote the paper; Zheng J, Yang C, Peng J and Liu N collected the data; Yang L revised the paper; Zhang XM designed the research project.
Supported bythe Project of Department of Science and Technology of Sichuan Province, No. 2016JY0105; Project of Medical Association of Sichuan Province, No. S20070; and the Project of City-University Science and Technology Strategic Cooperation of Nanchong City (North Sichuan Medical College), No. 20SXQT0324.
Conflict-of-interest statement:The authors have no conflicts of interest related to this article to declare.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Yi Zhou 0000-0002-1911-5380; Jing Zheng 0000-0002-2031-0845; Cui Yang 0000-0001-6751-9075; Juan Peng 0000-0002-7961-3533; Ning Liu 0000-0003-3587-5440; Lin Yang 0000-0001-8746-9255; Xiao-Ming Zhang 0000-0001-5327-8506.
S-Editor:Zhang H
L-Editor:A
P-Editor:Zhang H
 World Journal of Gastroenterology2022年27期
World Journal of Gastroenterology2022年27期
- World Journal of Gastroenterology的其它文章
- Crosstalk between dietary patterns, obesity and nonalcoholic fatty liver disease
- Regulatory T cells and their associated factors in hepatocellular carcinoma development and therapy
- Associations of gut microbiota with dyslipidemia based on sex differences in subjects from Northwestern China
- Prognostic significance of hemoglobin, albumin, lymphocyte,platelet in gastrointestinal stromal tumors: A propensity matched retrospective cohort study
- Contrast-enhanced ultrasound Liver Imaging Reporting and Data System: Lights and shadows in hepatocellular carcinoma and cholangiocellular carcinoma diagnosis
- Isolated gastric variceal bleeding related to non-cirrhotic portal hypertension following oxaliplatin-based chemotherapy: A case report
