Brain functional remodeling caused by sciatic nerve transposition repair in rats identified by multiplemodel resting-state blood oxygenation level-dependent functional magnetic resonance imaging analysis
Yu-Song Yuan,Hai-Lin Xu,Zhong-Di LiuYu-Hui KouBo JinPei-Xun Zhang
Abstract Lower extremity nerve transposition repair has become an important treatment strategy for peripheral nerve injury; however,brain changes caused by this surgical procedure remain unclear. In this study,the distal stump of the right sciatic nerve in a rat model of sciatic nerve injury was connected to the proximal end of the left sciatic nerve using a chitin conduit. Neuroelectrophysiological test showed that the right lower limb displayed nerve conduction,and the structure of myelinated nerve fibers recovered greatly. Muscle wet weight of the anterior tibialis and gastrocnemius recovered as well. Multiple-model resting-state blood oxygenation level-dependent functional magnetic resonance imaging analysis revealed functional remodeling in multiple brain regions and the re-establishment of motor and sensory functions through a new reflex arc. These findings suggest that sciatic nerve transposition repair induces brain functional remodeling. The study was approved by the Ethics Committee of Peking University People’s Hospital on December 9,2015 (approval No. 2015-50).
Key Words: brain connectivity; brain network; fALFF; MRI; nerve transposition repair; peripheral nerve injury; ReHo; remodeling
Introduction
Trauma-induced peripheral nerve injury is common,and has a generally poor prognosis. Peripheral nerve injury is found in 1.46-2.8% of traumatic injuries,and the incidence is increasing year by year (Li et al.,2020b). In addition to the severe loss of motor and sensory functions of the affected limbs,peripheral nerve injury can also be accompanied by afferent pain,significantly impacting physical,psychological and cognitive functions,as well as overall mental health (Yang et al.,2015a,b). There are different forms of nerve or neuronal injury,including long-segment nerve defect,nerve root avulsion injury,and central hemiplegia,which usually lead to functional loss of the limbs and are the most difficult to treat. Traditional treatment methods are ineffective,especially for central lesions,and even nerve transplantation does not effectively restore the function of the target organ. Nerve transposition repair is an ideal treatment option; choosing a suitable donor for nerve translocation to repair and innervate limbs may not only help form a functional reflex arc with the center,but also reduces complications (Kotani et al.,1971; Kawabata et al.,2001; Lam Van Ba et al.,2018; Zheng et al.,2018; Li et al.,2020a). However,even with an appropriate nerve repair method,the recovery of nerve function,to date,is not ideal. There is a therapeutic limit to repairing the nerve from the periphery determined by the tissue characteristics of the nerve itself. Treating a nerve injury through a central approach can improve the prognosis of peripheral nerve injury.
Brain neuroplasticity has been extensively studied in recent years. The brain can regain lost functions caused by injury through learning and training. Brain neuroplasticity can be divided into structural plasticity and functional plasticity (Dąbrowski et al.,2019; Chen et al.,2020). In structural plasticity,synapses and neurons establish new connections during learning and experience,thereby affecting behavior. In functional plasticity,a function controlled by an affected brain area can be taken over by an adjacent area through learning and training,and the injured region can regain some function through learning and training as well (Zhang and Chopp,2009).In recent years,resting-state blood oxygenation leveldependent functional magnetic resonance imaging (rs-fMRI) has become a promising method in brain science (Barkhof et al.,2014). Rs-fMRI is a non-invasive imaging method that can avoid task interference (Chao-Gan and Yu-Feng,2010). To clearly obtain visual information on brain functional areas post central remodeling induced by translocation repair,we designed a model of lower extremity nerve translocation repair. Herein,we investigated the features of functional remodeling of brain functional areas following sciatic nerve injury repair by nerve transposition in the lower limbs,with the aim of providing insight for future investigations and clinical treatments. To the best of our knowledge,this study is the first to describe brain remodeling processes following lower limb nerve transposition repair in rats.
Materials and Methods
Animals
Because male rats would become very large by the end of the study duration of nearly 1 year,which would make it difficult to carry out experimental procedures,only female rats were included in this study. A total of 36 specific-pathogen-free grade 6-week-old female Sprague-Dawley rats (weighing 200-220 g) were obtained from Beijing Weitong Lihua Experimental Animal Co.,Ltd. (license No. SCXK (Jing) 2016-0006). The generation of the animal model and breeding were carried out in the Animal Center of Peking University People’s Hospital under a 12-hour light/dark cycle,and 40% humidity. All experimental and feeding procedures were approved by the Ethics Committee of Peking University People’s Hospital on December 9,2015 (approval No. 2015-50). Experimental animal suffering was minimized in all experimental procedures to the best of our ability.
Groups and surgery
A total of 36 rats were randomly divided into the following two groups: the sciatic nerve transposition repair group (surgery group,n= 21) and the normal control group (control group,n= 15). In the surgery group,the right hind limb was termed the cross group,while the left hind limb was termed the denervation group during analysis.
Rats in the surgery group were placed in a prone position following isoflurane (Shenzhen Reward Life Technology Co.,Ltd.,Shenzhen,China) anesthesia (5% induction anesthesia,5% maintenance anesthesia,100 mL/min). The lower limbs and the rear half of the back were shaved. The skin was cut along the long axis of the left femur after disinfecting to expose the sciatic nerve. The sciatic nerve was freed and cut at the bifurcation. The distal stump of the left sciatic nerve was ligated after the left sciatic nerve was cut off. After covering the left sciatic nerve with muscle,the right sciatic nerve was exposed,disconnected at the deep surface of the piriformis muscle,and the proximal stump of the sciatic nerve was ligated. The back skin was cut along the line of the upper iliac crests. A 4-mm chitin conduit (Peking University People’s Hospital and Chinese Textile Academy,Patent No. 01136314.2) was used to cover the proximal end of the left sciatic nerve and the distal end of the right sciatic nerve with 10-0 suture,with a gap of about 2 mm between the nerve ends. The fascia and skin were sequentially sutured. Rats were placed in a new rat cage after the blood stains on the skin were wiped off. The control group received no treatment.
Neuroelectrophysiological testing
Six rats in each of the experimental and control groups were tested at 8 months after the operation. The skin was cut along the axial line of the right hind limb to expose the tibialis anterior and gastrocnemius muscles after isoflurane anesthesia (5% induction anesthesia,5% maintenance anesthesia,100 mL/min). The sciatic nerve and its branches were bluntly separated. Stimulation electrodes were placed at two locations in the common peroneal nerve,at 10 mm apart,and induction electrodes were placed on the tibialis anterior muscle. The stimulation parameters on the electrophysiological device (Synergy Electrophysiology Instrument,Oxford,UK) were 0.09 mA current intensity and 0.1 ms duration. The ∆tbetween the two incubation periods and length (L) of the neural stem between the two stimulation points were calculated. The motor nerve conductive velocity (MNCV) was measured inL/t,withtrepresenting the time between the two stimulations. The MNCV of the tibial nerve was measured by the same method.
Osmium acid staining
Eight months post-surgery,the transpositioned repaired nerves (n= 6 rats/group) were exposed along the surgical incision after nerve electrophysiological testing was completed. Then,3-mm nerve sections at the proximal and distal ends of the conduit were harvested,fixed with 4% paraformaldehyde,and stained with 1% osmium acid (Beijing Chemical Factory Co.,Ltd.,Beijing,China) for 12 hours. After rinsing for 6 hours under running water,a graded ethanol series was used for dehydration,and xylene was used for clearing. Samples were embedded in paraffin,sliced into 1-μm sections,and sealed with neutral resin after dewaxing. The sections were observed under the light microscope (Olympus Corporation,Tokyo,Japan) and imaged.
Wet muscle weight
The anterior tibialis and gastrocnemius tendon were severed from the ankle joint,peeled away from the far and near ends,and cut off at the proximal ends after electrophysiological testing was completed. The muscles were weighed on an electronic balance (FA2004,Shanghai Shunyu Hengping Scientific Instrument Co.,Ltd.,Shanghai,China) immediately after wiping the muscle surface with gauze.
Hematoxylin-eosin staining of muscle tissue
The central part of the anterior tibialis was hematoxylineosin-stained after measuring the wet weight. Muscles were fixed with 4% paraformaldehyde and stored at 4°C overnight. A graded ethanol series was used for dehydration,and xylene was used for clearing. Samples were embedded in paraffin and sliced into 5-μm sections. Sections were used for conventional hematoxylin-eosin staining and sealed with neutral resin after dewaxing. The slices were observed under the light microscope and imaged.
Magnetic resonance imaging data acquisition
At 12 months post-surgery,fMRI data acquisition was carried out on a 7.0T small animal magnetic resonance imager (PharmaScan 70/16 US,Bruker,Switzerland). Rats (n= 9 in the surgery group and 5 in the control group) were anesthetized with isoflurane gas (5% induction anesthesia,5% maintenance anesthesia,100 mL/min). The following scan sequences and parameters were used to obtain axial imaging information of the rat brain.
T2-weighted imaging: the scanning sequence was T2_TurboRare. Specific parameters: repetition time/echo time = 5600/33 ms; image size: 256 × 256; field of view: 35 mm × 35 mm; slice thickness: 0.55 mm; slices: 50; averages: 2; scan time: 5 minutes,58 seconds.
Rs-fMRI: The scanning sequence was T2star_FID_EPI. Specific parameters: repetition time/echo time = 2.000/20 ms; image size: 64 × 64; Field of view: 35 mm × 35 mm; slice thickness: 0.55 mm; slices: 50; averages: 1; repetition: 600; scan time: 20 minutes.
Fractional amplitude of low frequency fluctuations and regional homogeneity data analysis
Data pre-processing was performed with the spmratIHEP toolbox using statistical parametric mapping (SPM8; https://www.fil.ion.ucl.ac.uk/spm/software/spm8/) and restingstate fMRI Data Analysis Toolkit (REST) software (http://www.restfmri.net/forum). The regional homogeneity (ReHo) and fractional amplitude of low frequency fluctuations (fALFF) measurements were analyzed and compared between the control and surgery groups using spmratIHEP with SPM8 and REST software which,as described by Nie et al. (2010,2013,2014).
A linear model was used to remove any observed trends in the normalized images. To reduce the effects of low-frequency and high-frequency drift,the images were temporally bandpass-filtered. Kendall’s coefficient was calculated to assess the ReHo value (Nie et al.,2010,2013,2014).
Rs-fMRI data analysis
All functional image post-processing and data analysis were done with the spmratIHEP toolbox in SPM8 software,consisting of an fMRI rat standard brain template and atlas in Paxinos & Watson space (Liang et al.,2017),as previously described (Nie et al.,2010,2013,2014). Seed-based correlational analyses on a voxel-by-voxel basis were used to evaluate brain functional connectivity with spmratIHEP. The time course attributes were calculated and used to quantify the strength of the functional connectivity to establish a functional connectivity map.
Statistical analysis
All data are expressed as mean ± standard deviation (SD). The results of related data of neurological function testing were statistically analyzed with SPSS 17.0 software (SPSS,Chicago,IL,USA). An independent samplest-test was performed to assess differences in MNCV,fALFF and ReHo values. The cerebral regions with significant differences in fALFF and ReHo values between the two groups were selected,with a voxel-level height threshold ofP< 0.005 and a cluster-extent threshold of 20 voxels. Cronbach’sαof the MNCV was 0.05.One-way analysis of variance was used to assess differences in muscle weight that conformed with normality and homoscedasticity. Least significant difference test was used for further pairwise analysis. Non-parametric test (Kruskal-Wallis k samples) was conducted if the data did not conform with normality or homoscedasticity. Cronbach’sαwas set at 0.05.
Graph metrics between the surgery and control groups were compared with two-samplet-test. For global metrics,we compared the metrics at all thresholds. For nodal metrics,we compared the area under the curve first,and then compared the metrics at all thresholds. We also compared the differences in functional connectivity between the surgery and control groups using network-based analysis in MATLAB (https://www.mathworks.com) and BrainNet Viewer (https://www.nitrc.org/projects/bnv). Cronbach’sαwas 0.05.
Results
General observation of sciatic nerve transposition repair in rats
A total of five rats in the surgery group and four rats in the control group died prior to final testing. A brain tumor was detected by MRI in one of the rats in the surgery group,and was accordingly excluded. Six rats in each group received electrophysiological testing and histological staining at 8 months after surgery. Nine rats in the surgery group and five rats in the control group were tested by fMRI,12 months after surgery.
At 8 months post-surgery,the nerve suture area was covered with connective tissue with no neuroma or neuroedema. The conduits were partially absorbed. In the denervation group,the foot ulcer was found to be swollen,and normal foot anatomy could not be distinguished. The cross group showed obvious signs of atrophy and some autophagy (Figures 1and2).
Motor nerve conduction velocity in rats with sciatic nerve transposition repair
When the right common peroneal nerve and tibial nerve were stimulated in the surgery group,dorsiflexion and plantar flexion of the right hind limb was observed. The conduction velocities of the common peroneal and tibial nerves in the cross group were lower compared with the control group (P< 0.05;Table 1).
Regeneration of the sutured nerve in rats with sciatic nerve transposition repair
Osmium acid staining showed that compared with the control group,the myelin sheath of myelinated nerve fibers in the cross group was thinner,sparsely distributed,and lighter in color. The nerves in the cross group were regenerated to an extent,and many myelinated nerve fibers were visible (Figure 3).
Muscle wet weight in rats with sciatic nerve transposition repair
The cross and denervation groups had obvious signs of atrophy of the anterior tibialis and gastrocnemius. The anterior tibialis and gastrocnemius in the denervation group resembled a tendon,and the anterior tibialis and gastrocnemius muscles in the cross group were also indistinguishable in terms of their muscle tendon,tendon-abdominal joint,and abdominal muscle content. The wet weights of the anterior tibialis and gastrocnemius were significantly lower in the cross group compared with the control group (P< 0.05),but significantly greater compared with the denervation group (P< 0.05;Table 1).
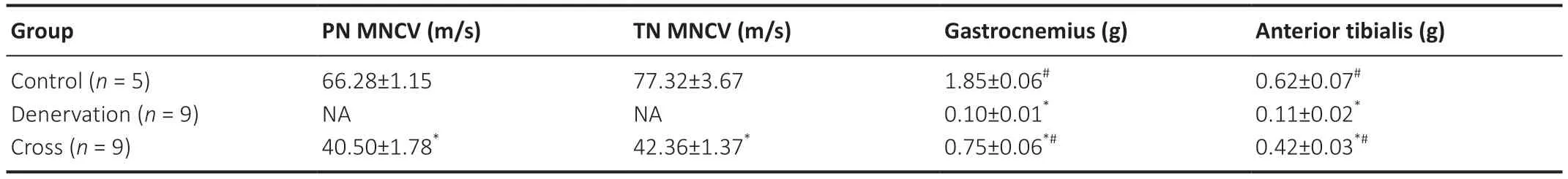
Table 1|Results of neuro-electrophysiological tests and muscle wet weights in rats with sciatic nerve transposition repair at 8 months post-surgery
Muscle fibers in the central part of the anterior tibialis in rats given sciatic nerve transposition repair
The muscle fibers in the control group were tightly arranged,with sharp edges and corners,and no pathological features were observed. The gaps between the muscle fibers in the cross group were increased and the diameter of the muscle fibers was smaller compared with the control group. Circular muscle fibers were seen,with less infiltration of connective tissue. In the denervation group,muscle fibers were filled with fat and connective tissue,and they showed signs of obvious atrophy (Figure 4).
Magnetic resonance imaging in different brain regions in rats given sciatic nerve transposition repair
fALFF value
The fALFF values of eight brain regions in the left hemisphere and 13 brain regions in the right hemisphere were significantly higher in the surgery group compared with the control group (P< 0.005;Table 2andFigure 5). The fALFF values of 10 brain regions in the left hemisphere and 6 brain regions in the right hemisphere were lower in the surgery group compared with the control group (P< 0.005;Table 2andFigure 5).
ReHo value
The ReHo values of eight brain regions in the left hemisphere and 11 brain regions in the right hemisphere were significantly higher in the surgery group compared with the control group (P< 0.005;Table 3andFigure 6). The ReHo values of 16 brain regions in the left hemisphere and 19 brain regions in the right hemisphere were lower in the surgery group compared with the control group (P< 0.005;Table 3andFigure 6).

Table 2|Fractional amplitude of low frequency fluctuation values in different brain regions in rats with sciatic nerve transposition repair at 8 months post-surgery

Table 3|Regional homogeneity values in different brain regions in rats with sciatic nerve transposition repair
Global metrics in different brain regions in rats with sciatic nerve transposition repair
A significantly different normalized characteristic path length (lambda) was found between the surgery and control groups at low range thresholds (P< 0.05). There was no significant difference in other indicators (Figure 7).
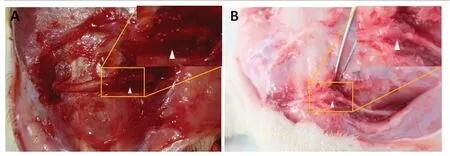
Figure 1|Observation of the surgical zone in rats given sciatic nerve transposition repair.
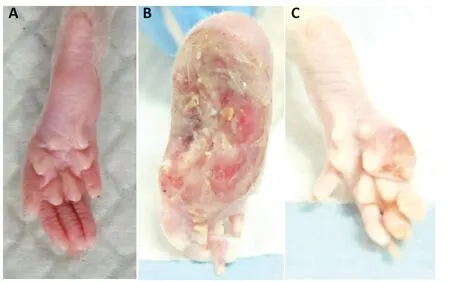
Figure 2|Observation of the hind limbs in rats with sciatic nerve transposition repair.
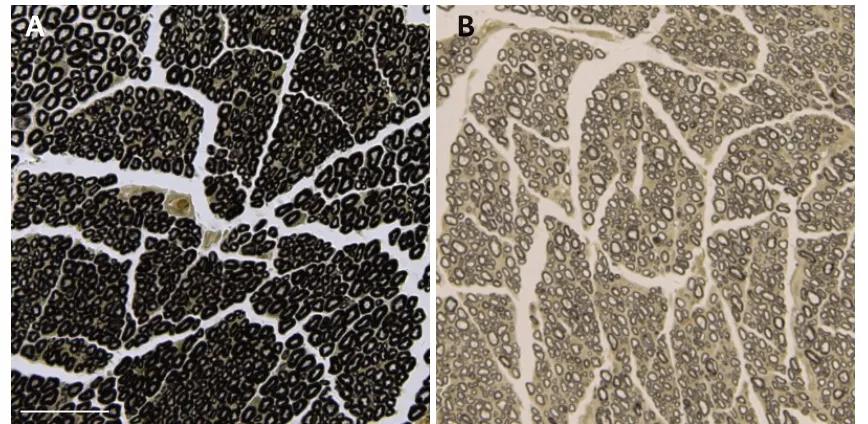
Figure 3|Morphology of myelinated nerve fiber sheaths in rats with sciatic nerve transposition repair at 8 months post-surgery (osmium acid staining).

Figure 4|Morphology of muscle fibers in the central part of the anterior tibialis of rats given sciatic nerve transposition repair at 8 months postsurgery (hematoxylin-eosin staining).

Figure 5|Resting-state fALFF maps of the whole brain compared between sciatic nerve transposition repair and normal rats at 8 months post-surgery.
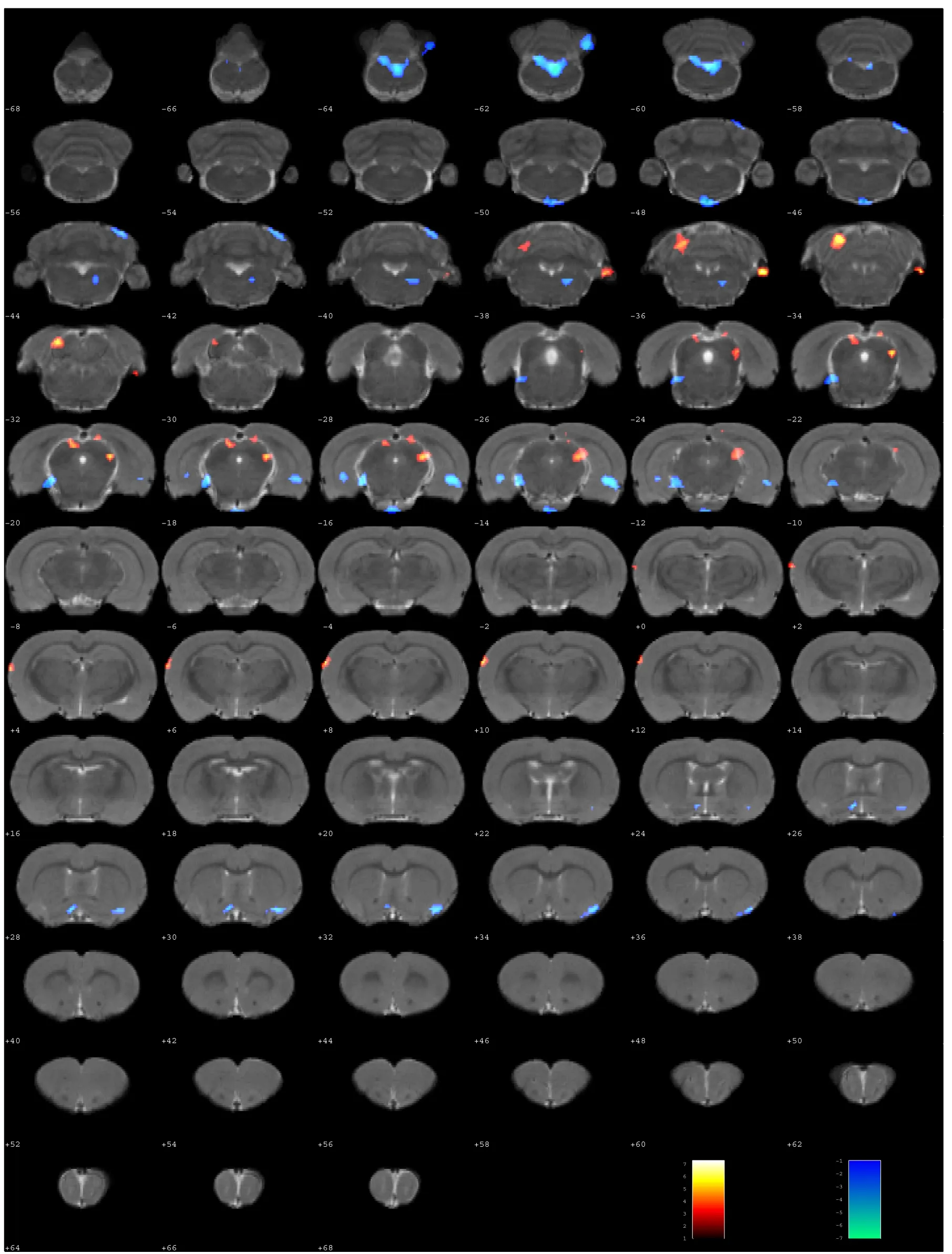
Figure 6|Resting-state ReHo maps of the whole brain compared between sciatic nerve transposition repair and normal rats at 8 months post-surgery.
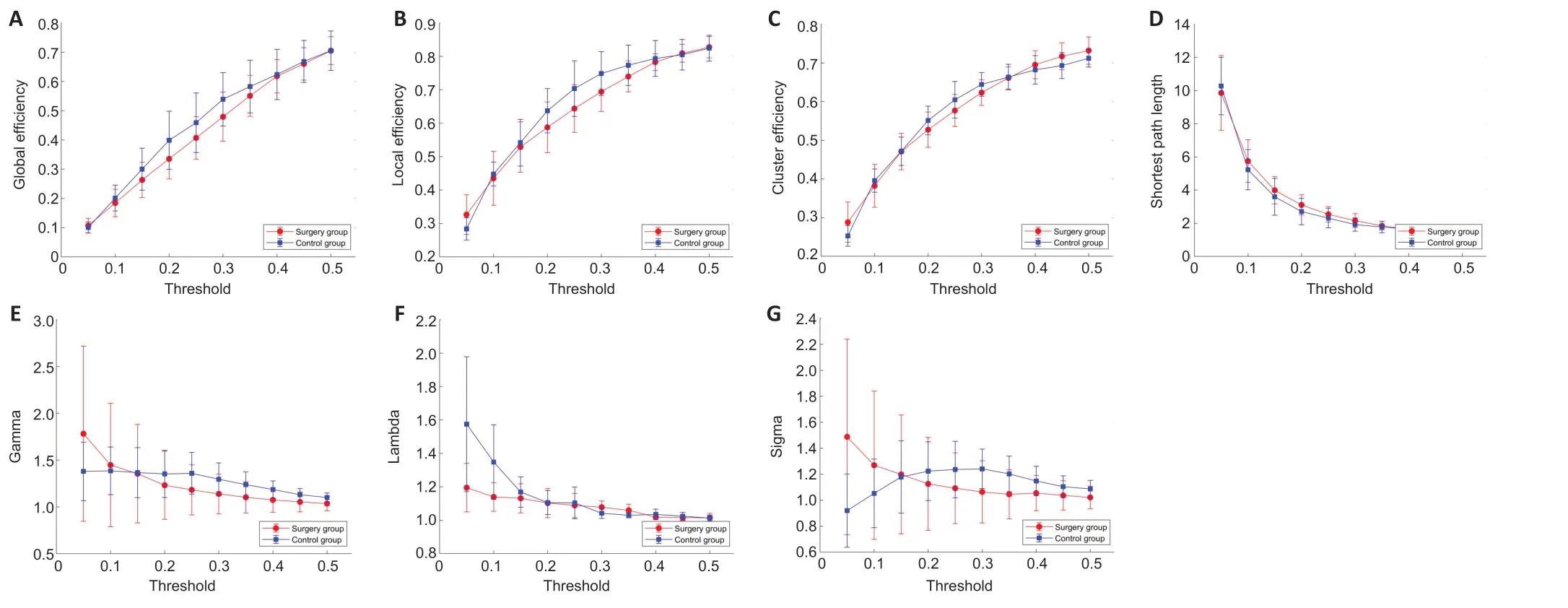
Figure 7|Global metric differences in different brain regions in rats with sciatic nerve transposition repair at 8 months postsurgery.
Local metrics in different brain regions in rats with sciatic nerve transposition repair
The right olfactory bulb,right thalamus and right temporal association areas showed significant differences between the control and surgery groups (P< 0.05). However,only the right temporal association cortex showed significant differences at a low threshold (P< 0.05;Figure 8).
Rich-club in different brain regions of rats with sciatic nerve transposition repair
There was no difference in rich-club,feeder or local strength between the control and surgery groups (P> 0.05;Figure 9).

Figure 8|Local metric differences in different brain regions in rats with sciatic nerve transposition repair.
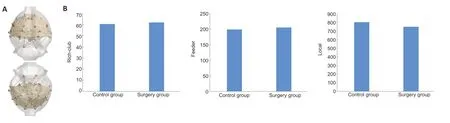
Figure 9|Overall topological properties of the brain in rats with sciatic nerve transposition repair.
Discussion
The human brain network is extremely complex and efficient,and is formed by multiple brain regions responsible for different functions based on the principles of compartmentalization and integration. This system enables internal functional coordination,monitoring,and feedback from the surroundings. The functional characteristics of the brain’s structural organization reveal the coordination of different functional brain regions. This requires efficient coordination in functional partitioning and functional integration to form a brain topological network,i.e. brain connectivity (Gorrostieta et al.,2012). Numerous studies have demonstrated that the loss of motor and sensory functions leads to disturbances in functional connections involving the sensorimotor cortex. As sensory-motor network activities change,some advanced functional networks,such as the default mode network,dorsal attention network,salience network,executive control network,and other cognitive association networks,may undergo direct or indirect changes (Gao and Lin,2012). These changes are not only limited to movement and sensory-related cortical connections,but also occur at a higher cognitive level (Auer et al.,2009; Makin et al.,2015; Feng et al.,2016; Bhat et al.,2017).Nerve repair therapy takes advantage of the brain’s intrinsic plasticity through neuronal remodeling,neurogenesis,and axonal regeneration (Ciumas et al.,2008). During motor learning,changes in temporal accuracy and flexibility involve multiple overlapping processes (Diedrichsen and Kornysheva,2015). Learning recruits new neurons that were not previously involved in the learning process (Costa et al.,2004). Bhat et al. (2017) found that compared with the control group,the functional connection of the anterior cingulate gyrus,right upper frontal gyrus,and left insular cortex in patients with unilateral brachial plexus ganglion injury was reduced. In patients with improved muscle strength following surgery,the functional connections of the left auxiliary motor cortex,medial frontal gyrus,and anterior cingulate cortex were enhanced (Bhat et al.,2017). Lu et al. (2016) found that compared with the normal control group,the functional connectivity of the supplementary motor area and multiple brain areas,including the anterior wedge,posterior cingulate gyrus and anterior cingulate gyrus,were decreased in patients with unilateral brachial plexus injuries. However,their findings remain controversial. Liu et al. (2013) found no difference in functional connectivity between patients with unilateral brachial plexus injury and a healthy control group,while Feng et al. (2016) found that the functional connectivity between the default mode network and executive control network in patients with unilateral total brachial plexus injury was greater than that in healthy controls.
Nerve transposition repair technologies are increasingly valued and considered an important treatment method for certain types of nerve injury (Yu et al.,2019; Xie et al.,2020). This repair technique constructs a new nerve reflex arc. Based on the method of contralateral brachial plexus nerve repair using the healthy C7 nerve root (Zheng et al.,2018),the left sciatic nerve was translocated to repair the right sciatic nerve. The nerve was found to have regenerated,based on histological observations,at 8 months post-surgery. The electrophysiological testing results showed that the nerve conduction (transmission) function of the nerve was restored to an extent,and that it was able to control the movement of the right hind limb. Some signs of autophagy were observed in the toes of the right hind limb,while the left hind limb looked significantly better,indicating that the sensory function of the right hind limb had also been restored to a degree (Tos et al.,2009). The muscle atrophy of the right hind limb was significantly less severe than that of the denervated muscle on the left,indicating that the repaired nerve could effectively innervate and protect the target organ. The results of the nerve function tests showed that following transposition repair,the nerves could effectively handle the motor and sensory functions of the receptor area,indicating that the central nervous system had indeed been remodeled.
The ALFF analysis method first passes the time series of each voxel through a 0.01-0.08 Hz band-pass filter to retain the blood oxygenation level-dependent signal in the low frequency band. This method reflects functional connectivity by measuring the spatiotemporal correlation between spatially separated brain regions (Yang et al.,2007). The ALFF method reflects the spontaneous activity of brain neurons by directly observing the amplitude of the baseline changes of the brain’s blood oxygenation level-dependent signal (Fox and Raichle,2007). Zou et al. (2008) improved the original ALFF method,defining the low frequency band (0.01-0.08 Hz) relative to the full-frequency power spectrum as the fALFF,effectively solving the problem linked to using ALFF in physiological noisesensitive contexts.
In this study,the fALFF values of the bilateral dentate gyrus,hippocampus,insular cortex,midbrain superior colliculus and thalamic lateral nucleus were higher in the surgery group than in the control group. This may indicate that the remodeling of these regions was caused by the responses of all brain regions involved in the default mode network and dorsal attention network. The higher fALFF values of the midbrain periaqueductal gray matter,midbrain tegmentum and pretectal area in the left hemisphere of the surgery group may be caused by the loss of target innervation. The higher fALFF values in the midbrain inferior colliculus,nucleus accumbens,olfactory cortex,basilar part of the pons,pontine tegmentum,retrosplenial cortex,sensory cortex and striatum in the right hemisphere indicate that the transposition repair not only changed the motor functions of the cortex,but also the sensory functions in this animal model.
The ReHo assumes that the time series of adjacent voxels in an active functional area are similar. The Kendall’s Coefficient of Concordance is used to measure this similarity (also called the ReHo value). The ReHo value reflects the synchronization of the local blood oxygenation level-dependent signal time series in the brain area,not the signal strength (Zang et al.,2004). Compared with the control group,the ReHo values of the bilateral insular cortex,midbrain inferior colliculus,midbrain superior colliculus,hippocampus and retrosplenial cortex were higher in the surgery group. This may suggest that these regions manipulate the whole brain to adapt to the changes caused by surgery. Accordingly,the regional homogeneity of the sensory brain regions of the right hemisphere,including the thalamic lateral nucleus,olfactory cortex and flocculonodular lobe,were also enhanced.
There was no difference in fALFF or ReHo value in the motor cortex in the whole brain between the two groups,which might be caused by the resting state. Differences might be revealed in the task state.
There was no difference in whole brain connectivity between the two groups,except for the lambda values at low range thresholds,demonstrating that the information delivery efficiency decreased after remodeling. However,these changes do not imply that the functional connectivity of the entire brain had changed,which would entail that sciatic nerve transposition repair surgery does not fundamentally change the efficiency of the functional connectivity of the brain.
There are some limitations to this study. Ideally,a group should have been added to exclude the effects of nerve damage. However,the changes in the right hemisphere of rats in the surgery group can represent the results of sciatic nerve transposition repair to a degree from fMRI data. The changes in the left cerebral hemisphere could be regarded as the result of paw denervation to an extent. Therefore,we reduced the number of groups based on animal ethics considerations. We will undertake a more detailed study in the future. Brain remodeling is a very complicated process. Here,we only examined a single time point in this cross-sectional study,which therefore cannot evaluate the dynamic processes inherent in brain remodeling. However,to the best of our knowledge,this is the first study of brain remodeling following the creation of a new reflex arc across the length of the spinal cord caused by nerve transposition repair,thereby shedding light on brain remodeling caused by nerve transposition repair of the lower extremities.
In summary,the brain regions in the dorsal attention network and default mode network may be remodeled at rest following nerve transposition repair,which may incur permanent changes to neurological functions. Brain regions function through complex network systems involving the entire brain. In other words,the sensorimotor cortex is not independent,and the remodeling of sensorimotor regions may cause a series of changes in other regions of the brain responsible for other complex functions,as similarly concluded by Markov et al. (Markov et al.,2013). The metabolic and other changes in the corpus callosum of the hemisphere that had lost the target organ could enhance the efficiency of information transfer between the two hemispheres. The regional homogeneity of the motor cortex and vision-related brain regions corresponding to the repaired limb were enhanced following nerve repair. Sciatic nerve transposition repair did not change the brain’s functional connectivity network nor its efficiency at a resting state,potentially due to the inactive neurons in the resting state.
The sciatic nerve transposition created a new reflex arc to make it possible for the brain functional zone to control a new target organ based,at least to a degree,on the functional remodeling of brain networks during the resting state. Functional remodeling in the task state and the structural changes in the brain will be investigated by diffusion tensor imaging in a future study,to clarify the spatio-temporal remodeling changes caused by nerve transposition repair.
Acknowledgments:We would like to thank Bin-Bin Nie (Institute of High Energy Physics,Chinese Academy of Sciences) and Gang Li (Institute of Materia Medica,Chinese Academy of Medical Sciences & Peking Union Medical College) for f-MRI data analysis assistance.
Author contributions:Study design,evaluation,and paper modification: PXZ; experimental implementation and data analysis manuscript writing: YSY,HLX,ZDL,YHK,BJ. manuscript writing: YSY,HLX. All authors approved the final version of the manuscript.
Conflicts of interest:The authors declare that they have no conflict of interest.
Financial support:This study was supported by the National Natural Science Foundation of China,Nos. 31771322,81671215 (to PXZ); the Beijing National Science Foundation,Nos. 7212121 (to PXZ); the NationalKey Research and Development Plan of China,No. 2018YFB1105504 (to PXZ); Shenzhen Science and Technology Plan Project,No.
JCYJ20190806162205278 (to PXZ); Sanming Project,No. SZSM202011001 (to PXZ); the Fundamental Research Funds for the Central Universities,Clinical Medicine Plus X - Young Scholars Project of Peking UniversityChina,No. PKU2020LCXQ020 (to YHK); the Key Laboratory of Trauma and Neural Regeneration (Peking University),the Ministry of EducationChina,No. BMU2019XY007-01; and the Ministry of Education Innovation Program of China,No. IRT_16R01. The funding sources had no role in study conception and design,data analysis or interpretation,paper writing or deciding to submit this paper for publication.
Institutional review board statement:The study was approved by the Ethics Committee of Peking University People’s Hospital on December 9,2015 (approval No. 2015-50).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Maria Marcella Lagana,National Institute for Research and Treatment,Italy; Alberto Ballestin,Jesús Usón Minimally Invasive Surgery Center,Spain.
Additional file:Open peer review reports 1 and 2.
- 中国神经再生研究(英文版)的其它文章
- A Drosophila perspective on retina functions and dysfunctions
- Celeboxib-mediated neuroprotection in focal cerebral ischemia: an interplay between unfolded protein response and inflammation
- Pramipexole,a dopamine D3/D2 receptor-preferring agonist,attenuates reserpine-induced fibromyalgia-like model in mice
- Effects of delayed repair of peripheral nerve injury on the spatial distribution of motor endplates in target muscle
- Neurorehabilitation using a voluntary driven exoskeletal robot improves trunk function in patients with chronic spinal cord injury: a single-arm study
- Gene and protein expression profiles of olfactory ensheathing cells from olfactory bulb versus olfactory mucosa

