Hypoxic preconditioning reduces NLRP3 inflammasome expression and protects against cerebral ischemia/reperfusion injury
Yi-Qiang Pang,Jing Yang,Chun-Mei Jia,Rui ZhangQi Pang
Abstract Hypoxic preconditioning can protect against cerebral ischemia/reperfusion injury. However,the underlying mechanisms that mediate this effect are not completely clear. In this study,mice were pretreated with continuous,intermittent hypoxic preconditioning; 1 hour later,cerebral ischemia/reperfusion models were generated by middle cerebral artery occlusion and reperfusion. Compared with control mice,mice with cerebral ischemia/reperfusion injury showed increased Bederson neurological function scores,significantly increased cerebral infarction volume,obvious pathological damage to the hippocampus,significantly increased apoptosis; upregulated interleukin-1β,interleukin-6,and interleukin-8 levels in brain tissue; and increased expression levels of NOD-like receptor family pyrin domain containing 3 (NLRP3),NLRP inflammasome-related protein caspase-1,and gasdermin D. However,hypoxic preconditioning significantly inhibited the above phenomena. Taken together,these data suggest that hypoxic preconditioning mitigates cerebral ischemia/reperfusion injury in mice by reducing NLRP3 inflammasome expression. This study was approved by the Medical Ethics Committee of the Fourth Hospital of Baotou,China (approval No. DWLL2019001) in November 2019.
Key Words: apoptosis; caspase-1; cell death; cerebral ischemia/reperfusion injury; gasdermin D; hippocampus; hypoxic preconditioning; NLRP3 inflammasome
Introduction
In China,the death rate associated with cerebrovascular diseases was recently reported at 149.49 per 100,000 population (Wang et al.,2020c). The only effective treatment for cerebral ischemic disease is the rapid restoration of the blood flow to the brain. However,reperfusion often aggravates brain injury,resulting in cerebral ischemia/reperfusion (I/R) injury (Kalogeris et al.,2012). Therefore,during the treatment of cerebral ischemia,the prevention and treatment of subsequent reperfusion injury must be performed simultaneously with the rapid recovery of cerebral tissue through perfusion. The pathophysiological process underlying cerebral I/R injury is complex. Currently,intracellular Ca2+overloading,oxygen free radical injury,excitatory amino acid toxicity,chemokines,and white blood cell interactions are considered to be underlying pathogenic factors that contribute to cerebral I/R injury (L et al.,2016; Min et al.,2020).
Hypoxic preconditioning (HPC) refers to the highly tolerant treatment of long-term lethal I/R injury through exposure to intermittent,repeated,short-term,non-lethal hypoxic stress. HPC has been reported to ameliorate I/R injury not only in the brain but also in the myocardial tissue (Tang et al.,2009). The potential mechanisms underlying HPC include autophagy induction and apoptosis inhibition (Zhang et al.,2017; Liu et al.,2020).
Inflammation is a biological response through which body tissues prevent the effects mediated by harmful stimuli (Chen et al.,2018; Mo et al.,2020). However,excessive inflammatory reactions can be harmful to neurons (Song et al.,2020; Zhang et al.,2020b). Inflammasomes are multiprotein complexes that are important components of the innate immune system and regulate inflammatory reactions. The NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome acts as a cytoplasmic pattern recognition receptor that can recognize various exogenous pathogens and sense endogenous risk signals,linking the innate immune response with the acquired immune response (Heneka et al.,2013; Coll et al.,2015). The NLRP3 inflammasome plays an important role in cerebral I/R injury (Wang et al.,2020a). However,whether the NLRP3 inflammasome is involved in the HPC-mediated protection against cerebral I/R injury remains unknown.
In this study,mice were treated with HPC followed by the generation of a cerebral I/R injury model to clarify the protective effects of HPC and to assess the involvement of the NLRP3 inflammasome in the potential underlying mechanisms.
Materials and Methods
Animals
A total of 45 male,specific-pathogen-free-grade BALB/c mice,aged 2 months and weighing 20-22 g,were purchased from Hunan SLYK Jingda Experimental Animal Co.,Ltd.,China [licence No. SCXK (Xiang) 2019-0004]. The experimental animal protocol was approved by the Medical Ethics Committee of the Fourth Hospital of Baotou,China (approval No. DWLL2019001; approval date: November 2019). All experiments were designed and reported according to the Animal Research: Reporting ofIn VivoExperiments (ARRIVE) guidelines.
To avoid the potentially confounding influence of estrogen,this study used only male mice. A total of 45 male mice were used,and three animals died during cerebral I/R injury modeling. All animals were maintained in a specific pathogenfree environment at 23 ± 2°C and relative humidity of 45-65% under a controlled 12-hour light/dark cycle. All mice had free access to food and water. During the first part of this experiment,the mice were randomly divided into three groups (n= 6 per group): HPC + 1 hour of ischemia followed by 12,24,and 36 hours of reperfusion (HPC + I/R 12-hour,HPC + I/R 24-hour,and HPC + I/R 36-hour groups,respectively). The follow-up experiments were conducted using 12-hour I/R conditions. Mice in the follow-up experiments were randomly divided into the following experimental groups (n= 6 per group): sham operation (control),HPC (HPC + sham),I/R,and HPC + I/R groups.
HPC
HPC was administered,as previously described (Cui et al.,2004). Briefly,the mice were placed in a 125-mL wide-mouth bottle containing fresh air. Immediately after the mice were placed in the bottle,the bottle mouth was closed with a rubber stopper,and the first observation of asthmatic breathing was taken as an index of the end of each hypoxic exposure. The tolerance times of the mice were recorded,and the mice were removed immediately; this procedure was considered one incidence of hypoxic exposure (H1),then transferred to another wide-mouth bottle with the same volume of fresh air. The second hypoxic exposure was recorded as H2,followed by the third (H3) and the fourth (H4) exposures using the same protocol. When the tolerance time of the second,third,and fourth hypoxic exposure reached two,four,and six times the tolerance time of the first hypoxia exposure,the hypoxic exposure was ceased.
Cerebral I/R models
One hour after HPC exposure,middle cerebral artery occlusion was performed under inhalation anesthesia with isoflurane (RWD,Shenzhen,China; 5% induction,1% maintenance),as previously described (Longa et al.,1989). A midline incision was prepared along the ventral side of the neck under a stereomicroscope (Olympus,Tokyo,Japan). The left common carotid artery,internal carotid artery,and external carotid artery were carefully exposed and separated from the vagus nerve. The distal end of the external carotid artery was ligated,and an arteriotomy was performed at the distal end of the external carotid artery. A nylon wire was inserted from the artery incision until it met with slight resistance. After 60 minutes,the occluding filament was gently removed to allow blood flow and reperfusion for 12,24,and 36 hours. Mice in the sham control group were subjected to the same operation,but no wire was inserted. Cerebral blood flow was monitored using a Doppler flowmeter (Yongan Jixin,Beijing,China). The effects of HPC on NLRP3 following different I/R conditions were evaluated (reperfusion of 12,24,and 36 hours). During surgery,the body temperatures of all animals were monitored and maintained between 37.0°C and 37.5°C using a heating pad. The mice in each group were sacrificed at the end of the indicted reperfusion period by decapitation under anesthesia with isoflurane. Brain tissues were cryopreserved,and the expression of NLRP3 was detected by western blot assay,as previously described (Song et al.,2019).
Evaluation of neurological function
According to the standards for evaluating the Bederson neurological function score (Bederson et al.,1986),the neurological function of mice was evaluated after 12 hours of reperfusion (Additional Table 1). Higher scores indicated more severe injury in the animals. A score of 0 indicated normal neurological function,whereas scores of 1-4 represented neurological dysfunction.
Triphenyl tetrazolium chloride staining
After 12 hours of reperfusion,the animals were anesthetized. After complete anesthesia,the heart was perfused with 10 mL normal saline; the whole brain tissue was frozen at -20°C for approximately 30 minutes. The cerebellum and olfactory bulb were discarded,and the remaining brain tissue was cut into five continuous slices coronally. The brain slices were placed in a closed container with 2% triphenyl tetrazolium chloride solution and incubated in the dark for 15 minutes at 37°C. During this period,the brain slices were flipped every 5 minutes to ensure even staining. The relative infarct area was calculated based on the following formula: relative infarct area (%) = infarct area/total area of the brain.
Hematoxylin-eosin staining
Neuronal morphology was detected using hematoxylin-eosin staining. After 12 hours of reperfusion,brain tissues were isolated and fixed in 4% paraformaldehyde overnight,followed by dehydration in 70%,80%,and 90% ethanol solution,a mixture of alcohol and xylene for 15 minutes,xylene I for 15,minutes and xylene II for 15 minutes. The sections were incubated in a mixture of xylene and paraffin for 15 minutes,followed by paraffin I and paraffin II,for 50-60 minutes,each. The tissues were paraffin-embedded and sectioned into 10-μm slices. The paraffin-embedded sections were dewaxed and hydrated. After being rinsed in distilled water,the slices were dyed with hematoxylin solution for 3 minutes,differentiated by hydrochloric acid alcohol differentiation solution for 15 seconds,washed slightly,and returned to eosin solution for 15 seconds. Images were obtained using a light microscope (Olympus),and pathological changes in the hippocampus were analyzed.
Enzyme-linked immunosorbent assay
After 12 hours of reperfusion,the animals were anesthetized,and brain tissues were collected for further use. The levels of cytokine expression in the brain tissue were detected using the indicated assay kits,according to the manufacturer’s instructions,as previously described (Yang et al.,2019): interleukin (IL)-8 assay kit (Cat# MM-0123M1,Meilian Bio,Shanghai,China),IL-6 assay kit (Cat# MM-0163M1,Meilian Bio),and IL-1β assay kit (Cat# MM-0040M1,Meilian Bio).
Terminal-deoxynucleotidyl transferase-mediated nick-end labeling staining
After 12 hours of reperfusion,the animals were anesthetized. Brain tissues were collected and fixed in 4% paraformaldehyde at 4°C overnight. The brain tissues were embedded in paraffin,and 3-μm sections were continuously sliced. The paraffin sections were placed in an oven at 65°C for 2 hours,incubated in xylene for 10 minutes,and then incubated in 100% ethanol,95% ethanol,80% ethanol,and purified water for 5 minutes each. The slices were transferred into a wet box,50 μg/mL protein K working solution was added,and the reaction was conducted at 37°C for 30 minutes. The sample was fully washed three times with phosphate-buffered saline (PBS). Residual PBS around the tissue was removed with absorbent paper,and an excessive volume of terminal-deoxynucleotidyl transferase-mediated nick-end labeling detection solution was dripped onto the slice and incubated at 45°C for 2 hours,followed by three washes with PBS. An anti-fluorescence quenching seal was used,and slices were examined under a fluorescence microscope (Olympus). The apoptotic rate was calculated using the formula: apoptotic rate (%) = Number of terminal-deoxynucleotidyl transferase-mediated nick-end labeling-stained cells (green)/number of DAPI-stained cells (blue) × 100.
Immunofluorescence
After 12 hours of reperfusion,the animals were anesthetized. Brain tissues were collected and fixed in 4% paraformaldehyde at 4°C overnight. The tissues were cryoprotected in 30% sucrose for 1 hour at 4°C and sectioned on a freezing microtome (Leica,Munich,Germany) at 20 μm. The slices were heated and boiled in citric acid buffer for 2 minutes and then washed with PBS after natural cooling. The slices were transferred into a wet box,incubated with 5% bovine serum albumin,and sealed at 37°C for 30 minutes. After removing the blocking solution,rabbit anti-NLRP3 antibody (1:100,Cat# bs-10021R,Bioss,Beijing,China) was added as the primary antibody and incubated at 37°C for 3 hours. The fluorescent goat anti-rabbit IgG-Fluor-488 (1:200; Cat# ZB-2301,ZSGB-BIO,Beijing,China) was added and incubated at 37°C for 30 minutes. Nuclei were stained with 4′,6-diamidino-2-phenylindole (Beyotime,Ningbo,China),and images were obtained using fluorescence microscopy. The fluorescence intensity of NLRP3 was calculated using ImageJ software.
Western blot assay
After 12 hours of reperfusion,the animals were anesthetized. Brain tissues were collected,and lysates were obtained,as previously described (Song et al.,2019). After 30 minutes on ice,the tissues were centrifuged at 4°C and 10,000 r/min for 10 minutes. The supernatant was carefully aspirated to obtain the total protein. The protein concentration was determined using a bicinchoninic acid kit (Beyotime,Beijing,China). The proteins underwent denaturation in a boiling water bath,and the samples were separated by sodium dodecyl sulfatepolyacrylamide gel electrophoresis (10% gel) for 1-2 hours,followed by wet transfer to a nitrocellulose membrane for 30-50 minutes. The membranes were blocked by 5% non-fat milk at room temperature for 2 hours and then incubated with the following primary antibodies at 4°C overnight: rabbit anti-NLRP3 antibody (1:100,Cat# bs-10021R,Bioss),rabbit anti-Caspase-1 antibody (1:1000,Cat# AF5418,Affinity,Cincinnati,OH,USA),rabbit anti-gasdermin D (GSDMD) antibody (1:1000,Cat# AF4012,Affinity),and rabbit anti-β-actin (1:100,Cat# bs-0061R,Bioss). The membranes were then incubated with goat anti-rabbit IgG/horseradish peroxidase antibody (1:200,Cat# bs-0295G-HRP,Bioss) at room temperature for 1-2 hours. An enhanced chemiluminescence exposure solution (Thermo Fisher Scientific,Inc.,Shanghai,China) was added to the membrane and visualized using a gel imaging system (Bio-Rad,Shanghai,China). The optical density of each band was analyzed by Quantity One Software (Bio-Rad).
Statistical analysis
All data were processed using SPSS 19.0 (IBM Corp.,Armonk,NY,USA). Data are expressed as the mean ± standard deviation (SD). Significant differences among groups were analyzed by one-way analysis of variance followed by Tukey’spost hoctest. A value ofP< 0.05 was considered significant.
Results
HPC prevents cerebral I/R injury
As shown inFigure 1A,four stages of HPC were examined in mice. The time of tolerance limits used for the second,third,and fourth hypoxia exposures were approximately two,four,and six times the tolerance time established during the first hypoxia exposure (F(3,20)= 89.68,P< 0.05),indicating that the HPC effect was suitable,and the adaptability of mice to a hypoxic environment was enhanced.
Figure 1Bshows the western blot analysis of NLRP3 protein expression in brain tissue after HPC combined with different I/R protocols. As shown inFigure 1B,NLRP3 expression levels increased with increased reperfusion time (F(2,15)= 3639,P< 0.05),which indicated that the NLRP3 inflammasome was involved in the regulation of HPC.
The Bederson neurological function score of the I/R group was 3.5 ± 0.58,which was significantly higher than those for the sham and HPC groups (scores of 0),indicating that the neurological function of the I/R mice was damaged and that limb activity was abnormal. The Bederson neurological function score of the HPC + I/R group was 2.5 ± 0.58,which was significantly lower than that of the I/R group,indicating that HPC was able to reduce the effects of I/R injury in mice. The results of triphenyl tetrazolium chloride staining (Figure 2) showed that the infarct area of the I/R group was remarkably larger than that in the sham group,whereas only a slight infarct area was observed in the HPC + I/R group (F(3,20)= 337.8,P< 0.05).
HPC prevents neuronal apoptosis in the hippocampus of cerebral I/R injury model mice
As shown inFigure 3,the hematoxylin-eosin staining results showed that the hippocampal morphologies of the control and HPC groups were complete,with regularly arranged cortical cells and a clear structure. After I/R modeling,the hippocampus was obviously damaged,including fuzzy structures,irregular arrangements,and the infiltration of inflammatory cells. Compared with the I/R group,the pathological changes observed in the HPC + I/R group were significantly improved. Terminal-deoxynucleotidyl transferasemediated nick-end labeling assay showed that the number of apoptotic cells was significantly increased in the I/R group than in the control group,whereas the apoptosis of the hippocampal cells was significantly decreased in the HPC + I/R group compared with that in the I/R group (F(3,20)= 55.21,P< 0.05;Figure 4).
HPC prevents cytokine release in the brains of cerebral I/R injury model mice
As shown inFigure 5,the cytokine contents,including IL-1β,IL-6,and IL-8,were measured in the brain tissues of mice using enzyme-linked immunosorbent assays. Compared with that of the control group,the IL-1β content of the I/R group significantly increased. HPC treatment significantly reduced the levels of IL-1β (F(3,20)= 117,P< 0.05),IL-6 (F(3,20)= 77.32,P< 0.05),and IL-8 (F(3,20)= 90.04,P< 0.05) compared with those in the I/R group.
HPC prevents the formation of the NLRP3 inflammasome in cerebral I/R injury model mice
As shown inFigure 6,the expression levels of caspase-1,GSDMD,and NLRP3 were detected by western blot assay to represent the expression of the NLRP3 inflammasome. The expression levels of caspase-1 (F(3,20)= 1113,P< 0.05),GSDMD (F(3,20)= 403.7,P< 0.05),and NLRP3 (F(3,20)= 756.2,P< 0.05) were significantly increased in the I/R group compared with those in the control group,whereas those in the HPC + I/R group were significantly reduced compared with those in the I/R group. The immunopositivity of NLRP3,as determined by immunofluorescence analysis,was similar to the results observed in the western blot assay. The immunopositivity of NLRP3 in the I/R group was significantly increased compared with that in the control group,whereas that in the HPC + I/R group was significantly reduced compared with that in the I/R group (F(3,20)= 982.2,P< 0.05;Figure 7).

Figure 1|Validation of hypoxic preconditioning and NLRP3 expression in I/R model mice.

Figure 2|Effect of HPC on the infarct area in mice subjected to cerebral I/R injury.

Figure 3|Effect of HPC on the hippocampal morphology in mice subjected to cerebral I/R injury,as detected by hematoxylin-eosin staining.

Figure 4|Effect of HPC on neuronal apoptosis in the brains of cerebral I/R injury model mice.
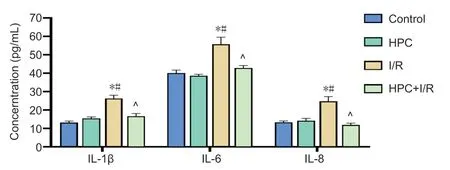
Figure 5|Effect of HPC on cytokine release in the brains of cerebral I/R injury model mice.
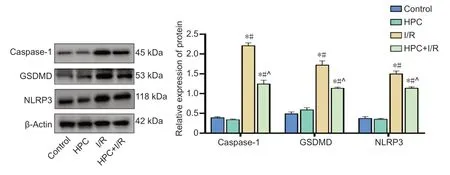
Figure 6|Effect of HPC on the expression of caspase-1,GSDMD,and NLRP3 in the brains of cerebral I/R injury model mice.

Figure 7|Effect of HPC on the fluorescence intensity of NLRP3 in the brains of cerebral I/R injury model mice,as detected by immunofluorescence.
Discussion
HPC can trigger the expression of a variety of protective factors,such as hypoxia-inducible factor-1α,erythropoietin,and vascular endothelial growth factor (Vellimana et al.,2020; Zhang et al.,2020a). These cytokines can improve the hypoxia tolerance of neurons through different mechanisms (Huang et al.,2014; Zhu et al.,2014). As an endogenous protective mechanism,HPC protects against neuronal injury (Fan et al.,2020). In this study,we first determined the successful establishment of HPC. Second,we found that NLRP3 expression increased with increased reperfusion time. Finally,we evaluated the functional effects of HPC for the protection against cerebral I/R injury. Our results indicated that HPC could reduce cerebral I/R injury and NLRP3 inflammasome expression levels in mice.
Excessive inflammatory reactions are detrimental to synaptic growth,which can result in the development of brain diseases (Amor et al.,2010; Wang et al.,2020b),such as Alzheimer’s disease and depression. Cerebral I/R injury can cause excessive inflammatory reactions in brain tissue,which is an important mechanism of brain reperfusion injury (Pei et al.,2015). A large number of inflammatory cells (leukocytes,microglia,and astrocytes) and inflammatory mediators (cytokines,chemokines,and adhesion molecules) participate in the inflammatory reaction (Sochocka et al.,2017). In this study,NLRP3 expression was upregulated following I/R injury,especially among mice that underwent 36 hours of reperfusion.
Pyroptosis is a newly discovered mechanism of programmed cell death,accompanied by inflammatory reactions. Pyroptosis shares morphological characteristics with both necrosis and apoptosis (Cookson and Brennan,2001). However,unlike apoptosis,during pyroptosis,many 1-2 nm pores form on the cell membrane,resulting in the loss of cell membrane integrity,disrupting the ability to regulate the entry and exit of substances. Eventually,the cell membrane dissolves completely,releasing the cellular contents and inducing an inflammatory reaction. Simultaneously,cells release IL-1β,resulting in the attraction of additional inflammatory cells,which expand the inflammatory response (Fann et al.,2018). In the present study,the IL-1β,IL-6,and IL-8 contents induced by I/R were significantly reduced by HPC. Moreover,neuronal apoptosis was also reduced by HPC. These results,together,imply that pyroptosis during cerebral I/R injury might be prevented by HPC. However,this finding must be verified by additional experiments.
GSDMD is known as a killer protein that mediates cell death and can independently mediate the release of inflammatory mediators,such as IL-1β,in addition to initiating cell membrane rupture and disintegration (He et al.,2015). Pro-caspase-1 is indirectly associated with the pattern recognition receptor NLRP3 via the adaptor protein adaptorassociated speck-like protein,which forms a macromolecular complex known as the caspase-1-dependent inflammasome. After activation by inflammatory mediators,such as IL-1β,inflammatory corpuscles further activate GSDMD and induce programmed cell necrosis (Saito et al.,2015). In studies in which the GSDMD gene was knocked out by gene-editing technology,macrophages were unable to initiate pyroptosis following induction by LPS and known inflammatory body agonists,indicating that GSDMD is a key pyroptosis gene (Kayagaki et al.,2015). The protein expression levels of caspase-1,GSDMD,and NLRP3 were detected by western blot assay,which demonstrated that HPC treatment reduced the expression levels of these three proteins. On the basis of the results from this study,we predict that HPC may inhibit the expression of inflammatory factors or other proteins that are active in the NLRP3 inflammasome pathway.
In our experiments,we identified serious injury to the brain tissue 12 hours after reperfusion,although a previous study suggested that 36 hours of reperfusion were necessary to induce severe injury (Huang et al.,2018). However,the death rate after 36 hours of reperfusion in our experiment was high. To reduce the death rate,we selected the 12-hour reperfusion protocol. In the present study,we identified an obvious protective effect of HPC against I/R injury. This study also had some limitations. This study used an HPC pre-treatment that is not feasible in the event of stroke-mediated I/R injury. In our future studies,we will plan to examine whether HPC can mediate the degree of I/R injury after infarction has occurred. Additionally,we only examined male mice in our study. Whether HPC exhibits similar functions in female mice remains unknown because estrogenic effects in female mice might influence the functions of HPC.
In conclusion,HPC prevented cerebral I/R injury in mice,likely through the reduction of NLRP3 inflammasome expression. This study suggested that HPC could serve as a potential measure to prevent cerebral I/R injury.
Author contributions:Study design and manuscript drafting: QP; experimental implementation and data analysis: YQP,JY,CMJ,RZ. All authors read and approved the manuscript and agree to be accountablefor all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflicts of interest:The authors declare that they have no competing interests.
Financial support:This work was supported by National Natural Science Foundation of China,No. 81771270 (to QP); Inner Mongolia Science Foundation of China,No. 2020MS08063 (to YQP); Health and Family Planning Scientific Research Plan Project of Inner Mongolia Autonomous Region of China,No. 201702138 (to YQP); Baotou Science and Technology Plan Project of China,No. 2018C2007-4-10 (to YQP); Baotou Medical and Health Science and Technology Project of China,No. wsjj2019036 (to JY); and Baotou Medical College Foundation of China,No. BSJJ201904 (to JY). The funding sources had no role in study conception and design,data analysis or interpretation,paper writing or deciding to submit this paper for publication.
Institutional review board statement:The study was approved by the Medical Ethics Committee of the Fourth Hospital of Baotou,China (approval No. DWLL2019001) in November 2019.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional file:
Additional Table 1:Longa’s neurological function score.
- 中国神经再生研究(英文版)的其它文章
- A Drosophila perspective on retina functions and dysfunctions
- Celeboxib-mediated neuroprotection in focal cerebral ischemia: an interplay between unfolded protein response and inflammation
- Pramipexole,a dopamine D3/D2 receptor-preferring agonist,attenuates reserpine-induced fibromyalgia-like model in mice
- Effects of delayed repair of peripheral nerve injury on the spatial distribution of motor endplates in target muscle
- Neurorehabilitation using a voluntary driven exoskeletal robot improves trunk function in patients with chronic spinal cord injury: a single-arm study
- Gene and protein expression profiles of olfactory ensheathing cells from olfactory bulb versus olfactory mucosa

