Improving cell transplantation by understanding and manipulating the phagocytic activity of peripheral glia
Lynn Nazareth,James St John,Jenny Ekberg
One of the key challenges in neuroscience is that the central nervous system (CNS; the brain and spinal cord),is largely unable to regenerate after injury. One factor contributing to this lack of repair is the accumulation of cellular and myelin debris at the site of injury. The debris is not efficiently phagocytosed and can persist for years after the initial injury,resulting in an inflammatory environment which inhibits axonal regrowth (Lutz and Barres,2014). The main cells responsible for phagocytosis in the CNS are microglia and astrocytes. While both these cells are competent phagocytes,their ability to clear cellular and myelin debris is diminished in CNS pathologies (Lutz and Barres,2014). In contrast to the CNS,the peripheral nervous system can regenerate unless the injury is complex or large. This is partly due to the ability of peripheral glia to rapidly phagocytose debris after an injury,followed by modulation of inflammation and secretion of growth factors that support axonal growth (Barton et al.,2017). The ability to promote regeneration has led to the use of peripheral glia in transplantation therapies to treat CNS injuries,particularly spinal cord injury. These peripheral glia are (1) Schwann cells,which surround most peripheral nerves and (2) olfactory ensheathing cells (OECs),which are the glia of the olfactory nerve. While these glia share many similarities,there have distinct differences. For example,a comparison of the Schwann cell and OEC transcriptomes showed that OECs express higher levels of factors relating to tissue repair than Schwann cells,including those involved in phagocytosis and degradation (Franssen et al.,2008). Understanding these differences may guide and improve transplantation therapies to repair the CNS.
Schwann cells myelinate large-diameter axons and also surround bundles of smaller,unmyelinated axons (termed Remak bundles). While injuries to peripheral nerves are infrequent,when they do occur,Schwann cells respond by transforming into a repair phenotype to phagocytose the debris arising from myelin and/or necrotic cells. While Schwann cells are the first responders after a peripheral nerve injury and commence the clearing of this debris,professional phagocytes such as macrophages and neutrophils are needed after larger injuries.
These additional phagocytes are recruited to the injury site following the release of proinflammatory cytokines and chemokines by the Schwann cells (Barton et al.,2017). The olfactory nerve is different from other peripheral nerves in that it constantly regenerates throughout life,due to the continual turnover of sensory olfactory neurons. This turnover means that cell debris from the neurons undergoing apoptosis (programmed cell death) and axons must be constantly removed. In addition,injury or infection of the olfactory nerve can lead to an increased amount of debris,this time arising from damaged neurons/axons and other cells undergoing necrosis (non-programmed cell death). Similar to Schwann cells,OECs can phagocytose the cell debris. Unlike Schwann cells,however,OECs continuously phagocytose debris (from olfactory neuron turnover),and do not recruit macrophages. Instead,it is the OECs that are the primary phagocytes in the olfactory nerve (Nazareth et al.,2015).
Phagocytosis of necrotic targets:Initial CNS trauma leads to cell necrosis and inflammation,resulting in further cell death in adjacent regions. Necrosis leads to the generation of necrotic bodies (a whole or part of a cell undergoing necrosis),which display molecules on the plasma membrane that phagocytic cells recognize,in particular the “eat me” signal phosphatidylserine that phagocytes bind to,allowing internalization of the necrotic body.
Both Schwann cells and OECs have been reported to display phagocytic receptors including the phosphatidylserine receptor (He et al.,2014),milk fat globule-epidermal growth factor 8 (Li et al.,2017),TAM receptors (Tyro3-Axl-Mertk) and Multiple epidermal growth factor-like domains 10 (MEGF-10) (Lutz et al.,2017),which have all been implicated in the recognition and phagocytosis of phosphatidylserinedisplaying dying cells. Further,cells undergoing necrosis also release cellular contents,termed damage-associated molecular patterns. Both Schwann cells and OECs also display Toll-like receptors that they may utilize to identify necrotic bodies (Vincent et al.,2007; Barton et al.,2017). Thus,Schwann cells and OECs are armed with an array of receptors for recognition and internalization of necrotic targets. When challenged with necrotic bodies,both Schwann cells and OECs rapidly (within 2 hours) engulf these targets (Nazareth et al.,2020),which is much faster than what has previously been reported for other glia and non-professional phagocytes (Loov et al.,2012; Schwegler et al.,2015). Phosphatidylserine blocking was shown to decrease OEC/Schwann cell-mediated phagocytosis of necrotic bodies by 40-50% (Nazareth et al.,2020) indicating that this is one of the major structures on the necrotic targets that these cells recognize.
Necrotic and myelin debris are processed by OECs and Schwann cells into endosomal/lysosomal compartments,albeit slower than for professional phagocytes:An important process of phagocytosis is the ability of a phagocyte to degrade the ingested cargo. Macrophages are proficient phagocytes that engulf large quantities of necrotic bodies and myelin,with the engulfed targets almost immediately broken down (within 15-30 minutes) (Nazareth et al.,2020). While Schwann cells and OECs degrade myelin debris within an hour after internalization,the processing of necrotic bodies requires more time. After engulfment,the necrotic bodies are trafficked to endosomes and lysosomes with OECs and Schwann cells requiring approximately 6 and 12 hours,respectively,to begin the degradation (Nazareth et al.,2020). Direct comparison of the phagocytic abilities between Schwann cells and OECsin vitroalso revealed OECs to be the more efficient phagocytes with a higher phagocytosis rate (i.e.,the number of targets phagocytosed over time) of both necrotic bodies and myelin debris (Nazareth et al.,2020).
Schwann cells produce more proinflammatory cytokines than OECs:To not cause adverse inflammation,it is key that the phagocytes degrade the cargo without excessive release of pro-inflammatory cytokines. This is especially crucial in the context of a potential therapy for CNS injuries. After phagocytosis of necrotic bodiesin vitro,Schwann cells produce the pro-inflammatory cytokines tumor necrosis factor alpha (TNF-α) and interleukin-6 within 1 hour,and continue to produce increasing amounts over 24 hours. In contrast,OECs release no detectable levels of TNF-α and very low levels of interleukin-6,and only after 24 hours (Nazareth et al.,2020). This is in accordance with the roles of Schwann cells and OECsin vivo. After an injury to a peripheral nerve,Schwann cells initiate the pro-inflammatory environment to recruit macrophages to aid repair. In contrast,in the primary olfactory nervous system,the continuous turnover of sensory neurons requires constant phagocytosis of axon debris,which the OECs perform without the aid of macrophages (Figure 1). As macrophages do not need to be recruited,and as the phagocytosis of axon debris is an ongoing continual process in the olfactory nerve,it is possible that OECs have evolved to not release large amounts of pro-inflammatory cytokines,thereby maintaining homeostasis regarding the inflammatory state of the olfactory nerve. In comparison,after macrophages commence phagocytosis of both necrotic bodies and myelinin vitro,they release large amounts of pro-inflammatory cytokines. Thus,both Schwann cells and macrophages (in particular the latter),but not OECs,release high levels of pro-inflammatory cytokines whilst phagocytosing myelin and necrotic bodies. These include TNF-α,which is known to exacerbate CNS injury (Van der Laan et al.,1996; Nazareth et al.,2020).
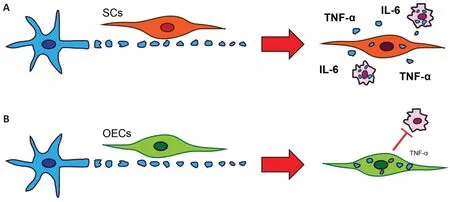
Figure 1|SCs and OECs have different phagocytic responses to necrotic bodies.
Conclusion and future perspective:While both OECs and Schwann cells are efficient phagocytes of necrotic bodies,there are key differences between the cell types. OECs can phagocytose larger quantities of necrotic bodies and myelin debris and degrade necrotic bodies more quickly than Schwann cells. In addition,OECs do not produce proinflammatory cytokines during this process,whereas Schwann cells do,and OECs appear to not attract macrophages. Thus overall,OECs are more efficient phagocytes and cause less inflammation,and are therefore likely better candidates for transplantation into the injured CNS,than Schwann cells. Transplanting OECs to repair CNS injuries may improve the removal of necrotic bodies and prevent the recruitment of proinflammatory macrophages/microglia to the injury site. However,to improve therapeutic outcomes it is also important to further determine the cellular and molecular mechanisms involved in this process,particularly when the glia are transplanted into the hostile,pro-inflammatory CNS injury site environment. Understanding the cellular and molecular mechanisms may also lead to the identification of novel drugs that could be used to stimulate the phagocytic activity of OECs and Schwann cells,further improving their therapeutic potential.
This work was supported by a Garnett-Passe and Rodney Williams Memorial Foundation Grant to JE,a National Health and Medical Research Council Grant to JS and JE (grant No. APP1183799),a Motor Accident Insurance
Commission Queensland Grant to JS and JE,a Perry Cross Foundation Grant to JE and JS,and a Clem Jones Foundation grant to JS and JE.
Lynn Nazareth,James St John,Jenny Ekberg*
Menzies Health Institute Queensland,Griffith University,Southport,Australia (Nazareth L,St John J,Ekberg J)Clem Jones Centre for Neurobiology and Stem Cell Research,Griffith University,Nathan,Australia (Nazareth L,St John J,Ekberg J)Griffith Institute for Drug Discovery,Griffith University,Nathan,Australia (St John J,Ekberg J)
*Correspondence to:Jenny Ekberg,PhD,j.ekberg@griffith.edu.au.
https://orcid.org/0000-0001-5151-4966(Jenny Ekberg)
Date of submission:February 8,2021
Date of decision:March 8,2021
Date of acceptance:April 21,2021
Date of web publication:July 8,2021
https://doi.org/10.4103/1673-5374.317969
How to cite this article:Nazareth L,St John J,Ekberg J (2022) Improving cell transplantation by understanding and manipulating the phagocytic activity of peripheral glia. Neural Regen Res 17(2):313-314.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Maria Easler,University of Padova,Italy.
- 中国神经再生研究(英文版)的其它文章
- A Drosophila perspective on retina functions and dysfunctions
- Celeboxib-mediated neuroprotection in focal cerebral ischemia: an interplay between unfolded protein response and inflammation
- Pramipexole,a dopamine D3/D2 receptor-preferring agonist,attenuates reserpine-induced fibromyalgia-like model in mice
- Effects of delayed repair of peripheral nerve injury on the spatial distribution of motor endplates in target muscle
- Neurorehabilitation using a voluntary driven exoskeletal robot improves trunk function in patients with chronic spinal cord injury: a single-arm study
- Gene and protein expression profiles of olfactory ensheathing cells from olfactory bulb versus olfactory mucosa

