Enhanced Arsenite Removal Using Bifunctional Electroactive Filter Hybridized with La(OH)3
ZHANG Shujing(张淑静), FANG Xiaofeng(方小峰), 2*, LIU Yanbiao(刘艳彪),2
1 Textile Pollution Controlling Engineering Center of Ministry of Environmental Protection, College of Environmental Science and Engineering, Donghua University, Shanghai 201620, China 2 Shanghai Institute of Pollution Control and Ecological Security, Shanghai 200092, China
Abstract: A bifunctional electroactive filter was rationally fabricated for simultaneous oxidation and sequestration of toxic trivalent arsentic As(Ⅲ). A novel nanoscale La(OH)3 modified electrochemical carbon nanotube(CNT)network filter was prepared by a facile electrodeposition strategy. The As(Ⅲ)decontamination kinetics and adsorption capacity were both found to increase with the flow rate(1.5-6.0 mL/min)and the applied voltage(0-2.5 V). The CNT filter hybridized with La(OH)3(CNT-La(OH)3)has demonstrated the ultra-high adsorption capacity of 750.2 mg/g for As(Ⅲ), which is ascribed to the combined role of sufficient adsorption sites, flow-through filtration and electric field. XPS analysis revealed that the As(Ⅲ)decontamination mechanism involved a two-step adsorption-oxidation process. The formation of inner-sphere La-O-As complexes, ligand exchange and electro-adsorption are all parts of the As(Ⅲ)adsorption process. The adsorbed neutrally-charged As(Ⅲ)was further oxidized to negatively-charged As(V)when aided by an electric field, which could be effectively sequestrated by La(OH)3. The CNT-La(OH)3 filter shows high stability under alkaline conditions and can be regenerated with dilute NaOH solution. In this study, all experiment results have demonstrated a promising and effective CNT-La(OH)3 electrochemical filter for As(Ⅲ)pollution minimization.
Key words: arsenite; carbon nanotube; La(OH)3; electrochemical filtration; adsorption-oxidation
Introduction
Arsenic(As)pollution in groundwater is an environmental issue of public concern.Excessive and long-term consumption of As-polluted water can cause serious health problems including skin lesions, neurological disabilities, and cancers[1].To reduce the health risk induced by As, the United States Environmental Protection Agency(USEPA)and European Union(EU)have regulated the contaminant level in drinking water below 10 μg/L and 6 μg/L, respectively.Various As removal techniques, such as adsorption[2], ion exchange[3], biological treatments[4], and electrochemistry methods[5], have been proposed to meet the strict drinking water purification levels.Among these techniques, adsorption is an attractive method for As uptake due to the advantages of the simple operation, high removal capacity, and low cost.Nowadays, diverse nano adsorbents such as alumina[6], iron hydroxides[7], manganese oxides[8], and bimetal or even ternary composite adsorbents[9], have been extensively used for As decontamination.Nonetheless, after adsorption, a complicated procedure for collecting and reusing of nano adsorbents is typically necessary.Besides, the adsorption capacity and adsorption kinetics in the traditional adsorption process are limited and need further improvement.
Electroactive membrane technology, which combines both the electrochemical and membrane separation processes, has gained wide acceptance from the scientific community[10].Innovative designs based on a range of organic and inorganic materials have been proposed so far, allowing the applied potential to be performed and spread over a portion of the entire membrane surface[11].Compared with the widely used polymeric membranes, the carbon nanotubes(CNTs)based membranes have emerged as a new generation of membrane materials by combining electrochemistry with conventional membranes processes.CNTs hold promise for many applications across a variety of industry sectors, such as a base material for electrodes and membranes, due to their high surface area, excellent electric conductivity, and desirable robustness[12].In a recent report, Liuetal.[13]have concluded the application of electroactive CNT-based membranes system under the electric field will contribute to enhancing removal kinetics and capacity.Furthermore, CNT-based membranes will allow for flow-through operation, allowing for maximum utilization of both the hybrid filter’s exterior and interior active sitesviaconvection-enhanced mass transport[14].For example, the flow-through convection design of a titanate-CNT filter was able to improve antimonite(Sb(III))adsorption kinetics by more than 3 times(0.305 mg/(g·h)vs.0.106 mg/(g·h))[15].
Recently, rare earth metals(REMs)have attracted extensive attention in the field of water treatment due to their favorable physical and chemical properties[16].Adsorbents prepared with REMs usually have higher removal efficiency and faster adsorption performance as compared to commonly used metals(e.g.aluminum, iron, and manganese)which can be due to more oxygen containing functional groups on their surface[17]and better catalytic properties[18].Lanthanum(La), as an environmentally and human-friendly REM, has piqued interest in the creation of new adsorbents[19].In the literature, the most common popular adsorption analysis for La-based adsorbents is on phosphate removal[20].As and phosphate are both members of nitrogen group, and have similar chemical structures.La-based materials are also considered as the superior adsorbents for the removal of As contamination.Combining nanoscale La-based adsorbents with an electroactive CNT membrane may be a novel technique for overcoming drawbacks of nano adsorbents and improving adsorption capability and kinetics.Nevertheless, the technique combining CNT membrane filtration and La-based materials adsorption has rarely been reported.
In this work, a novel electroactive membrane is produced with La(OH)3loaded on the CNT membraneviaa facile electrodeposition strategy.La(OH)3offers active sites for the removal of As; while CNT acts as the substrate and maintains the electrochemical reactivity.The electric field can enhance arsenite(As(Ⅲ))removal efficiency by electro adsorption and electromigration.Furthermore, highly toxic As(Ⅲ)can be oxidized to a less toxic arsenate(As(V))upon the application of an electric field.The CNT-La(OH)3network is characterized by various techniques.The effects of experimental parameters, such as the applied voltage, operation mode(batchvs.flow), and flow rate on the adsorption capacity and kinetics are evaluated.The As(Ⅲ)removal mechanism was further analyzed by quantitative determination of changes of As species in the effluent and X-ray photoelectron spectroscopy(XPS)analysis of a CNT-La(OH)3filter after As adsorption.In the analyses, we assume that(1)As(Ⅲ)can be effectively adsorbed by loaded nano La(OH)3due to high affinity towards As;(2)a flow-through filtration design can speed up the convection-enhanced mass transport of As(Ⅲ);(3)the electric field can suppress the desorption of surface As(Ⅲ)viaelectro adsorption and oxide As(Ⅲ)to As(V); and(4)these as-produced negatively-charged As(V)can be more favorable isolated by the positively-charged filterviaeletromigration.This research has developed a new and effective adsorbent for rapid and high-capacity As(Ⅲ)removal in a continuous-flow system.
1 Experiments
1.1 Chemicals and materials
All chemicals used were of analytical grade.Sodium arsenite(NaAsO2, ≥95.0%(mass fruction))was supplied by Sigma-Aldrich.Lanthanum nitrate hexahydrate((La(NO3)3·6H2O, 99.9% metals basis)), ammonium acetate(C2H7NO2, ≥98%),N-methyl-2-pyrrolidinone(NMP, ≥99.5%), sodium sulfate(Na2SO4, ≥99%), nitric acid(HNO3, 36%-38%), ethanol, sodium chloride(NaCl, ≥99.5%), sodium hydroxide(NaOH, ≥96%)and hydrochloric acid(HCl, 36%-38%)were purchased from Sinopharm Chemical Reagent Co., Ltd., China.Sodium bicarbonate(NaHCO3, ≥99.8%)and trisodium phosphate(Na3PO4, ≥96%)were supplied by Shanghai Aladdin Reagent Co., Ltd., China.Multiwalled CNTs with a tube diameter of 10-20 nm and a tube length of 10-30 μm were obtained from TimesNano Co., Ltd., Chengdu, China.All solutions were prepared with ultrapure water(18.25 MΩ·cm)produced by Milli-Q Direct 8 purification systems.
1.2 Synthesis of the functional composite filter
Fabrication of CNT filter: CNT powders were first treated by concentrated HNO3at 70 ℃ for 12 h to introduce oxygen-containing functional groups.Then, 20 mg CNT powder was dispersed into 40 mL NMP solutionviaprobe sonication for 45 min, followed by vacuum filtration onto a hydrophilic polytetrafluoroethylene(PTFE)membrane to obtain a CNT filter.
The CNT-La(OH)3filters can be fabricated by an electrodeposition route in a three-electrode system(i.e., a pre-formed CNT networks working electrode, a platinum counter electrode, and an Ag/AgCl saturated calomel electrode).A mixed nitrate solution(0.1 mol La(NO3)3·6H2O and 0.2 mol C2H7NO2in 100 mL ultrapure water)was used as electrolyte.The deposition process was performed at-1.0 Vvs.Ag/AgCl(exerted by a CHI 760E electrochemical analyzer)for 30 min under the condition of water bath temperature of 70 ℃.To optimize the La(OH)3loading, different electrodeposition time of 10, 20, 30 and 40 min were used, and the filters were named as CNT-La(OH)3-10, CNT-La(OH)3-20, CNT-La(OH)3-30, and CNT-La(OH)3-40, respectively.
1.3 Characterizations
The surface morphology of the pure CNT filters and CNT-La(OH)3filters were detected by the field emission scanning electrode microscope(FESEM, Hitachi S-4800, Japan).The surface chemical states and elemental compositions of CNT-La(OH)3filters before and after As(Ⅲ)decontamination were quantified by XPS(Escalab 250Xi, Thermo Fisher Scientific, USA).The loading content of La(OH)3was determined by thermogravimetric analysis(TGA)under air atmosphere with temperature ranging from 30 ℃ to 800 ℃ at a rate of 10 ℃/min.The point of zero charge(pHzpc)of CNT-La(OH)3filters before and after As(Ⅲ)filtration were measured using a zeta potential analyzer(JS94 H, Antonpa(Shanghai)Trading Co., Ltd., China).
1.4 As(Ⅲ)decontamination experiments
The As(Ⅲ)removal experiments were conducted in two operational modes:batch and recirculated filtration.For batch mode, the as-fabricated CNT-La(OH)3filter was placed in a flask containing 100 mL of 500 μg/L As(Ⅲ)solution and 10 mmol/L Na2SO4electrolyte solution.For recirculated filtration, the filter was placed into an electrochemistry-modified Whatman polycarbonate filtration casing(Fig.S1)in Appendix A.A schematic illustration of the filtration device was available in a previous report[21].One hundred milliliters of 500 μg/L As(Ⅲ)solution containing 10 mmol/L Na2SO4electrolyte was pumped through the CNT-La(OH)3filter anode and a perforated Ti sheet cathode sequentially and then returned.The effect of La(OH)3loading amount, the flow rate(1.5-6.0 mL/min), applied voltage(0-2.5 V), initial solution pH(3.0-11.0), and co-existing anions(e.g., sulfate, nitrate, chloride, silicate, carbonate, and phosphate)on As(Ⅲ)and total As(the sum of As(Ⅲ)and As(V)in solution)removal efficiency were systematically investigated.Aliquots of 2 mL were taken at specific time intervals and were analyzed by an AF-610E atomic fluorescence spectrometer(AFS, Beijing Haiguang Instrument Inc., China).Applied voltage was provided by DC power supply(DH1766A-1, China).Solution pH was adjusted by 1 mol/L H2SO4or NaOH solution.Exhausted filters were regenerated by chemical washing with 100 mL of 5 mmol/L NaOH solution.
2 Results and Discussion
2.1 Characterization of the CNT-La(OH)3 filter
The surface morphology of CNT and CNT-La(OH)3filters were explored by FESEM, as depicted in Fig.1.The pristine CNT had a smooth surface, while the CNT-La(OH)3had a rough morphology with a coating of nano-sized La(OH)3film on the CNT surfaces.The successful coating was further identified by the EDS mapping of the CNT-La(OH)3filter: C, La, and O, where La elements coincided with O elements.TGA analysis results have shown the residual weight increases with loaded La(OH)3on the CNT filter and the loading capacity of La(OH)3within an effective filtration area was 0.41 mg/cm2.Brunauer-Emmett-Teller(BET)surface area of a CNT-La(OH)3filter was 116.33 m2/g, higher than that of 89.50 m2/g for the pristine CNT filters.The slight increase can be ascribed to loaded La(OH)3.For the XPS results, four pairs of new peaks were observed on the CNT-La(OH)3and the superficial elemental atomic ratio was 52.48% C, 38.87% O, and 8.65% La, respectively.Details on the functional groups(i.e., the hydroxyl groups)on the CNT-La(OH)3filter surface will be discussed in the following section.Besides, the point of zero charges(pHzpc)of the CNT-La(OH)3filter was 8.47, which was within that of 9.40 for La(OH)3nanorods[22]and 3.20 for CNTs[23].All these results suggested that the CNT-La(OH)3filter was successfully fabricatedviathe electrodeposition technique.

Fig.1 FESEM characterizations of(a)CNT filter and(b)CNT-La(OH)3 filter; TEM characterization of(c)CNT-La(OH)3 filter and the corresponding EDS mapping scanning spectra of(d)C,(e)La, and(f)O
2.2 As(Ⅲ)removal performance
The role of the electric field and La(OH)3on As(Ⅲ)oxidation and sequestration was investigated.As displayed in Fig.2(a), for the pristine CNT filter, the poor As removal performance was obtained in the absence of applied voltage.Besides, the total As removal efficiency(13.7%)was close to that of As(Ⅲ)(21.7%).This phenomenon indicates that CNT filters possess a rather limited oxidative capability and poor affinity to both As species, which is similar to the results reported in Ref.[24].Interestingly, under the applied voltage of 2 V, the As(Ⅲ)removal efficiency increased to 88.6% whereas total As removal did not increase significantly(12.6%).It was believed that the electric field can improve and accelerate the As(Ⅲ)detoxication.Such a high As(Ⅲ)conversion performance has been previously reported[25].Meanwhile, the total As removal efficiency of the CNT-La(OH)3filter(88.8%)at 2 V was higher than that of the pristine CNT filter(12.6%).The enhancement showed that loaded La(OH)3had a positive effect on As sequestration.Overall, the electric field and La(OH)3play an indispensable role in As(Ⅲ)oxidation and sequestration.
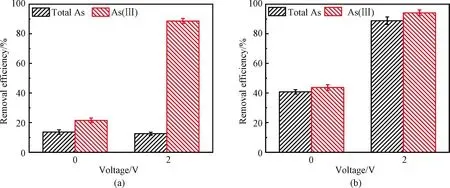
Fig.2 Comparison of As(Ⅲ)and total As removal efficiency without or with electric field using(a)CNT filter and(b)CNT-La(OH)3 filter
Considering that La(OH)3loading amount was an important parameter in dictating the As uptake, the total As removal efficiency at a functional of La(OH)3loading was performed by tailoring the electro-deposition time.As shown in Fig.3(a), the loading amount of La(OH)3was determined by TGA analysis.It was seen that the La(OH)3content increased with the increase of electrodeposition time.The La(OH)3weight percent increased from 25.5% to 41.3% with the deposition time increasing from 10 min to 40 min.The total As removal efficiency as a function of La(OH)3loading amount was further explored, as depicted in Fig.3(b).When the deposition time was extended from 10 min to 30 min, the total As removal efficiency increased from 47.6% to 88.8%, which may be ascribed to more La(OH)3deposited on CNT networks.However, when the deposition time further increased to 40 min, the total As removal efficiency tended to decrease to 77.6%.The inhibition effect can be attributed to the occurrence of agglomeration of adsorbents and blocking of active sites due to extended deposition[26].Based on the above analysis, the CNT-La(OH)3-30 filter was selected and used in subsequent investigations.

Fig.3 Comparison of CNT-La(OH)3 filters with different electrodeposition time(10, 20, 30, and 40 min)of(a)TGA curves and(b)total As removal efficiency
2.3 As(Ⅲ)adsorption kinetics
The effects of operation modes(batchvs.flow), flow rates(1.5-6.0 mL/min), and applied voltages(0-2.5 V)on As(Ⅲ)adsorption kinetics and capacity were investigated.The results are shown in Fig.4.For all the conditions, the adsorbed amounts reached a maximum with a reaction time of 2 h.To quantitatively describe sorption kinetics at the key operational parameters, the experimental As(Ⅲ)removal kinetics data were fitted using the pseudo-first-order model shown in Eq.(1)and the pseudo-second-order model shown in Eq.(2), which are given as
qt=qe(1-exp(-k1t)),
(1)
(2)
whereqe(mg/g)andqt(mg/g)are the adsorbed amount of As at equilibrium and at timet(h), respectively;k1(h-1)andk2(g/(mg·h))are the related adsorption rate constants.The corresponding parameters are summarized in Tables S1 and S2.According to the higher values of correlation coefficient(R2>0.99), the data were best fitted with a pseudo-second-kinetics model, which suggested that the adsorption process was dominated by chemical reaction[27].
As shown in Fig.4(a), compared with the conventional batch mode, As(Ⅲ)adsorption capacity and kinetics increased significantly in the filtration mode.Theqevalues of 9.64, 15.21 and 14.24 mg/g were achieved withkvalues of 0.008 9, 0.016 9 and 0.017 7 g/(mg·h)at a flow rate of 1.5, 3.0, 6.0 mL/min, respectively.Note theqevalues at 3.0 mL/min was similar to that of 6.0 mL/min due to limited adsorption sites on the CNT-La(OH)3networks.The batch mode yielded aqeof 6.45 mg/g withkvalues of 0.001 3 g/(mg·h), which were less than 70% of all recirculation conditions.The increased adsorption kinetics and capacity can be ascribed to the flow-through design, which can make full use of the active sites of the filter by convection enhanced mass transport instead of the diffusion-controlled mass transport[28].
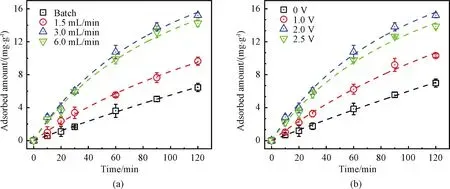
Fig.4 As(Ⅲ)adsorption kinetics on the CNT-La(OH)3 filter:(a)comparison of batch mode(black)and recirculated mode(flow rate 1.5-6.0 mL/min);(b)effect of applied voltage(0-2.5 V)on As(Ⅲ)adsorption
Figure 4(b)shows the effects of applied voltage(0-2.5 V)on As(Ⅲ)adsorption kinetics using a CNT-La(OH)3filter.In the range of 0-2.0 V, interestingly, the removal capacity and kinetics of As(Ⅲ)increased with applied voltage.The applied voltage increasedqefrom 6.98 mg/g at 0 V, to 10.33 mg/g at 1.0 V and to 15.21 mg/g at 2.0 V.Meanwhile,kincreased from 0.001 1 g/(mg·h)at 0 V to 0.005 8 g/(mg·h)at 1.0 V and to 0.016 9 g/(mg·h)at 2.0 V.A double function of the external voltage to the CNT-La(OH)3filter in the electrochemical filtration system has been proposed.The neutral As(Ⅲ)would get adsorbed on the surface of the CNT-La(OH)3filter, and oxidized to As(V).The electric filed enhances the near-surface transportviaelectromigration and improves the electrostatic interactions with the positively charged adsorption sites for negatively charged As(V).Besides, it could be possible that the electro adsorption reaction enhanced the deprotonation of surface As(Ⅲ)and suppressed the desorption[29].It has been reported that the pHzpcof metal oxides is determined by protonation and deprotonation of surface hydroxyl groups.As shown in Fig.S2, the shift of pHzpcto a lower pH range in the absence and presence of 500 μg/L As(Ⅲ)is the evidence of the formation of anionic negatively charged surface complexes[30].However, the total As removal capacity and kinetics cannot been further improved with increasing the applied voltage to 2.5 V.This may be due to the occurrence of other competing reactions such as water splitting accompanied with the production of oxygen and/or hydrogen bubbles on the surface of the electrode, leading to the blocking of active sites.Hence, an applied voltage of 2.0 V was selected as optimal for subsequent investigations.
2.4 Adsorption isotherm
At the optimal experimental conditions(applied voltage of 2.0 V, a flow rate of 3.0 mL/min, and pH of 7.0), we carried out the adsorption isothermal experiments to study the maximum adsorptive performance of CNT-La(OH)3filters in the recirculated mode for 12 h.The adsorption isotherm fitting curves are shown in Fig.5, where the points are experimental data and the dashed lines are the fittings to the Langmuir isotherm model shown in Eq.(3)rather than the Freundlich model(Fig.S3).
(3)
whereCe(mg/L)is the equilibrium As concentration in the solution,qmax(mg/g)is the maximum adsorption capacity, andKLis the Langmuir adsorption constant.As shown in Fig.5, the adsorption isotherm follows the model well(R2>0.99), indicating that the adsorption process is monolayer adsorption[31].Notably, the maximal adsorption capacities from the Langmuir model by the synthesized filter were 750.2 mg/g.We compared the maximum adsorption capacity of the CNT-La(OH)3filter with that of adsorbents reported in prior studies in recent years, and the results were summarized in Table 1.The comparison indicated that the As(Ⅲ)adsorption capacity of the filter is higher than that of adsorbent reported in earlier references.The ultra-high removal capacity may be contributed to the specific affinity of lanthanum, a large number of coordination sites of hydroxyl group[20], and the flow-through design combining the electric field[32].

Table 1 Comparison of the maximum As adsorption capacities of some adsorbents

Fig.5 Total As adsorption isotherms on CNT-La(OH)3 filter
2.5 XPS analysis
The XPS survey scan spectra of the synthesized CNT-La(OH)3filter before and after As(Ⅲ)filtration are illustrated in Fig.S4.The peaks in the XPS spectra of the fresh filter before adsorption indicated the appearance of C, O and La elements.The new As 3d peaks appeared after As filtration demonstrated the successful adsorption of As onto the CNT-La(OH)3filter.
The La 3d spectra of the CNT-La(OH)3filter before and after As(Ⅲ)filtration were shown in Fig.6(a), which mainly consist of the spin-orbit splitting of La 3d5/2and La 3d3/2.For the synthesized filter, the peaks of La 3d5/2were observed at 835.2 eV and 838.8 eV, and the peaks of La 3d3/2were observed at 852.1 eV.These peaks are similar to those of lanthanum hydroxide adsorbents[38].After As(Ⅲ)filtration, La 3d5/2and La 3d3/2characteristic peaks shifted to higher values(ca.0.7 eV).The electrons transfer in the valence band of La 3d and the formation of La-O-As inner surface complexes could explain this phenomenon[39].
With the purpose to further understand the mechanism between adsorbed As and the functional groups on the surface of filters, the O 1s XPS spectra of the CNT-La(OH)3filter before and after As(Ⅲ)uptake are analyzed.As shown in Fig.6(b), before As(Ⅲ)filtration, deconvolution of the O 1s XPS spectra of the fresh filter produced three peaks, which centered at 530.4 eV for crystal lattice(La-O-La), 531.8 eV for oxygen on the surface(La-O-H), and 532.9 eV for oxygen in the water molecules(H2O)[40].After As(Ⅲ)filtration in Fig.6(c), the peak with the binding energy of 531.2 eV appeared, which could be attributed to As-O.The ratio of La-O-H bonds decreased from 60.7% to 43.4%.This indicated that a portion of hydroxyl groups of lanthanum hydroxide might have interacted with As with the formation of La-O-As bonds through ligand exchange reaction[41-42].The results of XPS spectra analysis revealed that the adsorption enhancement might contribute to the inner-sphere complexation interaction.
The As 3d peak in the spectrum could be deconvoluted into two peaks: one at 46.3 eV for As(V)accounted for 70.7% and the other at 44.9 eV for As(Ⅲ)accounted for 29.3% in Fig.6(d).The result may be explained as follows: the applied electrical field can oxidize As(Ⅲ)to As(V), and the negatively charged As(V)is more favorable sequestered by the positively charged filter, and hence improves the total As removal efficiency.Furthermore, Jang and Dempsey[43]proposed that the process of As(Ⅲ)oxidation required a complexation process.Based on these results, the one possible pathway which was involved in the total As removal could be proposed: As(Ⅲ)was first adsorbed on the lanthanum hydroxide to form an As(Ⅲ)surface inner-complex, and then As(Ⅲ)was oxidized to As(V)by the electoral field followed by As(V)sequestration by the filter shown in Fig.7.

Fig.6 High resolution XPS of(a)La 3d spectra; O 1s spectra(b)before and(c)after As(Ⅲ)adsorption;(d)As 3d spectra after As(Ⅲ)uptake

Fig.7 Proposed mechanism of As(Ⅲ)adsorption-oxidation on the CNT-La(OH)3 nanohybrid filter
2.6 Effect of solution chemistry
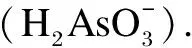
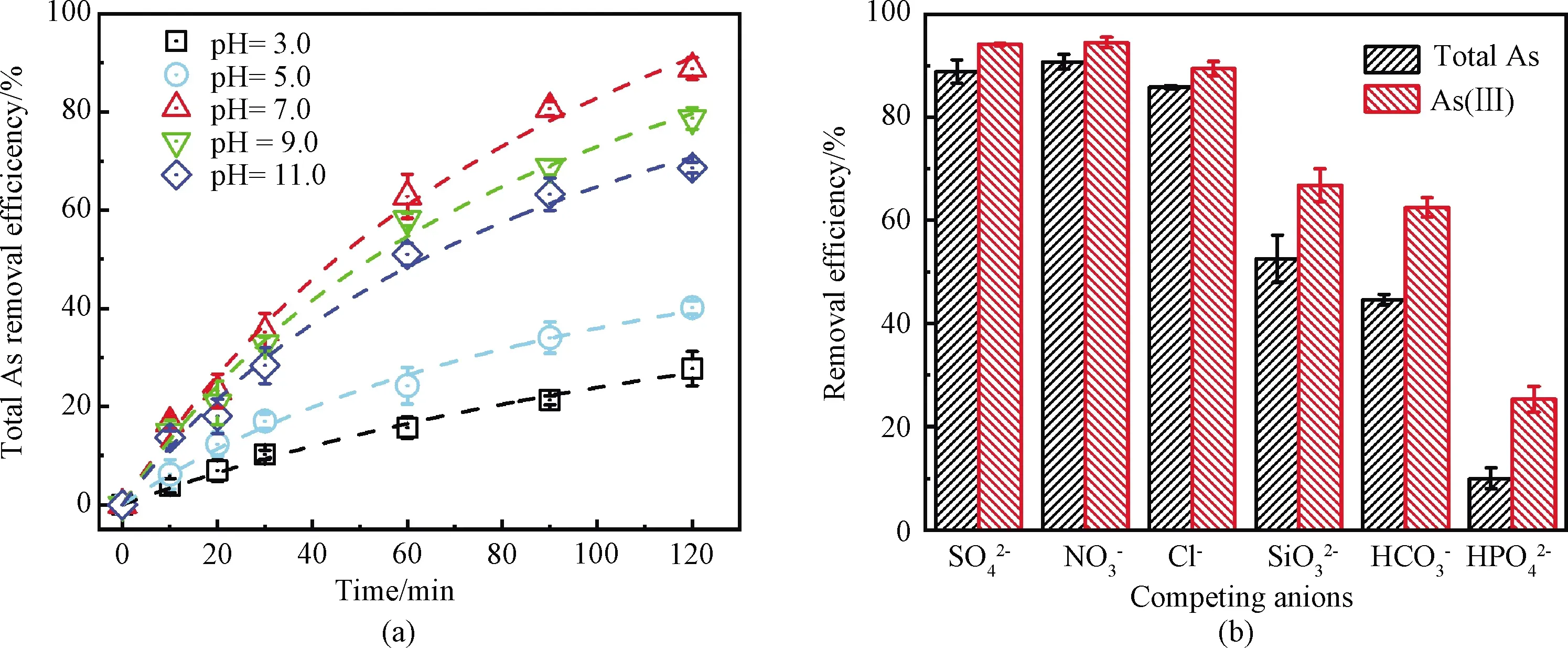
Fig.8 Total As removal performance on CNT-La(OH)3 filter under(a)different pH and(b)competing anions of 10 mmol/L
2.7 System stability evaluation
The proposed system efficacy was further evaluated under 500 μg/L As(Ⅲ)-spiked environmental water conditions.As shown in Fig.9(a), the total As removal efficiency of As(Ⅲ)-spiked tap water was 83.8% under optimal conditions, which was slightly decreased compared with that of ultrapure water(88.8%).In contrast, the total As removal efficiency declined to 62.7% in As(Ⅲ)-spiked lake water under similar conditions(a flow rate of 3 mL/min and an applied voltage of 2.0 V).It was believed that inorganic anions and natural organic matters in lake water usually posed negative impact on the removal of total As contaminants.
To investigate the leachability of La from La(OH)3-modified CNT filters, the La concentrations in the effluent were determined using the ICP-MS technique.In the adsorption experiments, almost no La was released to water(<1 μg/L).The result demonstrated that the CNT-La(OH)3filter was a stable material for As removal.Furthermore, the adsorption/desorption cycles were conducted five times to investigate the reusability of the CNT-La(OH)3filter.The Arsenic-loaded filter was regenerated by using 100 mL of 5 mmol/L Na(OH)solutions as the desorption agent[49].As shown in Fig.9(b), after the first cycle, the total As removal efficiency decreased from 88.8% to 76.0%.The effective adsorption amount of the fabricated filter for total As decreased as the number of regeneration cycle increased.However, the total As removal efficiency remained at 58.4% in the fifth regeneration.The decrease of removal efficiency could be due to the strong inner-sphere complexation bonds on the La(OH)3of the filter and it is difficult to reuse some of the active sites[50-51].Therefore, the regeneration process needs further investigation and optimization.
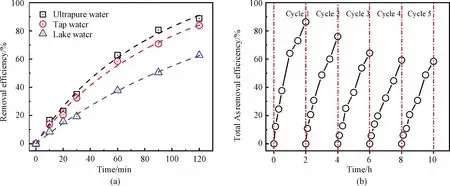
Fig.9 As(Ⅲ)removal performance of(a)different environmental matrix and(b)regeneration by chemical washing
3 Conclusions
In summary, CNT-La(OH)3filter was successfully prepared by a facile electrodeposition method and used for effective oxidation and sequestration of As(Ⅲ).An external potential not only can stabilize the adsorbed As(Ⅲ)and suppress desorption, but also caninsituoxide As(Ⅲ)to less toxic As(V).These produced As(V)can be further sequestered by La(OH)3.Through the synergistic effect of electrical conductivity, abundant active sites towards As and unique flow through design, an ultrahigh removal capacity of 750.2 mg/g was achieved by using the electroactive system based on a CNT-La(OH)3filter anode.The excellent As(Ⅲ)decontamination capability of the new electrochemical filtration system demonstrates the feasible application for As-contaminated wastewater.
Appendix A
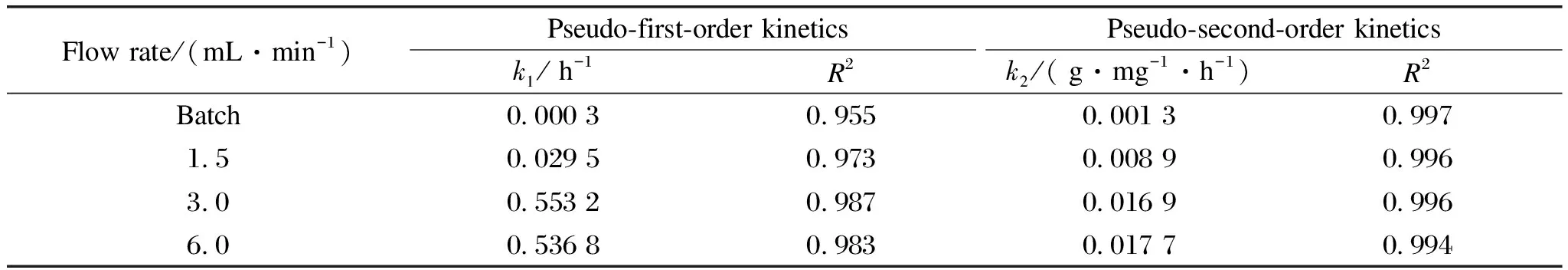
Table S1 Kinetic parameters of pseudo-first-order and pseudo-second-order fitting for As adsorption as a function of flow rate
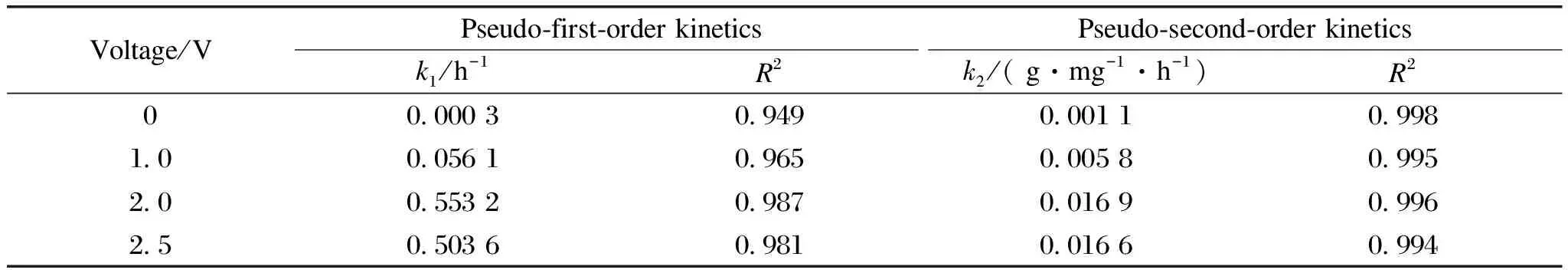
Table S2 Kinetic parameters of pseudo-first-order and pseudo-second-order fitting for As adsorption as a function of applied voltage

Fig.S1 Schematic illustration of the electroactive filtration apparatus:(a)a titanium anodic ring;(b)anode CNT-La(OH)3 filter;(c)a PTFE membrane support;(d)a perforated titanium shim cathode
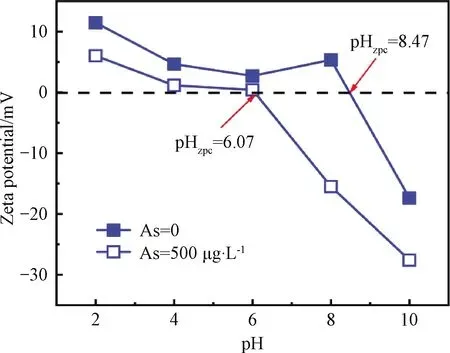
Fig.S2 Zeta potential of CNT-La(OH)3 filter as a function of pH and As(Ⅲ)concentration

Fig.S3 Isotherm curve of total As adsorption by CNT-La(OH)3 filter fitted by Freundlich model
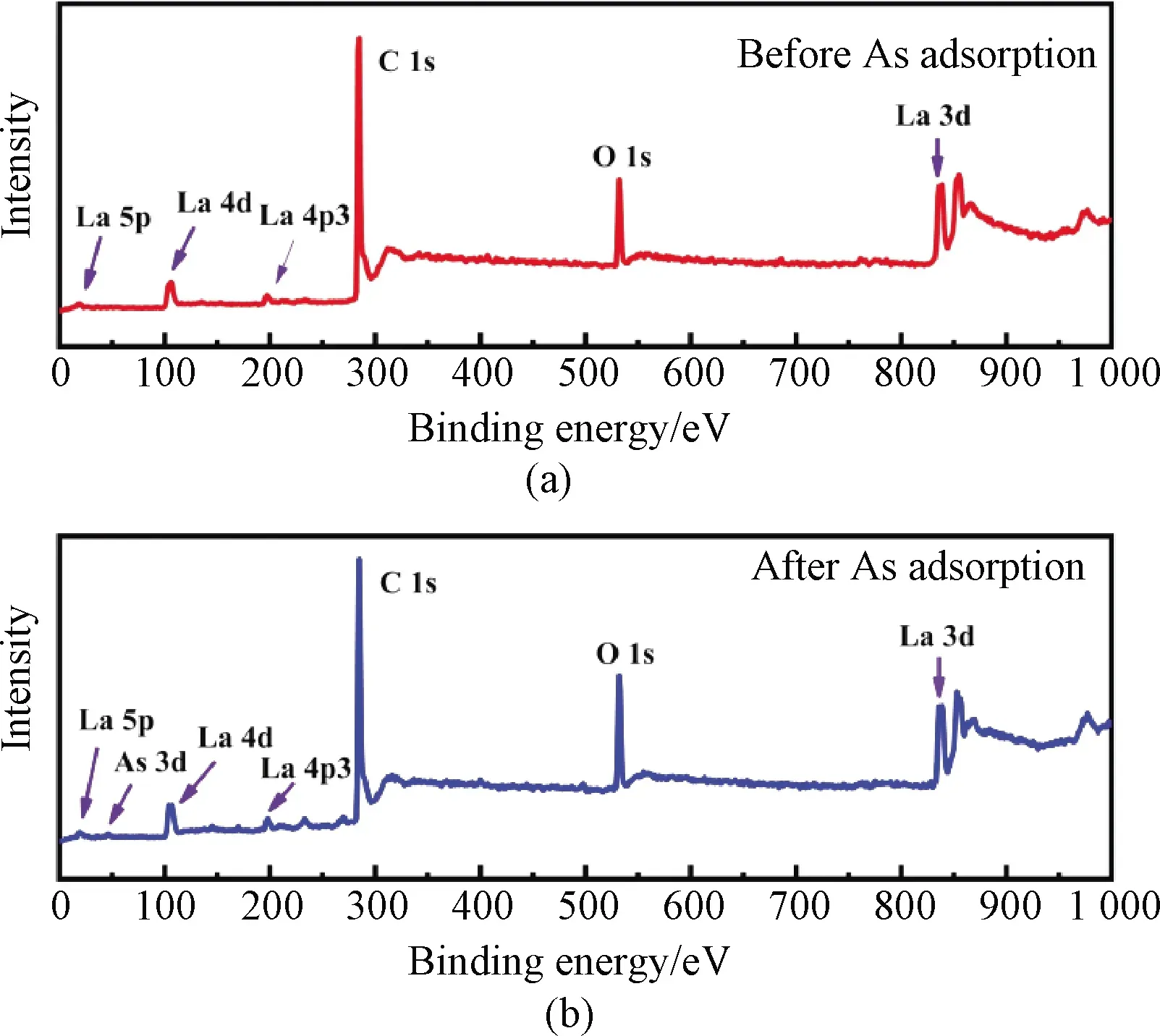
Fig.S4 XPS survey spectra of CNT-La(OH)3 filter(a)before and(b)after As adsorption
 Journal of Donghua University(English Edition)2022年3期
Journal of Donghua University(English Edition)2022年3期
- Journal of Donghua University(English Edition)的其它文章
- Three-Dimensional Model Reconstruction of Nonwovens from Multi-Focus Images
- Toughness and Fracture Mechanism of Carbon Fiber Reinforced Epoxy Composites
- Three-Dimensional Metacomposite Based on Different Ferromagnetic Microwire Spacing for Electromagnetic Shielding
- Blockchain-Based Architectural Framework for Vertical Federated Learning
- Optimal Control of Heterogeneous-Susceptible-Exposed-Infectious-Recovered-Susceptible Malware Propagation Model in Heterogeneous Degree-Based Wireless Sensor Networks
- Students’ Feedback on Integrating Engineering Practice Cases into Lecture Task in Course of Built Environment
