COVID-19 vaccine-associated myocarditis
Michael C Morgan,Lavannya Atri,Sean Harrell,Wael Al-Jaroudi,Adam Berman
Michael C Morgan,Lavannya Atri,Sean Harrell,Wael Al-Jaroudi,Division of Cardiology,Medical College of Georgia,Augusta,GA 30912,United States
Adam Berman,Baptist Heart,Baptist Medical Center,Jackson,MS 39202,United States
Adam Berman,Department of Population Health Sciences,Medical College of Georgia,Augusta,GA 30912,United States
Abstract Myocarditis is now recognized as a rare complication of coronavirus disease 2019(COVID-19) mRNA vaccination,particularly in adolescent and young adult males.Since the authorization of the Pfizer-BioNTech™ and Moderna™ mRNA vaccines targeting the severe acute respiratory syndrome coronavirus-2 (SARSCoV-2) spike protein,the Centers for Disease Control and Prevention (CDC) has reported 1175 confirmed cases of myocarditis after COVID-19 vaccination in individuals ages 30 years and younger as of January 2022.According to CDC data in June 2021,the incidence of vaccine-mediated myocarditis in males ages 12-29 years old was estimated to be 40.6 cases per million second doses of COVID-19 mRNA vaccination administered.Individuals with cases of COVID-19 vaccinemediated myocarditis typically present with acute chest pain and elevated serum troponin levels,often within one week of receiving the second dose of mRNA COVID-19 vaccination.Most cases follow a benign clinical course with prompt resolution of symptoms.Proposed mechanisms of COVID-19 vaccine myocarditis include molecular mimicry between SARS-CoV-2 spike protein and self-antigens and the triggering of preexisting dysregulated immune pathways in predisposed individuals.The higher incidence of COVID-19 vaccine myocarditis in young males may be explained by testosterone and its role in modulating the immune response in myocarditis.There is limited data on long-term outcomes in these cases given the recency of their occurrence.The CDC continues to recommend COVID-19 vaccination for everyone 5 years of age and older given the greater risk of serious complications related to natural COVID-19 infection including hospitalization,multisystem organ dysfunction,and death.Further study is needed to better understand the immunopathology and long-term outcomes behind COVID-19 mRNA vaccine-mediated myocarditis.
Key Words: COVID-19;SARS-CoV-2;mRNA vaccine;Myocarditis;Pericarditis
INTRODUCTION
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2),the novel virus responsible for the coronavirus disease 2019 (COVID-19) pandemic,has impacted the entire globe and continues to spread.On December 11,2020,the United States Food and Drug Administration granted an emergency use authorization (EUA) for the Pfizer-BioNTech™ COVID-19 vaccine in individuals 16 years of age or older.Seven days later,another EUA was released for the Moderna™ vaccine in adults 18 years of age or older[1].Since their introduction,the mRNA vaccines against the SARS-CoV-2 virus have been highly effective in preventing both symptomatic and asymptomatic infections along with COVID-19-related hospitalizations and death[2].Despite the great success of these vaccines,they have not come onto the public stage without controversy.In May 2021,the first case of myocarditis following mRNA vaccination was identified,and as of January 12,2022,the Vaccine Adverse Events Reporting System(VAERS) had received 2077 reports of myocarditis or pericarditis among people ages 30 and younger who received a COVID-19 vaccine with 1175 confirmed cases[3].This article aims to review the current literature regarding COVID-19 vaccine-related myocarditis.
EPIDEMIOLOGY AND CLINICAL PRESENTATION OF COVID-19 VACCINE ASSOCIATED MYOCARDITIS
While the possibility for developing myocarditis or pericarditis following COVID-19 vaccination is concerning,it is important to emphasize that the incidence of this adverse effect is rare.Since January 2022 there have been over 502 million doses of COVID-19 mRNA vaccines administered across the United States with less than 1175 confirmed cases of myocarditis or pericarditis[3].The primary group being impacted by this adverse event is the male adolescent and young adult population,ages 12-29[4-8].The principal window of risk for the development of COVID-19 vaccine-mediated myocarditis appears to be within a week of receipt of the vaccine and occurs most commonly following the second dose of an mRNA vaccine[4,5,7,9].Affected young men are predominately healthy individuals without a history of COVID-19 infection or comorbidities.Resolution of clinical symptoms usually occurs within 6 d with preservation of cardiac function,indicative of overall fast recovery with no short-term complications[4,5,8].
It is challenging to calculate the true incidence of vaccine-related myocarditis in the United States,as currently reported case series are not population-based.Based on crude data with both confirmed and unconfirmed cases reported to the VAERS,the CDC has estimated the incidence rates of myocarditis to be 40.6 casespermillion second doses of mRNA COVID-19 vaccines administered to males between 12 and 29 years old[1].Females in the same age group had an estimated incidence of 4.2 cases of myocarditispermillion second doses.In adults 30 years and older,rates of myocarditis were reported as 2.4 casespermillion second doses in males and 1.0 casepermillion second doses in females.As of December 31,2021,VAERS has processed 4317 reported events of COVID-19 vaccine-associated myocarditis and pericarditis across all age groups with the highest number of cases reported for both myocarditis and pericarditis in the age group 18-29 (Figure 1A)[9].

Figure 1 Coronavirus disease 2019 vaccine-mediated myocarditis and pericarditis cases reported to Vaccine Adverse Event Reporting System.
Investigators from Israel queried the database of the largest Israeli healthcare organization that contains data related to 2.5 million vaccinated individuals.They determined that post-vaccine myocarditis had an estimated incidence rate of 2.13 cases [95% confidence interval (CI): 1.56-2.70]per100000 individuals who had received at least one dose of the Pfizer-BioNTech™ vaccine[10].Additionally,the incidence increased to 10.69 casesper100000 individuals (95%CI: 6.93-14.46) among males between 16 and 29 years old.
To compare the three types of vaccines,a systematic review of 6 case reports and 2 case series with a total of 15 patients reported that 60% of the myocarditis-related COVID-19 vaccine cases were associated with the Pfizer-BioNTech™ vaccine,33% were associated with the Moderna™ vaccine,and 7% were associated with the Johnson &Johnson™ vaccine[5].Similarly,as of December 31,2021,VAERS indicated Pfizer-BioNTech™ had the highest number of cases reported for myocarditis and pericarditis,1615 and 1063 cases respectively (Figure 1B)[11].The clinical presentation of post-vaccine myocarditis is similar to other forms of myocarditis,most commonly featuring acute chest pain combined with other symptoms such as shortness of breath,fever,and palpitations[6,8,9,12,13].Evidence of myocardial injuryviaserum troponin elevations was present in all cases.Electrocardiogram(EKG) findings were varied but often showed ST segment elevations.Echocardiogram findings ranged from preserved ejection fraction to varying degrees of wall motion abnormalities.When cardiac magnetic resonance imaging (MRI) was performed,findings were consistent with acute myocarditis with late gadolinium enhancement being the most commonly cited abnormality.Figure 2 displays a cardiac MRI consistent with myocarditis following COVID-19 vaccination in a 21-year-old male.Notably,most cases resulted in normalization of symptoms,troponin levels,and EKG/echocardiogram abnormalities upon discharge or at follow-up (Table 1).The CDC Vaccine Safety Technical Work Group Report on August 30,2021,reviewed 98 cases with chest pain,pressure,and discomfort of which 56% of the cases met confirmatory criteria for myocarditis within 0-21 d of vaccination with elevated troponin,abnormal EKG findings,and abnormal MRI commonly found.It was determined that all of these included cases were discharged home,with 76% of them being discharged within 0-2 d[14].
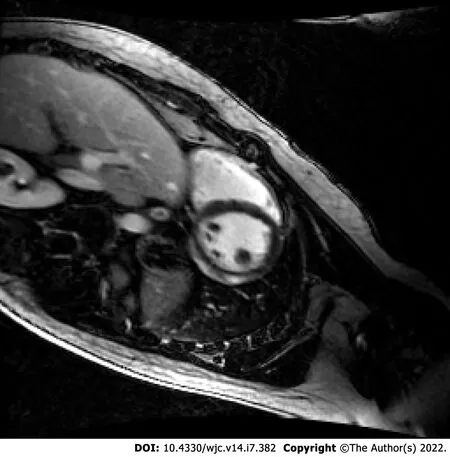
Figure 2 Cardiac magnetic resonance imaging of coronavirus disease 2019 vaccine-associated myocarditis.
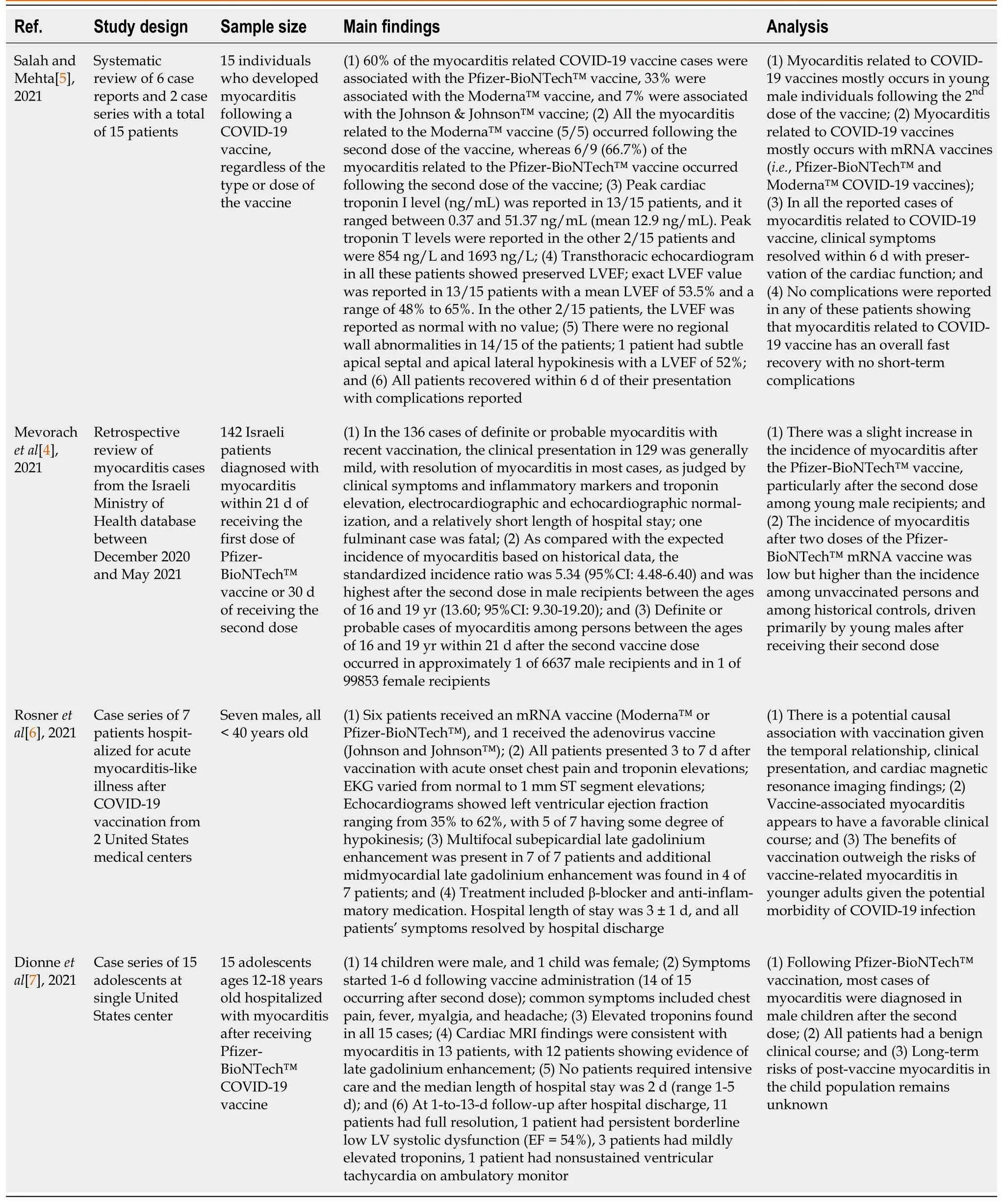
Table 1 Summary of coronavirus disease 2019 vaccine-associated myocarditis studies
POTENTIAL MECHANISMS OF COVID-19 VACCINE ASSOCIATED MYOCARDITIS
The mechanism underlying COVID-19 vaccine-mediated myocarditis is poorly understood.SARS-CoV-2 mRNA vaccines contain nucleoside-modified mRNA encoding for the virus’s spike protein encapsulated in lipid nanoparticles which aid in delivery of the mRNA into the cell.The cell then produces the spike protein,and a subsequent adaptive immune response ensues generating antibodies against the spike protein.The nucleoside modification of the mRNA aids in reducing the mRNA’s immunogenicity,however in some individuals with an unknown genetic predisposition,exposure to the mRNA may result in an overactivated immune responseviadendritic cells and Toll-like receptors of the innate immune system leading to proinflammatory immune cascades and cytokine activation[15].This inflammatory response is thought to play a role in COVID-19 vaccine-associated myocarditis.The role of mRNA in the development of vaccine-mediated myocarditis is further supported by the evidence that the incidence of myocarditis occurs at a much higher rate following mRNA vaccination compared to the adenovirus vector vaccine of Johnson and Johnson™[4].
Another potential mechanism for COVID-19 vaccine myocarditis is molecular mimicry between the spike protein of SARS-CoV-2 and self-antigens.Antibodies of the SARS-CoV-2 spike protein have been shown to cross-react with human proteins of similar structure including α-myosin in experimental studies[16].It appears more likely that the immune-mediated adverse effects of mRNA vaccination are due to the triggering of preexisting dysregulated pathways in certain predisposed individuals rather than the inherent immunogenicity of the vaccine itself[15].Polymorphisms in interleukin-6 have been suggested as an important genetic component for determining autoinflammatory dysregulation that may ensue upon exposure to SARS-CoV-2,however further study is needed to elucidate these theories[17].
Young men have been found to be most susceptible to the development of myocarditis outside the setting of COVID-19 vaccination as well.Kytöet al[18] have presented evidence that testosterone appears to play a major role in the pathogenesis of myocarditis identifying testosterone-mediated mechanisms such as inhibition of anti-inflammatory cell populations promoting cardiac inflammation,a preference towards a Th1 immune response,and increased transcription of cardiac fibrotic remodeling genes[18].Conversely,estrogen appears to play a protective roleviathe preference of a Th2 immune response,stimulation of inhibitory regulatory T cells,and inhibition of proinflammatory T cells[18].These mechanisms may contribute to why the young male population has the highest incidence of postvaccine myocarditis.
COVID-19 VACCINE ASSOCIATED MYOCARDITIS IN CHILDREN AND ADOLESCENTS
Cases of myocarditis have also been reported in the childhood population since the Pfizer-BioNTech™COVID-19 vaccine was authorized for emergency use on May 10,2021,for children ages 12 and older.A case series of 15 adolescents who developed myocarditis following administration of the Pfizer-BioNTech™ vaccine found that,similar to the adult population,the most commonly affected group were young males ages 12-18 years old following administration of the second dose[7].All patients in this study had an uncomplicated short-term clinical course,however the long-term prognosis of these adolescent patients remains unclear,emphasizing the importance of continued follow-up and monitoring.
A case series published by Marshallet al[19] reported myocarditis or pericarditis in 7 male adolescents ages 14-19 years old,all within 4 d of receiving the second dose of the Pfizer-BioNTech™COVID-19 vaccine[19].All 7 patients presented with elevated troponin levels.ST segment elevation was the most common EKG and was observed in 6/7 individuals.Echocardiogram results were normal in 5 of 7 patients;however,all patients had cardiac MRI findings consistent with acute myocarditis.Investigatory studies for other etiologies of myocarditis including respiratory pathogen panels,serum polymerase chain reaction (PCR) tests,and infectious serologies all returned negative,and multisystem inflammatory syndrome in children was excluded based on cardiac MRI findings.
EVALUATION AND MANAGEMENT OF COVID-19 VACCINE ASSOCIATED MYOCARDITIS
Given the increased incidence of myocarditis following mRNA vaccination in adolescent and young adult males,clinicians should have a high index of suspicion for myocarditis in this demographic who present with symptoms such as acute chest pain,shortness of breath,or palpitations.Initial evaluation should include obtaining an EKG,serum troponin levels,complete blood count with differential,chest x-ray,inflammatory markers such as C-reactive protein and erythrocyte sedimentation rate,brain natriuretic peptide,and an echocardiogram (Figure 3).If this initial workup supports a diagnosis of myocarditis,cardiology consultation should take place in conjunction with studies seeking to determine potential alternative etiologies of myocarditis.Consultation with infectious disease and/or rheumatology may also be considered to aid in this process[20].PCR testing for acute COVID-19 infection and SARS-CoV-2 antibody testing for prior COVID-19 infection are of particular importance.Obtaining enterovirus PCR along with a respiratory pathogen panel can assist in ruling out other potential viral etiologies (e.g.Coxsackievirus,Epstein-Barr virus,cytomegalovirus,respiratory syncytial virus,parvovirus) and autoimmune serologies such as antinuclear antibodies may be indicated depending on clinical presentation.Cardiac MRI may be utilized to aid in diagnosing suspected myocarditis without the need for obtaining invasive endomyocardial biopsy.

Figure 3 Clinical decision-making algorithm for diagnosis and management of suspected coronavirus disease 2019 vaccine-associated myocarditis.
The clinical management of COVID-19 vaccine-mediated myocarditis is largely supportive,and patients frequently exhibit rapid resolution of symptoms and normalization of cardiac biomarkers.Those with persistent mild symptoms and no signs of arrhythmia,left ventricular systolic dysfunction,or hemodynamic instability may benefit from therapy with nonsteroidal anti-inflammatory drugs,colchicine,or steroids.In more serious cases of myocarditis,such as those patients showing signs of hemodynamic instability,new-onset arrhythmia,or worsening systolic dysfunction,intravenous steroids or intravenous immunoglobulin may be considered[15].Patients with reduced ejection fraction should be placed on beta-blockers and angiotensin-converting enzyme inhibitors according to guideline-directed medical therapy.The majority of cases from prior reports resulted in a resolution of symptoms and abnormal cardiac studies prior to discharge following a short hospital stay with supportive care or a short course of nonsteroidal anti-inflammatory.Close monitoring and avoidance of strenuous exercise until a complete resolution of symptoms and normalization of cardiac biomarkers,EKG,and echocardiogram is an important measure,especially in the young age group who may be eager to return to a normal exercise routine.If a patient develops myocarditis following a first dose of mRNA vaccination,the CDC recommends the second dose be delayed and reconsidered later following the complete resolution of signs and symptoms[20].Of note,there are currently no randomized controlled trials examining the management of post-vaccine myocarditis which highlights the importance of the inclusion of cardiovascular specialists in the management and follow-up of these patients.
The concerted efforts of the biomedical community to develop safe and efficacious vaccinations in such a short time frame have been extraordinary.SARS-CoV-2 virus mRNA vaccines have been tremendously successful in curtailing the morbidity and mortality associated with COVID-19[21].Healthy adolescents and young adults are not immune to serious complications from COVID-19 infection and rising adolescent hospitalization rates from COVID-19 infection have been observed[22].Despite media attention regarding adverse effects of these vaccines,it is important to emphasize the low incidence in which these events occur.The benefits of vaccination to prevent both the spread and possible complications of COVID-19 infection including hospitalization,multisystem organ dysfunction,and death far outweigh the potential risk of post-vaccine myocarditis while this global pandemic persists.
The diagnosis of myocarditis is a serious one as cardiac myocytes do not regenerate,and an insult at a young age can lead to an increased risk of developing cardiac disease later on in life[23].While myocarditis has been linked to COVID-19 mRNA vaccination,it is important to compare the risk of developing myocarditis following vaccination to the risk following natural COVID-19 infection.A large study in Israel used data from the nation’s largest healthcare organization to determine the risk of myocarditis following vaccination after adequately matching vaccinated individuals to unvaccinated individuals[24].42 d after vaccination,they found a risk ratio of 3.24;95%CI: 1.55-12.44,and a risk difference of 2.7 eventsper100000 persons;95%CI: 1.0-4.6.To put this in context,they then determined the risk of myocarditis among SARS-CoV-2 infected individuals matched to uninfected individuals.COVID-19 infection was associated with a much higher risk of myocarditis with a risk ratio of 18.28;95%CI: 3.95-25.12 and a risk difference of 11.0 eventsper100000 persons;95%CI: 5.6-15.8[24].This largescale study supports the notion that the risk of myocarditis is much higher in the setting of natural COVID-19 infection compared to myocarditis following vaccination.Given the widespread transmissibility of this virus,it stands to reason that an individual should receive a vaccine to safeguard against the increased risk of myocardial injury associated with COVID-19 infection.
Underreporting of myocarditis in the adolescent and young adult male population is possible,as there may be a low index of suspicion in this relatively healthy age group.Mild cases of post-vaccine myocarditis are likely to go unreported as there is currently no routine screening protocol in place.However,as public awareness of post-vaccine myocarditis continues to grow,there may also be a potential for overreporting as well,emphasizing the need for effective surveillance systems to confirm suspected cases.
Based on the current available data,the CDC is continuing to recommend that patients aged 5 years and older be vaccinated against COVID-19,stating that the known risks and potential complications associated with COVID-19 infection far outweigh the rare chance of developing an adverse reaction to vaccination including myocarditis.Vaccine-mediated myocarditis has a low incidence,and while clinicians should be vigilant for its occurrence,this adverse effect should not deter vaccination efforts during this pandemic based on current data.Continued monitoring and reporting to the Vaccine Adverse Event Reporting System is strongly encouraged.Additional guidance from the American Heart Association for follow-up of patients with myocarditis emphasizes the use of cardiac MRI to examine the heartin vivo[25].
One of the major limitations of many of the studies describing post-vaccine myocarditis is the lack of follow-up data given the recency of vaccine approval and administration.There is minimal long-term data available to date which limits our ability to interpret long-term outcomes of patients who receive a diagnosis of COVID-19 vaccine-associated myocarditis.However the availability of long-term data will accumulate with time.Additionally,many of these studies were case reports compiledviacolleague communication rather than surveillance systems which allow for more complete diagnostic evaluation to exclude other potential etiologies of myocarditis.Many of the cases reported were presumed to be a result of the COVID-19 vaccine purely based on temporal association.While feasible,this assumption might result in the overestimation of myocarditis as a result of COVID-19 vaccination.Similarly,many studies used negative antibody tests to rule out active or prior COVID-19 infection which could be problematic due to false negatives or waning immunity.
With third dose eligibility for the Pfizer-BioNTech™ and Moderna™ mRNA vaccines recently expanding to include much of the adult general public,careful prospective observation of the rate of vaccine-mediated myocarditis following the third dose compared to rates following the second dose is needed.While there is limited data currently available,data obtained by the Israel Ministry of Health have reported lower rates of myocarditis following the third dose compared to the second dose.Proactive surveillance efforts have discovered 17 total myocarditis/perimyocarditis cases among ages 16-59 years following administration of over 2.5 million third doses of the Pfizer-BioNTech™ mRNA vaccine[26].
CONCLUSION
Vaccine-mediated myocarditis following vaccination against COVID-19 using mRNA vaccines is a rare,but potentially serious occurrence.Clinicians should be aware of the potential development of vaccinemediated myocarditis,particularly in young males.Early consultation with cardiologists and further investigationviaserum biomarkers and imaging with cardiac MRI may confirm the diagnosis.Longterm follow up of those patients developing vaccine-mediated myocarditis is necessary to assess the potential for chronic complications of this rare phenomenon.Despite the potential for vaccine-mediated myocarditis,vaccination continues to be recommended against COVID-19 in all eligible populations.
FOOTNOTES
Author contributions:Morgan MC and Atri L wrote the paper;Harrell S,Al-Jaroudi W and Berman A made critical revisions and added content to the manuscript;Berman A conceived the topic and provided oversight of the writing,editing and submission process.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:United States
ORCID number:Michael C Morgan 0000-0002-4780-5944;Lavannya Atri 0000-0002-0601-5575;Sean Harrell 0000-0002-1381-3049;Wael Al-Jaroudi 0000-0003-0890-4437;Adam Berman 0000-0002-9023-1130.
S-Editor:Fan JR
L-Editor:A
P-Editor:Fan JR
 World Journal of Cardiology2022年7期
World Journal of Cardiology2022年7期
- World Journal of Cardiology的其它文章
- Heart failure with reduced,mildly reduced,or preserved left ventricular ejection fraction: Has reasoning been lost?
- National trend of heart failure and other cardiovascular diseases in people living with human immunodeficiency virus
- Vitamin d deficiency and metabolic syndrome:The joint effect on cardiovascular and all-cause mortality in the United States adults
- Is there a window of opportunity to optimize trastuzumab cardiac monitoring?
- Heart failure in general and cardiac transplant patients with COVID-19
