Effects of stress of different duration on depression-like behavior and expression of CB1 and GluA1 in medial prefrontal cortex of rats
Yu-Xin Zhang, Hong-Mei Sun, Cong Gai, Cui-Cui Cheng, Lu-Ping Yang, Zhen-Yu Guo,Yu-Shan Gao, Die Hu
Beijing University of Chinese Medicine, Beijing 102488, China
Keywords:Depression mPFC CB1 GluA1
ABSTRACT Objective: To explore the changes of cannabinoid receptor (CB1) and AMPA receptor subunit GluA1 in the medial prefrontal cortex (mPFC) of depression model rats induced by chronic unpredictable mild stress (CUMS). Methods: 30 rats were randomly divided into three groups.The three-week model group was given a three-week CUMS model, the four-week model group was given a four-week CUMS model, and the control group was given no treatment. The behavior of rats were detected in each group, and the expression of CB1 and GluA1 protein in synaptosomes in mPFC brain region was detected by Western blot. Results: Compared with the control group, the surcose preference decreased, the feeding latency increased in the novel inhibition feeding test, and the immobility time increased in the forced swimming test in the four-week model group. However, after three weeks of CUMS, there was no obvious change in the behavior of rats compared with the control group, suggesting that four-week model of CUMS was successful. CUMS can reduce the expression level of CB1 and GluA1 protein in synaptosomes of mPFC brain area in four-week model group (P<0.05), but there was no significant difference between three-week model group and control group. Conclusion: Fourweek CUMS was more likely to lead to depressive-like behavior in rats, which may be closely related to the expression of CB1 and GluA1 in mPFC.
1. Introduction
Depression is a widespread, chronic mental illness that can affect thinking, mood and physical health and is characterized by depressed mood, lack of energy, insomnia and other physical symptoms, leading to severe cognitive impairment.[1,2] It is characterized by depressed mood, lack of energy, insomnia and other physical symptoms, leading to cognitive impairment in severe cases. The global prevalence of depression is now over 350 million,and in 2008 WHO ranked major depression as the third leading cause of disease burden worldwide and predicted that depression will be the leading risk factor for human morbidity and mortality by 2030[3] . Cannabinoid receptor 1 (CB1), one of the endogenous cannabinoid receptors, is widely distributed in the brain, mainly in the presynaptic membrane of neurons, and numerous experimental and clinical studies have shown that endogenous cannabinoids are important regulators of synaptic plasticity and function, and are closely related to the development of depression[4] . The ionotropic glutamate receptor α-amino-3-hydroxy-5-methyl-4-isobutazolyl propionate receptor mediates excitatory synaptic transmission in the central nervous system, and AMPA receptor subunit 1 (GluA1)is mainly present in the postsynaptic membrane and is involved in the regulation of synaptic plasticity, and its abnormal expression is a common mechanism in a variety of psychiatric disorders[5] .
Chronic unpredictability mild stress (CUMS) has been widely used to model depression, mainly by simulating chronic stress and stimuli in humans, resulting in depression-like behaviors.[6] .Therefore, in this experiment, CUMS modeling was adopted in rats to screen for a more stable CUMS modeling time, and was used to investigate the correlation between stress-induced depression and CB1 and GluA1 proteins in the mPFC brain region by measuring the expression levels of cannabinoid receptors in synaptosomes in the medial prefrontal cortex (mPFC) of the rat brain. The correlation between stress-induced depression and CB1 and GluA1 proteins in the mPFC brain region was investigated.
2. Materials and methods
2.1 Experimental animals
Thirty Sprague-Dawley (SD) rats, male, weighing (220-240 g),were purchased from Beijing Viton Lihua Laboratory Animal Technology Company, license No. SCXK (Beijing) 2016-0011.housed at the SPF Animal Experiment Center of Beijing University of Traditional Chinese Medicine, license No. SYXK (Beijing) 2016-0038. animal rooms were kept at constant temperature (23± 2℃) and relative humidity of 50% ~ 60% with 12h circadian rhythm control(8:00-20:00). The study was reviewed by the Experimental Animal Welfare Ethics Committee of Beijing University of Traditional Chinese Medicine and conformed to the national ethical regulations related to experimental animal welfare according to the 3R principles of experimental animal use, ethical review number: BUCM-4-2020080301-3004.
2.2 Main reagents and instruments
Rabbit polyclonal antibody CB1 (ImmunoWay, YT0687, 1:2000);Rabbit polyclonal antibodies GluA1 (abcam, ab31232, 1:2000),GAPDH (abcam, ab9485, 1:2000); Tanon-4500 chemiluminescence imager (Shanghai Tennant Technology Co., Ltd.).
2.3 Experimental methods
2.3.1 Animal grouping and model establishmentThirty healthy adult male SD rats (220-240 g) were acclimatized and housed for 7 days before the experiment, after which the rats were divided into three groups according to the random number table method, 10 rats in each of the control, three-week model and four-week model groups.
(1) Three-week model group: Three weeks of CUMS were given to establish a depression model, one long stress and one short stress were selected each day, and the same stress did not occur consecutively to prevent the animals from adaptation.
The stress methods for long stress are as follows: ① fasting: no feed for rats for 24h; ② water prohibition: remove the water bottles from the rat cage or place empty bottles without water for 24h; ③crowding: put 15-20 rats in the standard rat cage, and use different color markers on the tails of different groups of rats to distinguish them for 24h; ④ tilting: place the standard rat cage at an angle of 45 degrees for for 24h; ⑤ Continuous light: 24h continuous light was given to the rat feeding room.
The stress method of short stress is as follows: ① Ice water swimming for 5 min: put the rat into a bucket with 4℃ ice water,the water depth is about 45 cm, and it is necessary to ensure that the rat's tail cannot touch the bottom. ② Tail clamping 60s: put the rat into the fixture to expose the tail, using the clamps 1 cm from the tail heel, in order to make the rat make a sound as the standard; ③Restraint 2h: put the rat into the wire mesh, to ensure that the rat can not move in it; ④ Strobe 4h: strobe the rat through the strobe light,the speed is 1s/time; ⑤ White noise 4h: play white noise through the radio, the volume is kept at 100 decibels.
(2) Four-week model group: four weeks of CUMS modeling was given.
(3) Control group: normal feeding without any treatment.
2.3.2 Measurement of behavioral indicators
(1) General behavioral observation
The general behavior of each group of rats was observed during the survival period of the rats. The various behavioral aspects were also tested.
(2) Sugar water preference test[7,8] (sucrose preference test, SPT)
The SPT experiment was conducted on day 21-23 after the start of modeling for the three-week model group, and each rat was housed in a single cage; the SPT experiment was conducted on day 28-30 after the start of modeling for the four-week model group and the control group. 2 bottles of sugar water (1% concentration) were given to the rats on day 1 of the SPT experiment as an adaptation period, and 1 bottle of sugar water and 1 bottle of pure water each on day 2, and the positions of the two water bottles were switched after 12 h. At this stage, the water bottles need to be checked for leaks to avoid errors caused by leaking water bottles during the formal test. On the 3rd day of formal testing, the sugar water intake and pure water intake were weighed in 24h. Total intake = sugar water intake + pure water intake. Sugar water preference rate = sugar water intake/total intake × 100%. And compare the total water intake to exclude the effect from the variability of water intake of different rats. Total water intake = total intake (mg)/body weight (g).
(3) Novelty inhibited feeding test (NSFT)[7,8] (novelty inhibited feeding test, NSFT)
NSFT experiments were performed on day 24-25 after the start of modeling for the three-week model group, and on day 31-32 after the start of modeling for the four-week model and control groups.The rats were tested in the open field after fasting for 24 h. Food was placed in the center of the open field, and the rats were placed from the corner of the open field and their feeding latency was recorded.If the rat did not eat after 10 min, the feeding latency was counted as 600 s. The surrounding environment should be kept quiet during the test, and the rat was allowed to eat freely at the end of the test, and the food consumption (mg)/rat body weight (g) was recorded within 5 min to exclude the influence of the rat's appetite on the experiment.(4) Forced swimming test[7,8] (forced swimming test, FST)
The FST experiment was conducted on day 26-27 after the start of the modeling period for the three-week model group, and on day 33-34 after the start of the modeling period for the four-week model group and the control group. The experiment container was a transparent glass tank with a water depth of 30 cm, and the rats'tails could not touch the bottom of the barrel when swimming.During the test, the surrounding environment should be kept quiet and an opaque baffle should be placed between every two swimming containers so that the rats cannot observe the other rats.
(5) Experimental flow chart
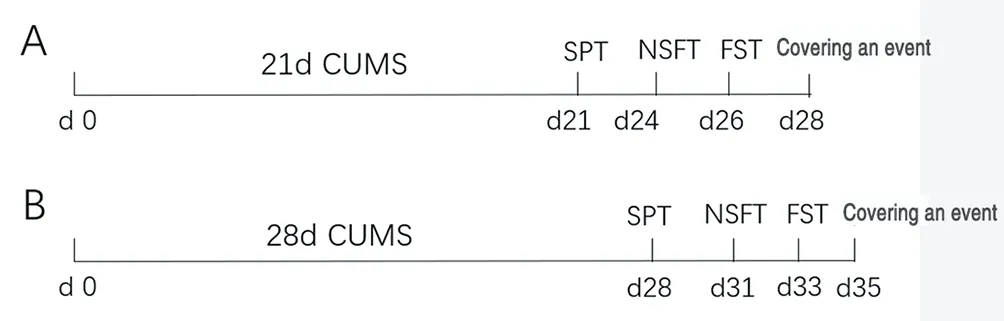
Figure 1 Flow chart of behavioral test and sampling after CUMS
2.3.3 Western blot experiment
The expression of synaptosomal GluA1 and CB1 proteins were detected. Rats were decapitated and placed in hexane for 20 s,then removed after quenching, and brain tissue was homogenized in the mPFC brain region. After homogenization, the brain tissues were allowed to fully bind to the buffer, and the supernatant was centrifuged for 10 min and continued to be centrifuged for 30 min.The precipitate was dissolved in HEPES-Lysis buffer, and then centrifuged at 25,000 g for 30 min at 4℃ after 30 min of standing.The synaptosomes were lysed with RIPA lysis solution, and the content of synaptosomal proteins was determined by BCA method.Using SDS-PAGE gel electrophoresis, 20 μg of protein was added to each lane, and electrophoresis was carried out until the strip was brought to the end of the bottom of the gel. 250 mA of constant current was used to transfer the protein to the PVDF membrane after closure solution was closed for 1 h. After adding the corresponding primary antibody working solution GluA1 (1:2000), CB1 (1:2000)was incubated overnight at 4℃, and the secondary antibody of the same source as the primary antibody was incubated for 1 h after TBST elution. The two substrates of enhanced chemiluminescence solution were mixed 1:1 and ECL luminescence solution was evenly added to the PVDF membrane and put into the chemiluminescence instrument for exposure. Grayscale value analysis was performed using Image J software. The final data characterizing the protein levels were the ratio of the values of the experimental group to the corresponding control group values[9] .
2.4 Statistical methods
Data were statistically analyzed using SAS 8.2 with one-way variance (ANOVA), and two-way comparisons between groups were performed using independent samples t-test with test level α=0.05 and two-sided test. The experimental results were expressed as mean± SEM (± sem).
3. Results
3.1 Behavioral results
3.1.1 General behavioral observationsBefore the start of modeling, all the rats in each group had smooth hair, normal diet and water, no significant difference in body weight,lively and active, and good mental condition. After three weeks of CUMS, the rats in the model group had rough hair and lower body weight than those in the control group. After four weeks of CUMS modeling, it was observed that the rats in the four-week model group had dull hair, depressed, not active and drowsy, and their body weight decreased compared with the rats in the control group.
3.1.2 Results of sugar water preference experiment
Compared with the control group, there was no significant difference in the sugar-water preference rate and total water consumption in the three-week model group. In the four-week model group, the rate of sugar-water preference was significantly lower than that of the control group ( P<0.05 ), but there was no significant difference in the total water consumption between the two groups(P>0.05) (see Table 1 for results). It is suggested that four weeks of CUMS modeling can lead to symptoms of pleasure deficit in rats.
3.1.3 Results of novel inhibition feeding experiments
There was no significant difference in the feeding latency of the rats in the three-week model group compared with the control rats,while the feeding latency of the rats in the four-week model group was significantly longer than that of the control rats (P<0.01), and the difference in total food consumption in five minutes between the three groups was not statistically significant (P>0.05) (see Table 1 for results) . It was suggested that four weeks of CUMS led to anxiety-like behavior in rats.
3.1.4 Results of forced swimming experiment
The results showed that there was no significant difference between the three-week model group rats and the control group rats in the immobility time within 5 minutes, while the four-week model group rats had prolonged immobility time within 5 minutes compared with the control group, and the difference was statistically significant(P<0.05) (see Table 1 for the results). It is suggested that four weeks of CUMS modeling can lead to desperate behavior in rats.
3.2 Expression of CB1 and GluA1 proteins in mPFC brain regions of rats
In the mPFC brain region, there was no significant difference in CB1 and GluA1 protein expression levels in the synaptosomes of rats in the three-week model group compared with the control rats,while both CB1 and GluA1 expression levels were significantly lower in the four-week model group (P<0.05) (see Table 2, Figure 2).
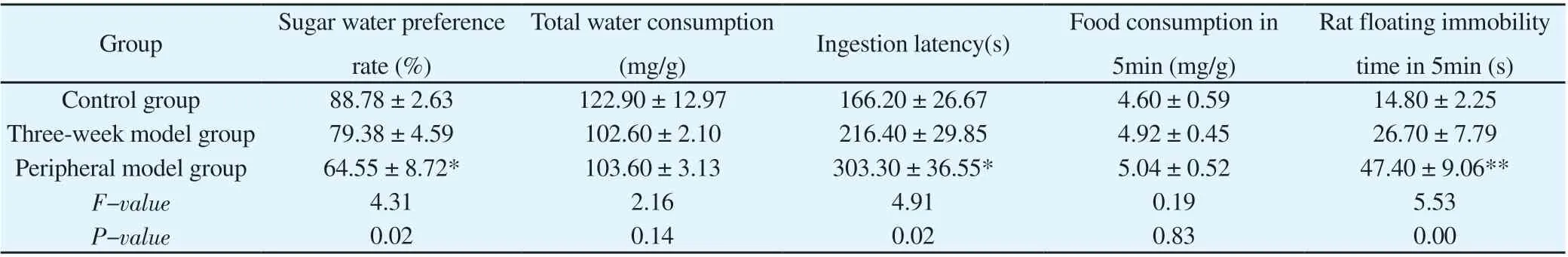
Table 1 Behavioral data

Table 2 Relative expression levels of CB1 and GluA1 protein in each group
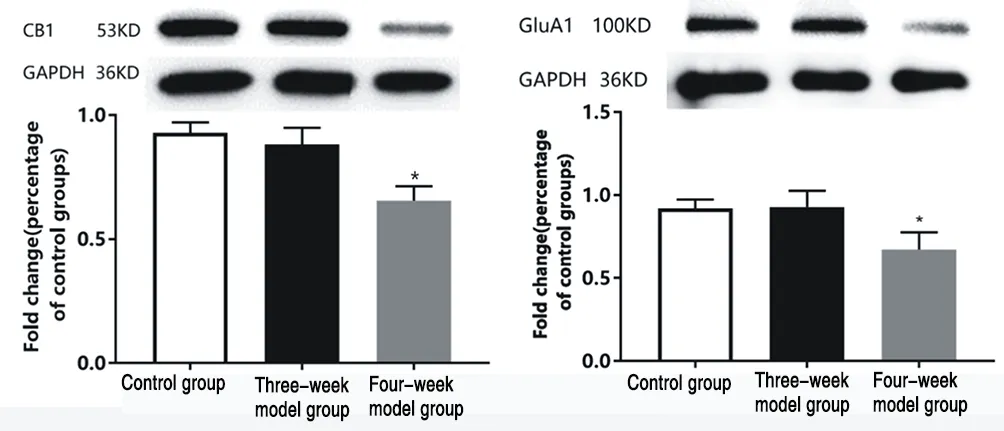
Figure 2 Expression of CB1 and GluA1 proteins in PrL brain region of rats in each group
4. Discussion
Depression is modeled in various ways: stress models, surgical models, pharmacological models and transgenic models.[10-12]Among them, CUMS modeling has been widely used in the study of depression-related mechanisms because it can simulate the main symptoms of depression in humans.[13] The CUMS model has been widely used to study the mechanisms of depression because it can simulate the main symptoms of depression in humans. A statistical study of CUMS depression models in the last decade observed that the duration of modeling is usually three to four weeks.[14-16]Therefore, in the present study, the three-week CUMS model and the four-week CUMS model were used to screen for the most stable modeling duration. It was observed that after four weeks of CUMS,the rats experienced reduced sugar-water preference, increased feeding latency in the novel inhibition feeding test, and increased immobility time in the forced swimming test compared to the control group, with statistically significant differences (P<0.05), whereas no significant differences were observed in the three-week stress compared to the control group, suggesting that the rats developed significant depression and anxiety-like behaviors after four weeks of CUMS modeling, and the rats were depressed. The modeling was successful.
The mPFC brain region is involved in the pathogenesis of several psychiatric disorders, and its reduced brain volume, altered synaptic structure and function, and neuronal atrophy within the brain region are associated with depression.[17,18] The group also found that abnormalities of GABAergic neurons in the mPFC brain region are associated with depression caused by chronic stress.[7] The mPFC is the main area of the cortex responsible for executive functions,and when its function is impaired, the emotional processing,cognitive performance, neurotransmission, autonomic regulation,and neuroendocrine responses associated with mood disorders are impaired.[19] . Increased stress susceptibility in rats with damage to the mPFC brain region compared to normal rats can be observed in experimental studies, probably due to the activation of brain regions involved in neuroendocrine and autonomic responses[20] . Therefore,the present study focused on the effects of chronic stress on the expression levels of CB1 and GluA1 proteins in the mPFC brain region of rats by observing the effects of chronic stress.
The endocannabinoid system has received increasing attention for its involvement in a variety of different brain physiological processes, including the regulation of mood, motivation, and cognitive function, and the production of endocannabinoids can promote neuroplasticity[21] . In addition, animal and clinical studies have shown that endogenous cannabinoid signaling dysfunction produces depression-like behaviors[22] . Endogenous cannabinoid activity can be modulated through CB1 receptors in the CNS,and changes in endogenous cannabinoid concentrations and CB1 receptor binding affinity and their density have been found in PFC brain regions of depressed patients[23] . The present experiment also confirmed that the onset of depression in rats induced after four weeks of CUMS was associated with reduced CB1 expression levels in mPFC brain regions.
Changes in the strength of synaptic transmission between neurons are critical for information processing in the central nervous system,and the ionotropic glutamate receptor AMPA receptors mediate much of the rapid excitatory synaptic transmission in the mammalian brain. AMPA receptors can rapidly insert into and move out of the postsynaptic membrane, and this dynamic mode of transport is thought to be a major mechanism of synaptic plasticity, learning,and memory.[24] GluA1, an AMPA receptor subunit, is present in the postsynaptic membrane of excitatory synapses and is involved in the regulation of synaptic plasticity, and its abnormal expression is a common mechanism for psychiatric disorders such as depression,schizophrenia, and chronic drug addiction.[25] . The present experiment also confirmed that the onset of depression induced in rats after four weeks of CUMS was associated with reduced levels of GluA1 in mPFC brain regions, thus affecting synaptic plasticity.
It was found that CB1 may be involved in glutamatergic signaling,and in an animal model of alcohol dependence and depression,discontinuation of antidepressant treatment resulted in decreased CB1 receptor expression in PFC brain regions and abnormal expression of glutamate receptors GluN1, GluA1, and mGlu5.[26] . Intraperitoneal administration of the CB1 receptor antagonist AM251 in rats during adolescence resulted in abnormal expression of glutamate receptor subunits, thereby affecting the maturation of glutamatergic neurons[27] . Injection of tetrahydrocannabinol leads to changes in AMPA subunit components in the nucleus accumbens and triggers long term depression (LTD), leading to changes in synaptic plasticity, which can be reversed by AM251.[28] This process can be reversed by AM251. However, no reports have been found to elucidate how CB1 affects glutamatergic signaling and synaptic plasticity in depression.
In summary, the animal model of depression established using four weeks of chronic stress was more stable. In addition, this study explores the effects of abnormal CB1 and GluA1 expression in mPFC brain regions associated with depression caused by chronic stress from the perspective of basic research, which provides new ideas for future research and treatment of depression.
Authors' Contribution Yuxin Zhang performed the experimental manipulation, data collection and processing, and wrote the article; Hongmei Sun contributed to the writing review and supervision; Cong Gai and Cui-Cui Cheng performed the data analysis. Luping Yang, Zhenyu Guo and Yushan Gao participated in the execution of the experiments;Die Hu was responsible for proposing the experimental protocol and article revision. All authors designed the experiments, wrote the manuscript, and revised the paper.
 Journal of Hainan Medical College2022年10期
Journal of Hainan Medical College2022年10期
- Journal of Hainan Medical College的其它文章
- Study on the medication rules of traditional Chinese medicine in the treatment of sleep disorder after stroke based on data mining
- Intervention effect and mechanism of Jisheng Shenqi Decoction plus Panax notoginseng and Bionjia on CCl4-induced hepatic fibrosis rat model
- Effects of acupuncture combined with Kaijingtongmai Decoction on ATP sensitive potassium channel related proteins Kir6.1 and Kir6.2 in myocardial infarction rats
- Exploring the molecular biological mechanism of Shugan Jianpi Decoction in the treatment of depression-related breast cancer based on network pharmacology
- Clinical effect of enriching qi, activating blood circulation, clearing away dampness and heat combined with western medicine in the treatment of idiopathic membranous nephropathy: A meta-analysis
- Effects of LPS-induced cholangitis on the cytoskeleton morphology of bile duct epithelium and the intervention mechanism of Dahuang Lingxian formula for these changes
