Randomized controlled study of a diagnosis and treatment plan for moderate coronavirus disease 2019 that integrates Traditional Chinese and Western Medicine
XIA Wenguang,ZHENG Chanjuan,ZHANG Jixian,HUANG Min,LI Qinglin,DUAN Can,LI Zhengliang,FAN Cunyu,ZOU Yilong,XU Bo,YANG Fengwen,LIU Qingquan
XIA Wenguang,ZHENG Chanjuan,HUANG Min,LI Qinglin,DUAN Can,LI Zhengliang,Rehabilitation Department,Hubei Provincial Hospital of Integrated Traditional Chinese and Western Medicine,Wuhan 430000,China
ZHANG Jixian,FAN Cunyu,ZOU Yilong,XU Bo,Respiratory Department,Hubei Provincial Hospital of Integrated Traditional Chinese and Western Medicine,Wuhan 430000,China
HUANG Min,Endocrinology Department,Hubei P rovincial Hospital of Integrated Traditional Chinese and Western Medicine,Wuhan 430000,China
YANG Fengwen,Traditional Chinese Medicine Department,Tianjin University of Traditional Chinese Medicine,Tianjin 300000,China
LIU Qingquan,Emergency Department,Beijing Hospital of Traditional Chinese Medicine Affiliated to Capital Medical University,Beijing 100000,China
Abstract OBJECTIVE:To investigate the clinical efficacy and safety of a diagnosis and treatment plan for moderate coronavirus disease 2019 (COVID-19) that integrates traditional Chinese (TCM) and western medicine.METHODS:One hundred twenty patients with moderate COVID-19 were randomized 1∶2 to the control group (n=40) and experimental group (n=80).Both groups received conventional western medicine treatment,and the experimental group also received TCM decoction.Over a 2-week period from diagnosis,we observed the time to clinical recovery (TTCR),rate of improvement onlung computed tomography (CT) imaging,time to defervescence,cough remission time,hospital discharge rate,average hospitalization stay,modified Medical Research Council (mMRC) scale score,clinical cure rate,laboratory findings,incidence of progression to severe or critical disease,and adverse events.RESULTS:Among 120 enrolled patients,108 completed the study.The baseline data did not differ between the experimental and control groups (all P >0.05).After treatment,the TTCR,rate of lung CT imaging improvement,time to defervescence,cough remission time,hospital discharge rate,average hospitalization stay(among discharged patients),mMRC scale score,clinical cure rate,and rates of normal values for laboratory findings were better in the experimental group than in the control group (P <0.05 or <0.01).The incidence of progression to severe or critical disease and the incidence of adverse events did not differ between the two groups (P >0.05).CONCLUSION:The diagnosis and treatment plan integrating Chinese and western medicine showed improved clinical efficacy compared with western medicine alone for patients with moderate COVID-19 and is worthy of clinical promotion and application.
Keywords:COVID-19;SARS-CoV-2;integrative medicine;diagnosis and treatment plan
1.INTRODUCTION
Coronavirus disease 2019 (COVID-19) is an emerging acute infectious disease of the respiratory tract that is highly contagious,1-3with an incubation period of 1-14 d(most commonly 3-7 d).In December 2019,many COVID-19 cases emerged in Wuhan,Hubei province,from where the disease swiftly spread throughout China and other countries.The outbreak of COVID-19 quickly led to a global pandemic.The virus that causes COVID-19,severe acute respiratory syndrome coronavirus 2(SARS-CoV-2),is a novel betacoronavirus (βCoV).Current research suggests that the genome of SARSCoV-2 shares more than 85% homology with the bat SARS-related-CoV,but their genetic characteristics differ significantly.4For the first several months of the COVID-19 pandemic,no vaccines or specific medications were available for COVID-19,5and thus,patients could only be given symptomatic and supportive treatment.
Traditional Chinese Medicine (TCM) has played an essential role in the treatment of infectious disease epidemics for thousands of years in China and eventually developed a mature theoretical system for epidemics.COVID-19 is classified as an epidemic disease according to the terms of TCM,as the etiology is a pestilential pathogen,the disease location is the lungs,and its pathogenesis is characterized by “dampness,toxin,heat and deficiency”.Indeed,the pestilential pathogen invades the lungs of people,especially those with weak spleen and stomach and deficiency ofYang Qi,causing complex and variable early symptoms due to its characteristics of aggressiveness and changeability.The National Health Commission and the State Administration of Traditional Chinese Medicine (in China) jointly issued the “Diagnosis and Treatment Protocol for COVID-19” to provide guidance for combinational therapy.In the present study,we aimed to investigate the clinical efficacy and safety of this diagnosis and treatment plan for moderate COVID-19 that integrates TCM and western medicine.
2.MATERIALS AND METHODS
2.1.Ethics considerations
We performed a randomized controlled trial between 28 January to 29 February,2020 in the Hubei Provincial Hospital of Integrated Traditional Chinese and Western Medicine.The trial was registered with the Chinese Clinical Trial Registry (No.ChiCTR2000029461) and the Chinese Medicine Clinical Trial Registry (No.ChiMCTR2000002959).The trial was approval by the Ethics Committee of the Hubei Provincial Hospital of Integrated Traditional Chinese and Western Medicine(approval No.2020006).Participation was voluntarily,and all patients provided verbal agreement (due to the emergent nature and severity of the epidemic,only verbal consent and recording were possible).
2.2.Sample size and randomization
Our sample size calculation indicated that a total of 111 evaluable patients (allowing for a 10% drop-out rate),with 74 in the treatment group and 37 in the control group,were needed to provide a power of 90% for detecting a difference in the meantime to clinical recovery (TTCR)of 1.5 d between the two groups at a 5% level of significance,assuming the standard deviation was 2.12.This difference in mean TTCR and standard deviation were based on results from our prior clinical observation.
We enrolled 120 patients in the present study to achieve better validity.
Patients were randomly assigned 2 ∶1 to the experimental group and the control group according to a computer-generated randomization procedure.The allocation was carried out via a concealed process using sealed,fully opaque,numbered envelopes.The evaluators were blinded to the interventions administered in each group.Because the smell and color of TCM decoction are unique,the patients knew their group assignment,and no placebo control group was established in this study.
2.3.Clinical classification of COVID-19
According to the “Diagnosis and Treatment Protocol for COVID-19 (Trial Version 5,6)”,6,7the following clinical classifications of COVID-19 were applied in this study:(a) mild cases:the clinical symptoms are mild with no sign of pneumonia on imaging;(b) moderate cases:presence of fever and respiratory symptoms with radiological findings of pneumonia;(c) severe cases(cases meeting any of the following criteria):respiratory distress (≥30 breaths/min);oxygen saturation ≤ 93% at rest;arterial partial pressure of oxygen (PaO2)/ fraction of inspired oxygen (FiO2) ≤300 mm Hg (l mm Hg=0.133 kPa);or obvious lesion progression >50% within 24-48 h on chest imaging;and (d) critical cases (cases meeting any of the following criteria):respiratory failure requiring mechanical ventilation;shock;or other organ failure requiring intensive care unit (ICU) care.
2.4.Participants
All participants were diagnosed with COVID-19 according to the “Diagnosis and Treatment Protocol for COVID-19 (Trial Version 5,6)”6,7and hospitalized in the Department of Respiratory Medicine of Hubei Provincial Hospital of Integrated Traditional Chinese and Western Medicine between January 28 and February 29,2020.The inclusion criteria were:age 18-80 years and a diagnosis of moderate COVID-19.Patients were excluded if any of the following conditions were present:cognitive impairment or mental illness;severe or critical COVID-10;severe lung,liver,kidney,or blood disease;primary disease of the endocrine system;increased intracranial pressure;tumor;and pregnancy.Patients were removed from the final analysis according to the following elimination criteria:incomplete data that affected the evaluation of efficacy and safety;poor compliance and/or withdrawal from the study;and exacerbation of disease with emergence of serious adverse events or complications.
2.5.TCM syndrome differentiation
We applied the standards for TCM syndrome differentiation from the “Diagnosis and Treatment Protocol for COVID-19 (Trial Version 5,6)”:6,7(a) Colddamp constraint in the lung pattern (clinical manifestations:fever,low cough and sputum,or yellow sputum,suffocation,shortness of breath,bloating,and constipation.The tongue is dark red and fat,the coating is greasy or yellow,and the pulse is slippery or stringy);(b) Damp-heat accumulation in the lung pattern (clinical manifestations:low or no fever,slight chills,fatigue,heavy sensation in the head and body,muscle soreness,dry cough with little sputum,sore throat,thirst without desire to drink water,or accompanied by chest tightness,no sweating or poor sweating,or vomiting with anorexia,diarrhea,or sticky stool.The tongue is reddish,and the coating is white,thick and greasy or thin and yellow.The pulse is slippery or soggy);and (c) Cold-damp obstructing the lung pattern (clinical manifestations:low fever,dry cough with little sputum,fatigue,chest tightness,nausea,or stomach discomfort.The tongue is pale or light red,and the coating is white or white greasy.The pulse is soggy).
2.6.Interventions
All patients in both groups received conventional western medicine treatment,and those in the experimental group also used TCM decoction.Both treatments followed the guidelines of the “Diagnosis and Treatment Protocol for COVID-19 (Trial Version 5,6)”.6,7
The conventional western medicine treatment included:(a) bed rest,strengthening supportive treatment,treatment to ensure adequate warmth,and close monitoring of vital signs,oxygen saturation,water and electrolyte balance,and internal environment stability;(b)effective oxygen therapy administered in a timely manner according to the oxygen saturation level;(c)antiviral therapy:α-interferon atomized inhalation (5 million U in 2 mL sterilized water,twice daily),Arbidol Hydrochloride (0.2 g,three times a day),and intravenously injected Ribavirin (0.6 g in 250 mL of 0.9%sodium chloride,twice daily);and (d) antibacterial treatment:antibacterial drugs given in a timely manner upon observation of evidence of secondary bacterial infection.
The TCM treatment included combinations for the following conditions:(a) Cold-damp constraint in the lung pattern,recommended prescription:Mahuang(Herba Ephedra Sinica) 6 g,Kuxingren (Semen Armeniacae Amarum) 15 g,Shigao (Gypsum Fibrosum)30 g,Yiyiren (Semen Coicis) 30 g,Cangzhu (Rhizoma Atractylodis Lanceae) 10 g,Huoxiang (Herba Agastaches Rugosa) 15 g,Qinghao (Herba Artemisiae Annuae) 12 g,Huzhanggen (Radix Polygoni Cuspidati)20 g,Mabiancao (Herba Verbenae Officinalis) 30 g,Lugen (Rhizoma Phragmitis) 30 g,Zhizi (Fructus Gardeniae) 15 g,Gualou (Fructus et Semen Trichosanthis) 30g,Dahuang (Radix Et Rhizoma Rhei Palmati) 6g (decocted later),Juhong (Exocarpium Citri Rubrum) 15 g,and Gancao (Radix Glycyrrhizae) 10 g;(b)Damp-heat accumulation in the lung pattern,recommended prescription:Binglang (Semen Arecae)10 g,Caoguo (Fructus Tsaoko) 10 g,Houpu (CortexMagnoliae Officinalis) 10 g,Zhimu (Rhizoma Anemarrhenae) 10 g,Huangqin (Radix Scutellariae Baicalensis)10 g,Chaihu (Radix Bupleuri Chinensis)10 g,Chishao (Radix Paeoniae Rubra) 10 g,Lianqiao(Fructus Forsythiae Suspensae) 15 g,Qinghao (Herba Artemisiae Annuae) 10 g (decocted later),Cangzhu(Rhizoma Atractylodis Lanceae) 10 g,and Gancao(Radix Glycyrrhizae) 5 g;(c) Cold-damp obstructing the lung pattern,recommended prescription:Cangzhu(Rhizoma Atractylodis Lanceae) 15 g,Chenpi(Pericarpium Citri Reticulatae) 10 g,Houpu (Cortex Magnoliae Officinalis) 10 g,Huoxiang (Herba Agastaches Rugosa) 10 g,Caoguo (Fructus Tsaoko) 6 g,Mahuang (Herba Ephedra Sinica) 6 g,Qianghuo(Rhizoma et Radix Notopterygii) 10 g,Shengjiang(Rhizoma Zingiberis Recens) 10 g,and Binglang (Semen Arecae) 10 g.The recommended dosing was:one total dose daily,boiled in 400 mL water and taken twice in the morning and evening.
2.7.Endpoints
The primary efficacy endpoints were:(a) TTCR defined as previously described:8the time at which the body temperature,breathing rate,and oxygen saturation returned to normal ranges and cough had been alleviated or resolved for at least 72 h (normalization standards:infrared thermometer measured body temperature ≤ 36.6 ℃,armpit temperature ≤ 37.2 ℃,or rectal temperature ≤ 37.8 ℃;breathing rate ≤ 24 times/min;and oxygen saturation >94% in resting state);and (b) improvement on lung computed tomography (CT) images was defined as previously described.9Briefly,the lung CT images of COVID-19 patients showed:unilateral or bilateral lungs with localized inflammatory infiltrates,characterized by subpleural patchy,lumpy,segmental,or subsegmental ground-glass opacities (GGO).Progression was characterized by an increased number and expanded range of lesions gradually invading multiple segments of the bilateral lungs and consolidation among some lesions.
The secondary efficacy endpoints included:(a) time to defervescence10(in those with fever at enrollment):the duration of body temperature recover to normal level for at least 72 h;(b) cough remission time11(in those with moderate or severe cough at enrollment):the number of days from diagnosis until cough became mild or disappeared;(c) hospital discharge rate;(d) average hospitalization stay (in days) of discharged patients;(e)modified Medical Research Council (mMRC) scale score12(classified into four grades of 0-4 from mild to severe according to severity of dyspnea);(f) clinical cure rate (in reference to the “Diagnosis and Treatment Protocol for COVID-19 (TrialVersion5,6)”,6,7including normal body temperature for at least 3 d,significant improvement of respiratory symptoms,obvious absorption of inflammatory lesions on lung CT images,and two consecutive negative tests for respiratory SARSCoV-2 nucleic acid (specimens taken at an interval of at least 1 d);(g) laboratory findings from morning venous blood for C-reactive protein (CRP),serum amyloid A(SAA),erythrocyte sedimentation rate (ESR),white blood cell (WBC) count,neutrophil percentage(NEUT%),lymphocyte percentage (LYMPH%),lactate dehydrogenase (LDH),creatine kinase-MB (CK-MB),aspartate transaminase (AST),alanine transaminase(ALT),blood urea nitrogen (BUN),D-dimer,and procalcitonin (PCT);(h) incidence of severe or critical cases (according to the clinical classifications for COVID-19 described above);and (i) adverse events consisting primarily of nausea,vomiting,gastric discomfort,diarrhea,constipation,headache,dizziness,sleep disorder,and others.
2.8.Statistical analysis
SPSS 25.0 for Windows (SPSS,Inc.,Chicago,IL,USA)was used for all statistical analyses,and statistical significance was defined as a two-tailedP<0.05.Quantitative data are presented as mean ± standard deviation ().Count data are presented as frequency and percentage.For intra-group comparisons of ranked data,Wilcoxon signed rank test was used.For comparisons of quantitative indexes between groups,two-samplet-test or a suitable non-parameter method was applied.χ2test or Fisher exact probability test was used for comparisons of qualitative indexes between groups.
3.RESULTS
Among 151 COVID-19 patients recruited for this study,31 were excluded (21 did not meet the inclusion criteria,6 refused to participate in the study,and 4 could not be included for other reasons).Thus,120 patients were included and randomized 2∶1 to the experimental group(n=80) and the control group (n=40).In the experimental group,7 patients withdrew from the study(1 due to exacerbation of the disease and 6 due to poor compliance),and in the control group,5 patients withdrew from the study (3 due to aggravation of the disease and 2 due to poor compliance).Finally,108 patients completed the study,including 73 [in the experimental group and 35 in the control group (Figure 1).No significant differences in the baseline information and laboratory findings were observed between the two groups (allP>0.05,Tables 1 and 2).
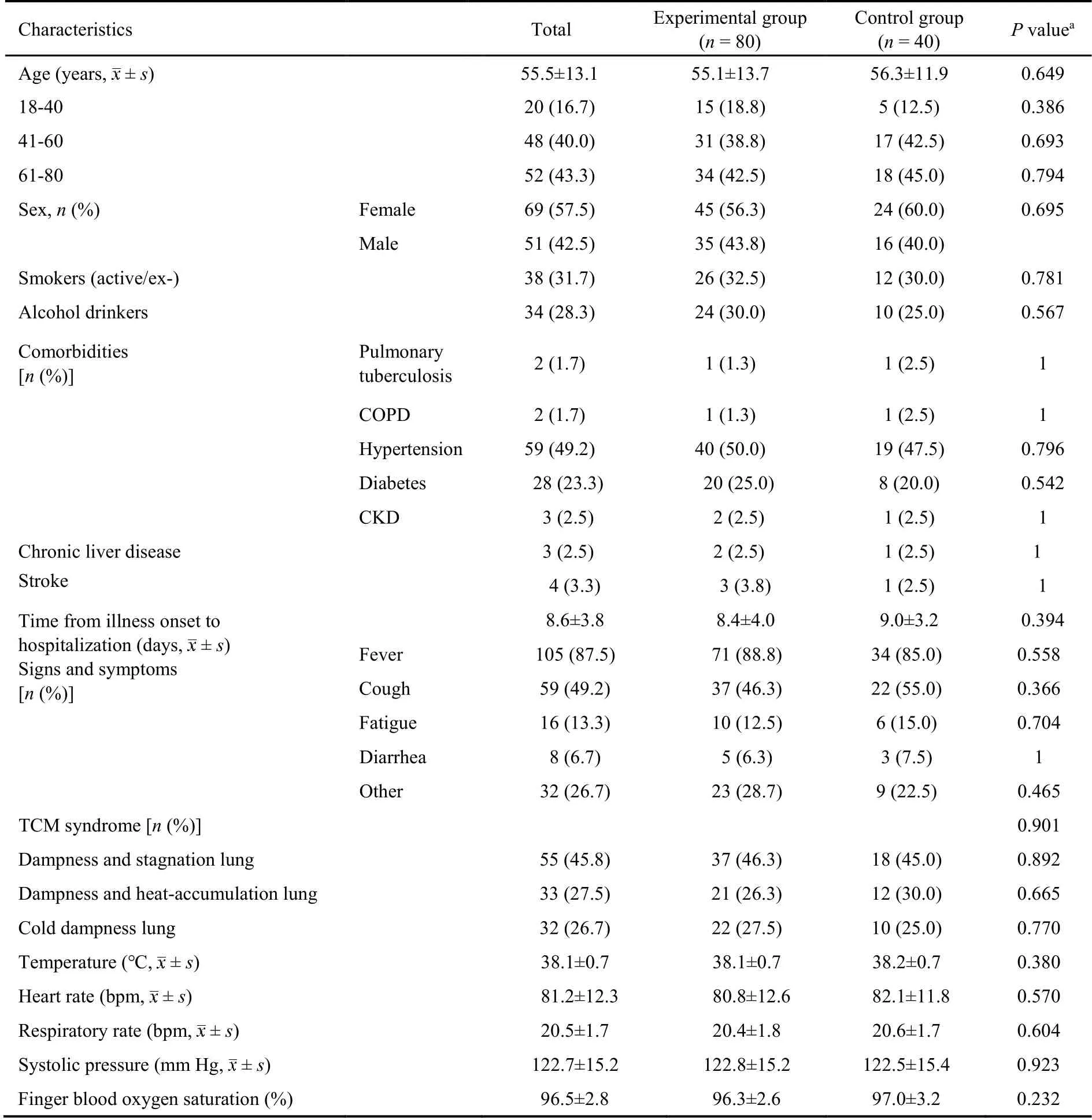
Table 1 Baseline characteristics of the patients with moderate COVID-19
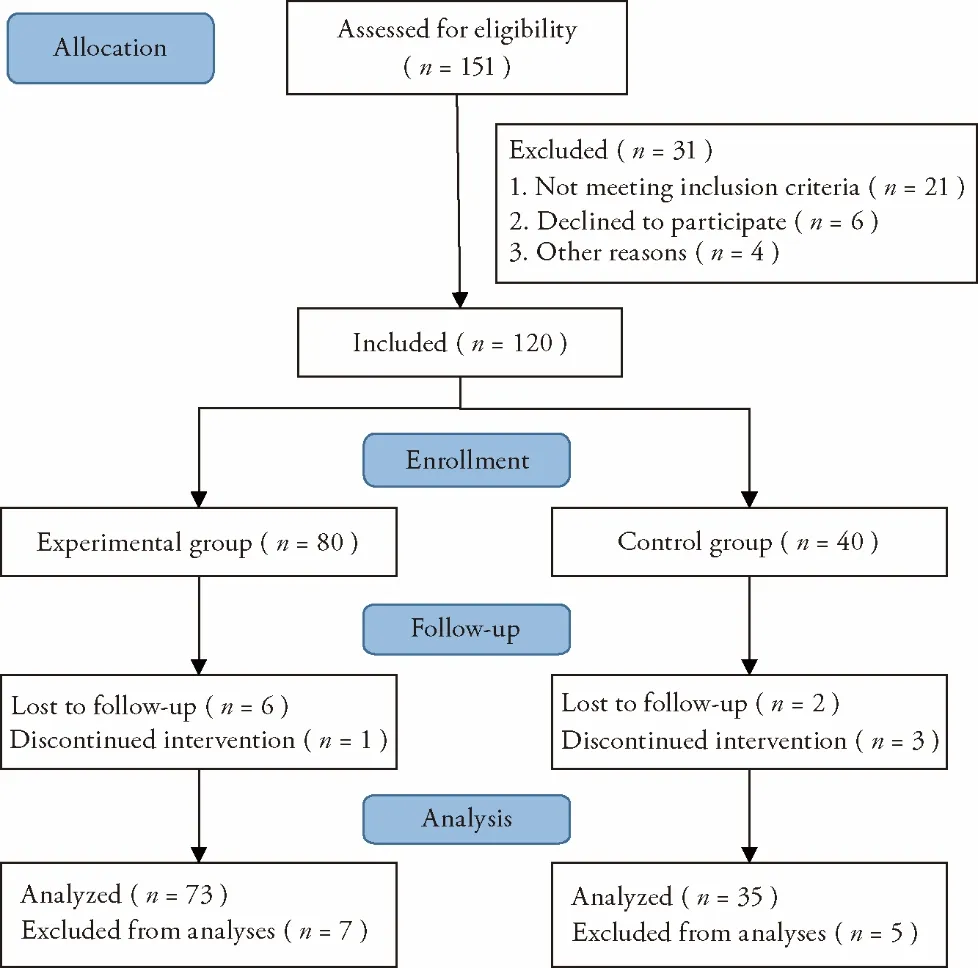
Figure 1 Consort flow for the conducted study
For the primary efficacy endpoints,the experimental group had a shorter TTCR (P<0.01) and a higher rate of improvement on lung CT imaging (P<0.05) compared with the control group (Figures 2 and 3).Among the efficacy secondary endpoints,the time to defervescence,cough remission time,and average hospitalization stay(among discharged patients) were lower in the experimental group than in the control group (allP<0.01,Figure 2).Additionally,the clinical cure rate was greater in the experimental group than in the control group (P<0.01),whereas the incidence of progression to severe or critical cases did not differ between the two groups (P>0.05,Figure 3).Both groups showed improved scores on the mMRC scale after treatment (P<0.05),and betweengroup comparison indicated that the improvement in the mMRC scale score was greater in the experimental group than in the control group (P<0.05,Table 3).In both groups,hospital discharge began from day 5 after admission,and the mean time of discharge in the experimental group was 3 d ahead of that in the control group.Moreover,the hospital discharge rate on the 14th day from diagnosis was higher in the experimental group than in the control group (P<0.05,Figure 4).The percentages of patients with normal values for WBC count,LYMPH%,SAA level,CRP level,ESR,CK-MB level,LDH level,ALT level,and AST level were greater in the experimental group than in the control group (P<0.05 orP<0.01,Table 4).
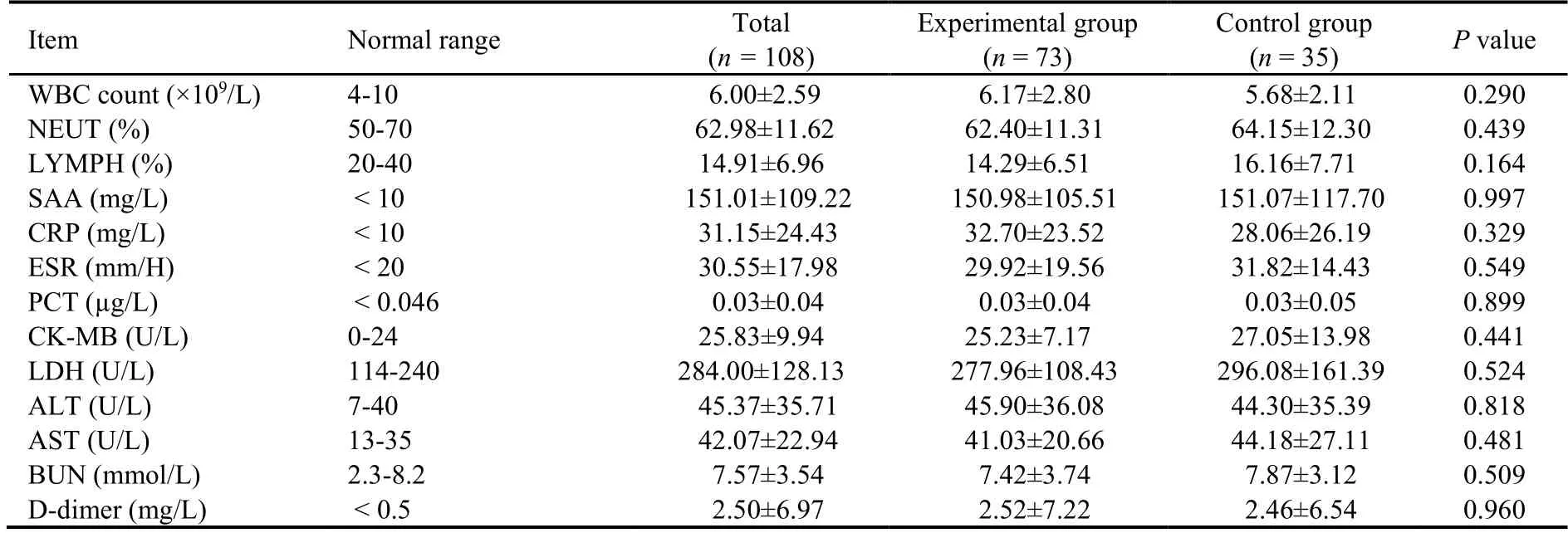
Table 2 Comparison of laboratory findings on admission between the experimental and control groups of patients with moderate COVID-19
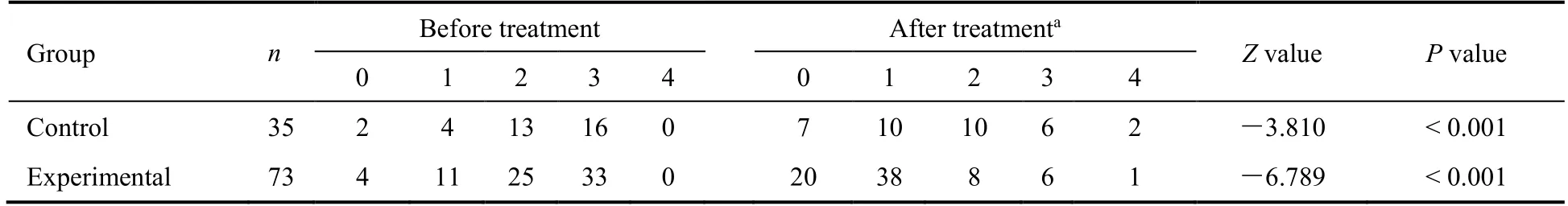
Table 3 Comparison of mMRC scale scores between the experimental and control groups of patients with moderate coronavirus disease 2019
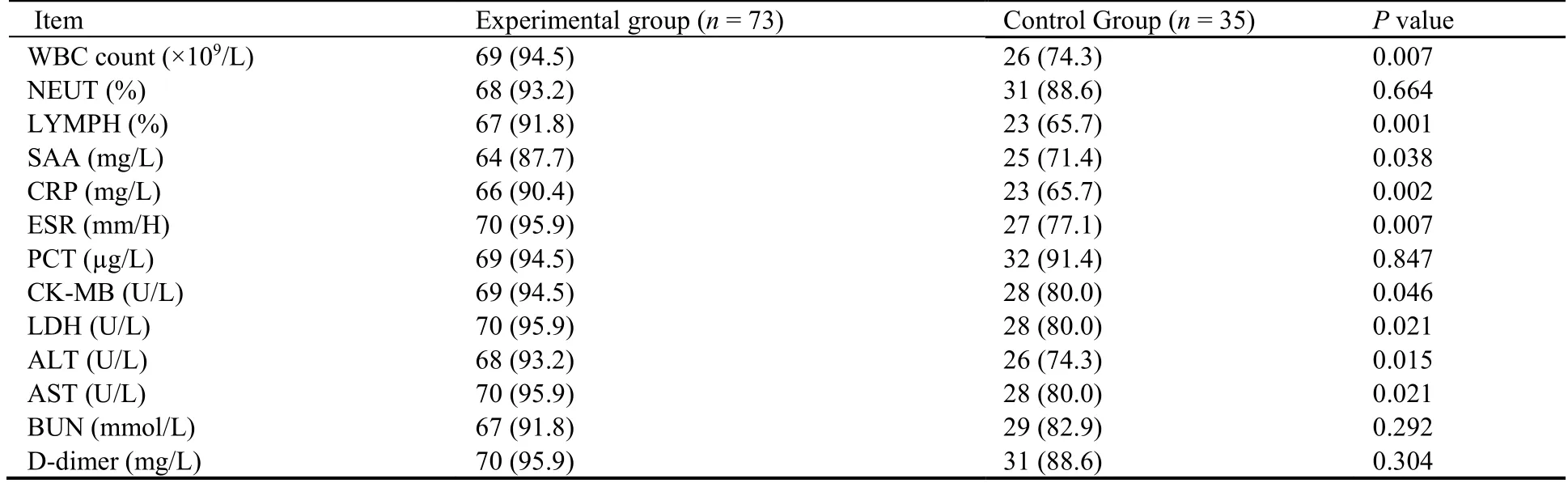
Table 4 Comparison of the percentages of patients with normal values after treatment between the experimental and control groups of patients with moderate COVID-19 [n (%)]

Figure 2 Comparison of efficacy endpoints for coronavirus disease 2019 patients who received combination treatment versus control treatment
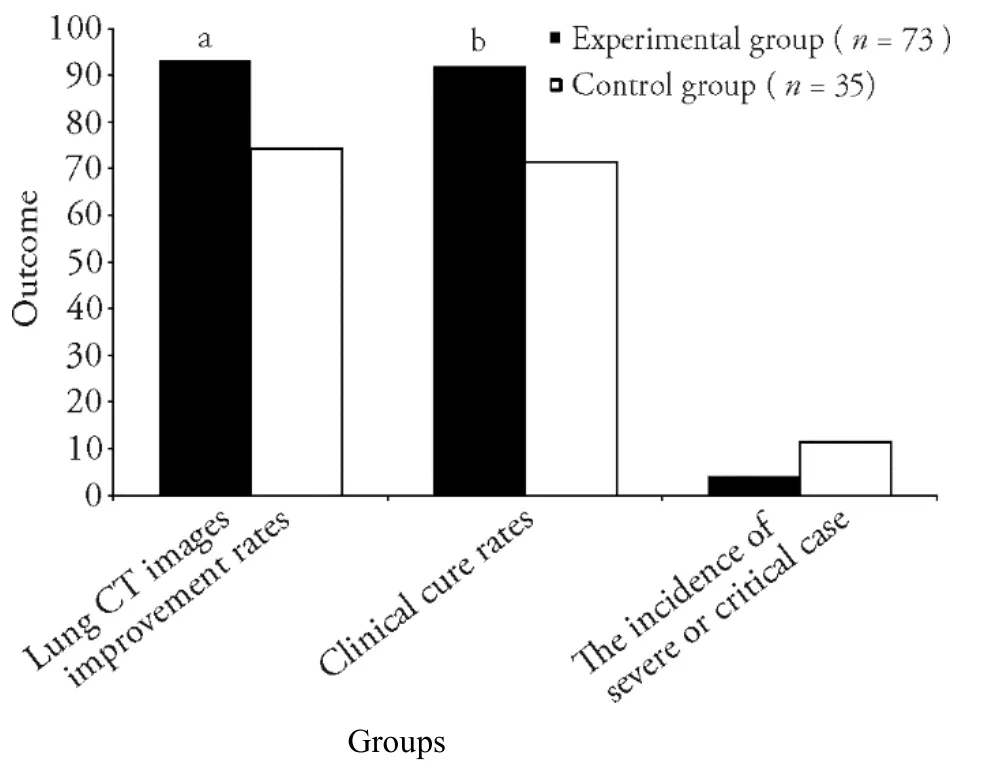
Figure 3 Comparison of efficacy endpoints for coronavirus disease 2019 patients who received combination treatment versus control treatment
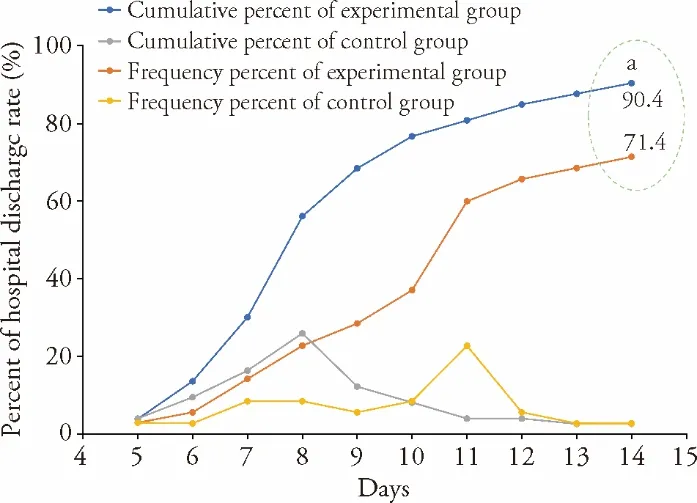
Figure 4 Hospital discharge rates in the experimental and control groups over 2 weeks following coronavirus disease 2019 diagnosis Experimental group:patients received Western Medicine treatment plus Traditional Chinese Medicine;control group:patients received only Western Medicine treatment.Significant differences compared with control group were designated as aP <0.05.
No severe adverse events occurred in either group.The adverse events observed in the experimental/control groups were:gastric discomfort (5/2),diarrhea (3/2),nausea (1/1),and dizziness (1/1).The incidence of adverse events did not differ between the two groups (P>0.05).All included patients maintained the original treatment without requiring any other special intervention,and all adverse events gradually resolved.
4.DISCUSSION
The results of the present study support the good clinical efficacy and safety of the “Diagnosis and TreatmentProtocol for COVID-19” jointly issued by the National Health Commission and the State Administration of Traditional Chinese Medicine (in China) for the diagnosis and treatment of patients with moderate COVD-19.In comparison to the control treatment with the western medicine approach only,the integrated treatment achieved better results in terms of theTTCR,lung CT imaging improvement rate,clinical cure rate,and other efficacy endpoints.Moreover,the incidence of progression to severe or critical COVID-19 as well as the incidence of adverse events did not differ between the experimental and control groups.
Most patients with moderate COVID-19 have significantly an increased SAA level,and given that acute inflammatory reactive proteins are more a sensitive parameter than CRP,14early testing of the SAA level was proposed as useful for the diagnosis of COVID-19.Research has found that about 80% of COVID-19 patients have normal liver and renal function along with no evidence of myocardial injury,whereas patients with severe COVID-19 are more likely to have abnormal levels of indicators for myocardial,liver,and renal function in early the stage.A recent theory proposes that“cytokine storm”15is the mechanism of the inflammatory state that leads to injury of multiple organs such as the lungs,heart,liver,and kidneys as well as coagulation system disorders in patients with severe COVID-19.While abnormal levels of these indicators are rare in moderate COVID-19 patients,their later appearance may indicate a poor prognosis.Thus,dynamic observation of these indicators could aid the early recognition of deterioration of the disease.In the present study,the ALT and AST levels in the experimental group were significantly lower than those in the control group,which may reflect the protective effects of specific components of the TCM decoction in the liver.Additional research is needed to verify this possibility.
The mechanisms by which TCM treatment ameliorates the severity of COVID-19 have yet to be elucidated.From the perspective of modern medicine,possible mechanisms for TCM may be related to the complex combination of components in TCM decoction acting on patients via multiple targets and multiple pathways to jointly exert antiviral,anti-inflammatory,and proimmunity activities that reduce lung injury,protect related organs,and alleviate side effects.Additional research is needed to determine the exact mechanisms of TCM in COVID-19.Meanwhile,the results of the present study demonstrate that dynamic observation of long-term lung function,lung CT images,and patients’quality of life are valuable in the management of COVID-19 patients.
Our study has several limitations.First,the generalizability of the results may be limited because this trial was performed at a single center with a small sample.Second,the patients knew their group assignment due to the unique smell and color of the TCM decoction,and no placebo control group was established.Finally,this study lacked long-term observation of lung CT images,respiratory symptoms,the incidence of reinfection with SARS-CoV-2,and other indicators of treatment efficacy.In conclusion,the present study revealed that the integrated TCM and Western Medicine treatment approach can significantly improve the clinical cure rate,shorten the course of disease,and alleviate clinical symptoms among patients with moderate COVID-19.Thus,the integrated “Diagnosis and Treatment Protocol for COVID-19” from the National Health Commission and the State Administration of Traditional Chinese Medicine may offer significantly improved clinical efficacy for patients with moderate COVID-19 compared with the western medicine treatment alone and is worthy of clinical promotion and application.
5.ACKNOWLEDGMENTS
The authors express their deepest appreciation to all the patients who volunteered to participate in the study as well as to the research doctors and nurses.
 Journal of Traditional Chinese Medicine2022年2期
Journal of Traditional Chinese Medicine2022年2期
- Journal of Traditional Chinese Medicine的其它文章
- Acupoint application therapies for essential hypertension:a systematic review and Meta-analysis
- Biosynthesis of titanium dioxide nanoparticles using Hypericum perforatum and Origanum vulgare extracts and their main components,hypericin and carvacrol as promising antibacterial agents
- Protective effect of resveratrol on rat cardiomyocyte H9C2 cells injured by hypoxia/reoxygenation by regulating mitochondrial autophagy via PTEN-induced putative kinase protein 1/Parkinson disease protein 2 signaling pathway
- Efficacy of aqueous extract of flower of Edgeworthia gardneri (Wall.)Meisn on glucose and lipid metabolism in KK/Upj-Ay/J mice
- Effect of manipulation on cartilage in rats with knee osteoarthritis based on the Rho-associated protein kinase/LIM kinase 1/Cofilin signaling pathways
- Baicalin inhibits inflammation of lipopolysaccharide-induced acute lung injury via toll like receptor-4/myeloid differentiation primary response 88/nuclear factor-kappa B signaling pathway
