Low soil carbon saturation deficit limits the abundance of cbbLcarrying bacteria under long-term no-tillage maize cultivation in northern China
YIN Tao ,QIN Hong-ling ,YAN Chang-rong ,LIU Qi ,HE Wen-qing
1 College of Resources and Environment,Qingdao Agricultural University,Qingdao 266109,P.R.China
2 Institute of Environment and Sustainable Development in Agriculture,Chinese Academy of Agricultural Sciences,Beijing 100081,P.R.China
3 Key Laboratory of Subtropical Agro-ecology,Institute of Subtropical Agriculture,Chinese Academy of Sciences,Changsha 410125,P.R.China
4 Key Laboratory of Prevention and Control of Residual Pollution in Agricultural Film,Ministry of Agriculture and Rural Affairs,Beijing 100081,P.R.China
Abstract The responses of cbbL-carrying bacteria to different levels of soil carbon saturation deficits (SCSD) under tillage managements are largely unknown. We assessed the influence of SCSD on the abundance and diversity of cbbLcarrying bacteria under long-term no-tillage with residue retention (NT) and conventional tillage without residue retention(CT) cultivation systems in maize. We found SCSD was smaller under NT than under CT in the 0-15 cm soil layer. The abundance and the Shannon diversity of cbbL-carrying bacteria in the NT treatment were lower than in the CT treatment.Soil carbon saturation and cbbL gene abundance showed a significant positive correlation,but there was no correlation between soil carbon saturation and cbbL gene diversity. However,the long-term NT practice decreased cbbL-carrying bacteria diversity and altered the community structure of the cbbL-carrying bacteria. Our results indicated that low SCSD limited the abundance of cbbL-carrying bacteria,but there was no relationship between low SCSD and diversity of cbbLcarrying bacteria. We suggest that further studies of cbbL-carrying bacteria carbon sequestration rates and capacity should be based on the effect of management practices on cbbL-carrying bacteria abundance and diversity. Our study has important implications for the relationship between the biological and physicochemical mechanisms in CO2 fixation.
Keywords: conservation tillage,soil carbon saturation,cbbL-carrying bacteria,abundance and diversity
1.Introduction
Autotrophic bacteria play a crucial role in the carbon cycling of terrestrial ecosystems and are widely distributed throughout various habitats (Giriet al.2004;Nanbaet al.2004;Nakaiet al.2012). They are common in agricultural ecosystems (Geet al.2016),and have a potential global carbon sequestration rate of 0.6-4.9 Pg C yr-1(Yuanet al.2012a;Geet al.2013). Autotrophic bacteria can sequester CO2viasix pathways,of which the most common one in managed soils is the Calvin-Benson-Bassham cycle (CBB)(Longet al.2015;Wuet al.2017). The key enzyme in the CBB cycle is ribulose-1,5-bisphosphate carboxylase/oxygenase (RubisCO),which has four distinct holoenzyme forms (I,II,III,and IV) (Tabita 1999). Form I RubisCO,encoded by thecbbLgene,is the most abundant form(Tabitaet al.2008). ThecbbLgene is used as a functional marker to analyze the diversity of autotrophic bacteria in farmland ecosystems (Liaoet al.2020). Several studies based oncbbLgene detection have shown that autotrophic bacteria are sensitive to changes in the soil environment resulting from various tillage practices (Selesiet al.2005;Yinet al.2010;Geet al.2016).
During the last three decades,conservation tillage has gradually been adopted by farmers in semi-arid regions of northern China (Chimsahet al.2020;Yinet al.2020). Conservation tillage is considered as a viable and effective option for improving soil fertility by increasing soil organic carbon (SOC) levels (Madejonet al.2009;Roperet al.2010). However,some long-term conservation tillage treatments have not increased SOC stocks at equilibrium,suggesting that soil carbon was already at or near saturation (Stewartet al.2007;Novaket al.2020). Other long-term conservation tillage practices have resulted in decreased SOC stabilization efficiency in soils with high carbon content but not in low carbon soils (Stewartet al.2009;Corbeelset al.2016). These results suggest that soils with a low soil carbon saturation deficit (SCSD) have a decreased SOC stabilization efficiency with respect to carbon inputs. The evaluation and corroboration of soil carbon saturation in previous studies were mainly based on physicochemical processes(Sixet al.2002;Stewartet al.2009). A recent study has found that biological mechanisms may contribute to soil carbon saturation,because the mineral-associated SOC in the soil carbon saturation process is largely microbially generated (Craiget al.2021). No studies to our knowledge have explored whether soil carbon saturation feedbacks inhibit the growth of carbon-fixing bacteria. However,previous studies have shown that external organic compounds (such as extracellular free organic carbon (EFOC)) generally inhibit the cell growth of autotrophic bacteria,thereby reducing the abundance of autotrophic bacteria and carbon fixation yield (Wanget al.2017,2018). Thus,it is not known whether soil carbon saturation increases the inhibition of autotrophic bacteria by EFOC. Some studies have demonstrated a relationship between soil nutrient level and autotrophic bacteria. These studies have shown that the abundance and diversity of autotrophic microorganisms is greater in nutrient-poor soil than in nutrient-rich soil (Freemanet al.2010;Yuanet al.2012b;Tanget al.2015). Taken together,these results suggest that SCSD may be an important factor affecting the carbon sequestration by autotrophic microorganisms. To our knowledge,there has been no test of the influence of SCSD on the abundance and diversity of autotrophic bacteria.
The objective of this study was to evaluate whether SCSD influenced the abundance and diversity ofcbbLcarrying bacteria under long-term no-tillage agriculture with residue retention (NT) versus conventional tillage agriculture without residue retention (CT). We hypothesized that low SCSD limited the abundance and diversity ofcbbL-carrying bacteria. To test this hypothesis,we (1) investigated the SCSD under long-term NT versus CT,(2) investigated the abundance,diversity,and composition (phylum to genus level) ofcbbL-carrying bacterial communities under the two tillage treatments,and (3) assessed the relationship between SCSD andcbbL-carrying bacteria.
2.Materials and methods
2.1.Site description
This study was conducted at the Field Experimental Station,Agro-Environment and Crop Water Productivity,Ministry of Agriculture and Rural Affairs,at Shouyang,Shanxi Province,China (37°51´N,113°05´E,1 130 m a.s.l.). The area is characterized as having a semiarid continental temperate monsoon climate,with a mean annual precipitation of 470 mm and a 25-year mean annual temperature of 8.1°C. A single crop of maize (ZeamaysL.) grown between late April and early October is the main crop cultivated in the region. The soil type is cinnamon sandy loam,classified as a Calcaric-Fluvic Cambisol (IUSS and WRB 2006). Soil properties,determined using methods described in Section 2.3,in the 0-20-cm layer at the beginning of the study were as follows:SOC,24.4 g kg-1;available potassium,0.10 g kg-1;available phosphorus,0.008 g kg-1;total nitrogen (TN),1.08 g kg-1;pH (soil:water,1:2.5),8.4.
2.2.Field experimental design
Prior to the start of the experiment,the site had been in a single spring maize cropping system since 2004.A completely randomized design was used in the field experiment,and each treatment was repeated three times. The experiment included NT and CT treatments.In the NT treatment,the whole crop residue (at the rate of 5.25 t ha-1) was applied to cover the soil surface after maize harvest. In the CT treatment,all crop residues were removed,and the plots were ploughed after the autumn harvest,using a moldboard to a depth of 15-20 cm.
The spring maize seed cultivar Qiangsheng 31 was sown at a rate of 55 556 plants ha-1,with a row spacing of 0.6 and 0.3 m between plants within rows,using a handheld hole-sowing/fertilization machine (3 cm in diameter),with a sowing depth of approximately 3-5 cm.Maize was seeded between 25-30 April and harvested between 5-10 October for 15 years. Urea (CO(NH2)2)(150 kg N ha-1) and diammonium phosphate [(NH4)2HPO4](75 kg P2O5ha-1) were used as fertilizers. In each treatment,insects and weeds were controlled by applying an insecticide (40% dimethoate) and an herbicide (2,4-D butylate),at rates of 0.4 and 0.9 a.i.kg ha-1,respectively.
2.3.Soil sampling and analysis
After the harvest in 2019,soil samples were collected at depths of 0-5,5-10 and 10-15 cm using a shovel. In each plot,for each soil layer,we collected five samples,following a W scheme (one sample was taken at each angle or tip of the W),which were mixed to obtain one composite sample per soil layer per plot. The samples were placed in an icebox and immediately transported to the laboratory. Any maize residues were removed with the help of tweezers,and subsamples were stored at-80°C for DNA extraction.
Soil temperature was measured in the field at depths of 5,15,and 25 cm between the rows using a temperature logger (HIOKI LR 5011,HIOKI,Japan) during maize growth period. Soil moisture content of the collected samples was measured by weighing soil before and after drying fresh soil at 105°C to constant weight. Soil pH was measured in a soil:water ratio of 1:2.5. SOC was determined using the K2CrO7-H2SO4oxidation procedure with external heating,and TN was measured by the Kjeldahl method (Bao 2005). Microbial biomass carbon(MBC) was measured using the CHCl3fumigationextraction method (Wuet al.1990). Dissolved organic carbon (DOC) in the KCl extracts was determined using a total carbon analyzer (Phoenix 8000,Tekmar-Dohrmann Co.,Germany). Soil bulk density (BD) was determinedviathe core method (Reinsch and Grossman 1995). All soil properties are shown in Table 1.

Table 1 Physiochemical properties of the soils sampled under different tillage treatments in a semi-arid area in Shanxi Province,China,cultivated with maize
Soil texture was determined using a modified version of the standard hydrometer method,without removal of carbonates or organic matter (Gee and Bauder 1986),on a 30 g subsample dispersed in 100 mL of 5% sodiumhexametaphosphate solution for 18 h. Subsamples of the silt and clay fractions were oven-dried and ground(<200 μm),for determination of the soil carbon content.
The soil carbon protective capacity (g C kg-1soil) was calculated using the association between soil texture and mineral (silt+clay) carbon content (g C kg-1soil),developed by Sixet al.(2002),as follows:

We then estimated SCSD (%) based on SOC content for all sites and horizons,as described by Sixet al.(2002),as follows:

2.4.DNA extraction
We extracted DNA from 0.50 g of soil,using the FastDNA Spin Kit (MP Biomedicals,Santa Ana,CA,USA),according to the manufacturer’s protocol. The purity and yield of extracted DNA were verified by 1% agarose gel electrophoresis and ultraviolet absorption in a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific,Waltham,MA,USA).
2.5.Gene quantification by real-time PCR
Quantification of the bacterial 16S rRNA gene and thecbbLgene was performed with real-time quantitative PCR,using the primers 799F/1492R and k2f/v2r (Tolli and King 2005). The reaction mixtures had a volume of 10 μL,containing 5 μL 2×SYBR PremixExTaq(TaKaRa Bio Inc.,Shiga,Japan),0.5 μL of each primer (Invitrogen,China),1 μL of soil DNA template (5 ng μL-1),and 3 μL of sterilized water,amplified in an ABI Prism 9700 real-time PCR system (PerkinElmer,Applied Biosystems,USA).The thermal protocol for both bacteria andcbbLgenes were as follows:30 s at 95°C,followed by 40 cycles of 10 s at 95°C,40 s at 62°C,and 30 s at 72°C. Each sample was run in triplicate. A standard curve was generated using ten-fold serial dilutions of plasmids containingcbbLand 16S rRNA gene inserts,and was carried out in parallel with that for the soilcbbLand 16S rRNA genes (Yuanet al.2015). The copy numbers of thecbbLand 16S rRNA gene in the reaction mixture were automatically calculated using SDS 2.3 Software(Applied Biosystems,Foster City,CA) within the realtime PCR system,with reference to the standard curve generated within each run.
2.6.Sequencing and analysis
ThecbbLgene was PCR-amplified from the total extracted DNA,using the primer set k2f/v2r. The PCR productsfrom different replicates for each sample were pooled,purified using a Gel-Extraction kit (Tiangen,China),and cloned intoEscherichiacoliDH5α using the pGEM-T easy vector system (Promega,Mannheim,Germany),followed by screening for colonies with the insert.Randomly selected clones (approximately 100 clones for each sample) were screened for the presence of inserts,using the vector-specific primers T7 and SP6,and then sequenced through BGI (Wuhan,China). The sequences ofcbbLclones were checked for close relatives and taxonomic assignment to knowncbbLsequences,using the basic local alignment search tool. Sequence identity of >97% was defined as an operational taxonomic unit(OTU). Representative nucleotide sequences of these OTUs were aligned with closely related known sequences from the NCBI database using ClustalX 1.83,and neighbor-joining trees forcbbLwere generated using MEGA 6.0. The representative nucleotide sequences of these OTUs were deposited in GenBank under accession numbers MK 133929-MK 134001. A bootstrap analysis of 1 000 replicates was used to estimate the stability of tree topologies. The coverage rate was computed as C=[1-(n/N)]×100,wherenrepresents the number of OTUs containing one individual sequence andNis the total number of sequences. The Shannon-Wiener index(H) was used to compare the biodiversity ofcbbL-carrying bacterial communities under different soil management practices and was calculated using the following formula:H=-∑Ai×lnAi,whereAiis Hp/100.
2.7.Statistical analysis
Univariate general linear modeling was used to test the effects of treatment,soil depth,and their interactions on the dependent variable. We applied one-way ANOVA and calculated least significant differences (LSD) to detect statistical differences across soil depths within the same treatment. The independent samplest-test was used to detect statistical differences between treatments within the same soil depth,applying a significance level ofP<0.05. All statistical analyses were performed using the software package IBM SPSS Statistics 21.0 (SPSS Inc.,Chicago,USA). Curve fitting was conducted with Origin version 2019b (OriginLab,Northampton,MA,USA).
We used CANOCO 5.0 (Microcomputer Power,Ithaca,NY,USA) to analyze the relationship between thecbbLcarrying bacterial community structure and environmental factors. Based on the results of the detrended correspondence analysis (DCA),redundancy analysis(RDA) was used for correlation analysis,and the Monte Carlo permutation test was used for significance analysis(P<0.05).
3.Results
3.1.Soil carbon saturation deficit
After 15 years conservation tillage,the SCSD was significantly influenced by treatment,soil depth,and their interaction (allP<0.01;Fig.1). In the 0-5,5-10,and 10-15 cm soil layers,the SCSD of the CT treatment was significantly larger than that of the NT treatment by 336.2,69.5,and 21.7%,respectively (allP<0.05;Fig.1).In NT,the SCSD increased significantly with increase in soil depth (P<0.05). In CT,the SCSD of the 5-10 cm soil layer was significantly lower than that of the 0-5 and 10-15 cm soil layers,respectively (P<0.05;Fig.1).

Fig. 1 Soil carbon saturation deficit as influenced by tillage treatment and soil depth. NT,no-tillage with residue retention;CT,conventional tillage without residue retention. Univariate general linear modeling was used to test the effects of treatment,soil depth,and their interaction on SCSD. The independent samples t-test was used to detect the statistical differences between tillage treatments within each soil depth. Different lowercase letters indicate significant differences among soil depth within the same treatments (P<0.05). Error bars represent standard errors (n=5). * and ** represent statistically significant difference at P<0.05 and P<0.01,respectively.
3.2.Abundance of bacterial 16S rRNA,cbbL,and ratio of cbbL to 16S rRNA
The abundance of the 16S rRNA gene was only significantly influenced by soil depth (P<0.05;Fig.2-A),while the abundance of thecbbLgene and the ratio ofcbbLto 16S rRNA were significantly influenced by tillage treatment,soil depth,and their interaction (allP<0.001;Fig.2-B and C).
In the 0-5 and 5-10 cm soil layers,the NT treatment showed a higher abundance of the 16S rRNA gene than the CT treatment. However,this difference was not statistically significant (P>0.05;Fig.2-A). In the 10-15 cm soil layer,the abundance of 16S rRNA in NT was significantly lower than that in CT (P<0.05;Fig.2-A).In addition,the abundance of 16S rRNA decreased with increasing soil depth in NT;however,for CT,the differences among different depths were not statistically significant (P>0.05;Fig.2-A).
Compared with the 16S rRNA gene,the abundance of thecbbLgene showed different trends in response to tillage practices. At depths of 0-5,5-10,and 10-15 cm,there was a significantly lower abundance of thecbbLgene in the NT treatment than in the CT treatment (P<0.05;Fig.2-B). In CT,thecbbLgene abundance increased with increasing soil depth,and significant differences were observed at the soil depth of 10-15 cm (P<0.05;Fig.2-B).Similar trends were observed for the ratio of thecbbLto 16S rRNA genes,with significant differences between NT and CT for all soil depths (P<0.05;Fig.2-C).
3.3.Community structures of cbbL-carrying bacteria
ThecbbL-carrying bacterial communities differed between the NT and CT treatments and were mostly composed of the generaThiodictyon,Cyanobium,Mesorhizobium,Bradyrhizobium,Starkeya,Curpriavidus,Thermomonospora,Azospirillum,andDevosia(Fig.3-A).In addition,the average value of unclassified genera accounted for 27.6% in the topsoil layers (0-5,5-10,and 10-15 cm) under NT and 27.0% in the topsoil layers (0-5,5-10,and 10-15 cm) under CT. In the topsoil layers under NT and CT,the dominant genera wereThiodictyonandCyanobium,accounting for 57.0 and 6.7%,46.7 and 14.2%,respectively,of the totalcbbL-carrying bacteria,(Fig.3-A).
Several genera exhibited substantial variation in abundance under the NT and CT treatments. For example,the relative abundances ofThiodictyonwere greater under NT than under CT,while the relative abundances ofCyanobiumwas greater under CT(Fig.3-B).
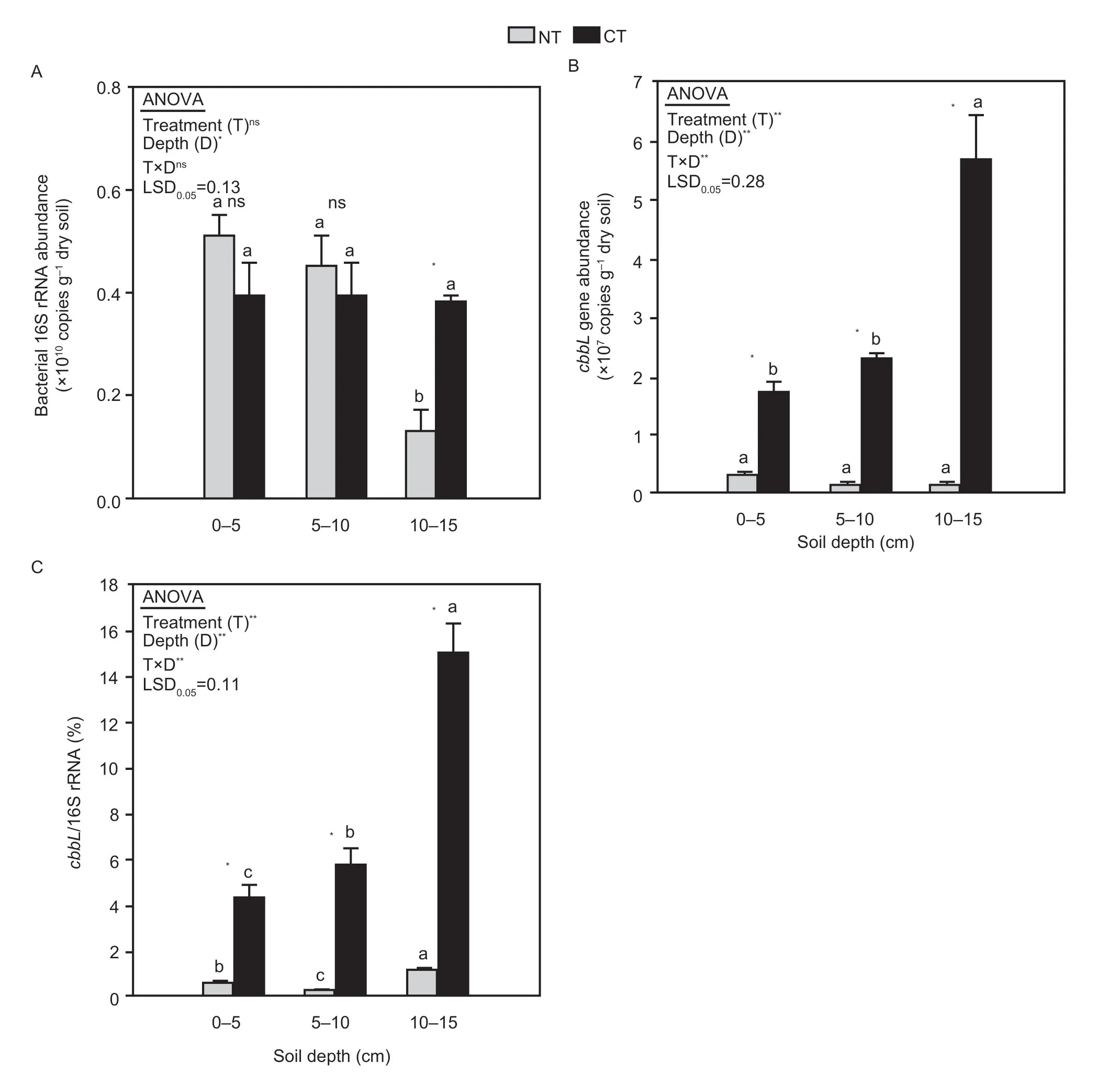
Fig. 2 Abundance of bacterial 16S rRNA and cbbL and ratio of cbbL to 16S rRNA,as influenced by tillage treatment and soil depth. A,bacterial 16S rRNA abundance. B,cbbL gene abundance. C,ratio of cbbL to 16S rRNA. NT,no-tillage with residue retention;CT,conventional tillage without residue retention. LSD0.05 is the least significant difference (P<0.05).Different lowercase letters indicate significant differences between different soil layers in the same treatment (P<0.05).Independent samples t-test was used to detect the statistical differences between tillage treatments within the same soil depth (P<0.05). Error bars represent standard errors (n=5).* and **,indicate significant differences at P<0.05 and P<0.01,respectively;ns,not significant.
3.4.Phylogenetic analysis of cbbL-carrying bacteria
A phylogenetic tree was constructed with the 73cbbLcarrying bacterial OTUs retrieved from the NT and CT treatments,and 16 knowncbbLsequences from the NCBI database (Fig.4). The coverage rates of clone library sequences for three soil samples were 88.0% for NT and 84.3% for CT. The OTUs mainly belonged toα-Proteobacteria(31.5%),γ-Proteobacteria(12.3%),andCyanobacteria(34.2%). In addition,21.9% of thecbbLcarrying bacterial OTUs were grouped in the unclassified cluster. Overall,phylogenetic analysis showed that there was no significant difference in the diversity ofcbbLcarrying bacteria between the two treatments.
3.5.Diversity analysis of cbbL-carrying bacterial communities
Over the 0-15 cm soil depth,thecbbL-carrying bacteria were distributed in 36 and 47 OTUs in the NT and CT treatments,respectively (Fig.5-A and B). In NT,the exclusive OTUs decreased with increasing soil depth;while in CT,a more even distribution under the different soil layers was observed (Fig.5-A and B).

Fig. 3 Changes in relative abundance at the genus level of cbbL-carrying bacterial. A,relative abundance of cbbL-carrying bacterial at the genus level in different soil depths under NT and CT. B,change in relative abundance (relative abundance in NT-relative abundance in CT) at the same soil layer under NT and CT. NT,no-tillage with residue retention;CT,conventional tillage without residue retention. 5,10,15 after NT and CT represent 0-5,5-10,and 10-15 cm soil layers,respectively. Phylogenetic groups accounting for <1% of all classified sequences are summarized in the artificial group ‘Others’.

Fig. 4 Phylogenetic tree of cbbL sequences from no-tillage with residue retention (NT) and conventional tillage without residue retention (CT)treatments in semiarid soil. 5,10,15 after NT and CT represent 0-5,5-10,and 10-15 cm soil layers respectively.GenBank accession numbers of clone sequences are MK 133929-MK 134001.

Fig. 5 Diversity of cbbL-carrying bacteria. A and B,the overlaps of cbbL-carrying bacteria within different soil depths under NT and CT treatments based on operational taxonomic units (OTUs) (97% similarity). C,Shannon-Wiener index of cbbL-carrying bacterial communities under different soil depths in NT and CT treatments. NT,no-tillage with residue retention;CT,conventional tillage without residue retention. LSD0.05 is the least significant difference (P<0.05). Different lowercase letters indicate significant differences between different soil layers in the same treatment (P<0.05). The Independent samples t-test was used to detect the statistical differences between tillage treatments within the same soil depth (P<0.05). Error bars represent standard errors (n=5).* and **,indicate significant differences at P<0.05 and P<0.01,respectively;ns,no significance.
The Shannon-Wiener index value significantly differed between CT and NT for all soil depths (allP<0.05;Fig.5-C). In CT,at the depths of 0-5,5-10,and 10-15 cm,the values were higher than those in NT by 22.9,75.0,and 36.4%,respectively.
3.6.Links between the selected soil properties and the cbbL-carrying bacteria
The first RDA axis (horizontal) explained 45.3% of the total variance incbbL-carrying bacteria,while the second axis (vertical) explained 26.7% of the total variance (Fig.6). According to the results of the Monte Carlo permutation test,soil moisture (P=0.001) had the highest correlation withcbbL-carrying bacteria,followed by BD (P=0.003). Other parameters,such as pH,soil temperature,DOC,MBC,SOC,and TN,were not significantly correlated withcbbL-carrying bacteria.The genusThiodictyonwas mostly positively associated with the observed soil physicochemical properties,whileCyanobiumwas negatively related with all the observed soil physicochemical properties.
3.7.Relationship between soil carbon saturation deficit and cbbL gene abundance
We found a significant increasing exponential relationship between the SCSD and the abundance of thecbbLgene(Fig.7-A). With an increase in SCSD,the abundance of thecbbLgene increased considerably. When the SCSD was small,the abundance of thecbbLgene was also small. There was no relationship between the SCSD and the Shannon-Weiner index (Fig.7-B).

Fig. 6 Redundancy analysis of the dominant phyla of cbbLcarrying bacteria (blue arrows) and the selected soil properties(red arrows) under different tillage treatments. BD,SOC,TN,MBC,and DOC represent soil bulk density,soil organic carbon,total soil nitrogen,microbial biomass carbon,and dissolved organic carbon,respectively.

Fig. 7 Relationship between soil carbon saturation deficit and cbbL gene abundance (A) and Shannon-Weiner index (B). * indicate significant differences at P<0.05.
4.Discussion
4.1.Impacts of different tillage treatments on soil carbon saturation
It is well known that long-term conservation tillage improves the nutrient status of topsoil and produces a new equilibrium soil carbon content over time (Novaket al.2020). Consequently,long-term conservation tillage has produced a different SCSD to conventional tillage(Corbeelset al.2016). Our results showed that NT had a lower SCSD than CT,irrespective of the soil depth(Fig.1-A-C). The lower SCSD under NT could be mainly attributed to the lower levels of physical disturbances,higher input of organic matter,and greater decomposition of crop residues by soil microbes (Alvarez and Steinbach 2009). Some studies on soil carbon saturation behavior of different soil fractions showed that soil carbon saturation is sequential or hierarchical (Guldeet al.2008;Stewartet al.2009;Duet al.2014). With an increase in carbon input,soil carbon saturation first appears in mineral-associated SOC,and then in labile soil carbon pools (Sixet al.2002;Guldeet al.2008). However,mineral-associated SOC is composed of either direct microbial production(i.e.,microbial cellular and extracellular compounds) or byproducts of microbial microbe-mediated decay (Lianget al.2017,2019). Therefore,further research is needed on the abundance,diversity and community structure of microorganisms in relation to soil carbon saturation under long-term tillage practices.
4.2.Impacts of different tillage treatments on the abundance and diversity of cbbL-carrying bacteria
Changes in soil physicochemical properties have been reported as key factors driving variation in the abundance and diversity ofcbbL-carrying bacteria in different tillage treatments (Geet al.2016). Our results showed that the abundance of 16S rRNA was higher in the surface layer (0-10 cm) of NT than in CT,but the abundance ofcbbLgenes was significantly higher in CT,with relatively low soil nutrient levels than in NT,with higher soil nutrient levels (Fig.2-A-C;Table 1).Consistent with previous studies,high abundance ofcbbL-carrying bacteria in nutrient-poor soils suggests the soil autotrophic microorganisms are more important in nutrient-poor environments (Xiaoet al.2018;Zhaoet al.2018;Nel and Cramer 2019). These findings highlight the contribution of the CO2-fixation process to SOC sequestration in agricultural soils.
The results of this study showed that long-term NT treatment reduced the diversity ofcbbL-carrying bacteria in the 0-15 cm soil layer compared to the CT treatment(Fig.5-A-C). Previous studies have shown that soil pH is a major driver of variation in bacterial diversity (Zhaoet al.2018;Zhouet al.2019),but some studies have shown that soil pH and bacterial diversity are not correlated in arid and semi-arid regions (Maestreet al.2015),which is consistent with our findings. This is because the linear increase in bacterial diversity with pH was found mainly in soils with pH 3.5-6.5 (Lauberet al.2009),while the soil pH of our study was in the range of 7.3-7.5 (Table 1).In addition to soil pH,soil moisture is considered to be the main factor driving changes in bacterial community diversity in arid and semi-arid regions (Chenet al.2015;Zhaoet al.2021). Our results indicate that soil moisture content is highly correlated withcbbL-carrying bacteria(Fig.6). Thus,our results imply that soil moisture may be a more critical regulator than pH ofcbbL-carrying bacteria in arid and semi-arid agroecosystems.
4.3.Impacts of different tillage treatments on the community structure of cbbL-carrying bacteria
In general,the community structure ofcbbL-carrying bacteria responds to changes in soil factors caused by farm management practices (Zenget al.2016;Zhouet al.2019;Liet al.2020). Our research showed that the dominant genera ofcbbL-carrying bacteria in NT and CT treatments wereThiodictyonandCyanobium,respectively. However,compared to CT,NT treatment increased the relative abundance ofThiodictyonbut decreased the relative abundance ofCyanobium(Fig.3-B). This is mainly becauseCyanobium,which belongs toCyanobacteriais a phototrophic autotrophic microorganism (Fig.4) that requires light for its growth process (Cuellar-Bermudezet al.2015). Long-term NT reduced light in the subsoil and therefore limited the growth ofCyanobium(Wuet al.2014). This was verified by our data,where the relative abundance ofCyanobiumunder the NT treatment decreased with increasing soil depth,while under the CT treatmentCyanobiumshowed no significant variation with increasing soil depth (Fig.3-A).
Our study shows that the genus with the highest relative abundance ofcbbL-carrying bacteria in both NT and CT isThiodictyon(Fig.4),which belongs toγ-Proteobacteriaand is a purple sulfur bacterium(Storelliet al.2013). Previous studies have shown thatThiodictyonstrains have high rates of CO2assimilation in both light and dark environments in the chemocline of Lake Cadagno (de Wit and van Gemerden 1987;Storelliet al.2014). This was verified by our results,where an increase in the relative abundance ofThiodictyonoccurred with increasing soil depth under both the NT and CT treatments(Fig.3-A). Unlike the results of previous studies,in paddy soils,the dominantcbbL-carrying bacteria areα-Proteobacteria(Yuanet al.2012b),while in arid soils,α-Proteobacteria,β-Proteobacteriaand uncultured bacteria are more common (Tanget al.2015). This could be mainly due to differences in soil types,ecosystems,and tillage practices (Luet al.2019;Xiaoet al.2019). Additionally,it has also been suggested thatThiodictyonstrains play an important role in the fixation of inorganic carbon (Camachoet al.2001;Storelliet al.2014). Combined with our results of an increase in the relative abundance ofThiodictyonunder both NT and CT conditions,it is implied thatThiodictyonmay play an important role for carbon fixation in semi-arid farmland ecosystems with high soil inorganic carbon content,and this suggestion needs further study.
4.4.Relationship between the abundance and diversity of cbbL-carrying bacteria and soil carbon saturation
A series of recent studies have shown that microorganisms may contribute to soil carbon saturation processes dominated by mineral-associated organic carbon and additionally that CBB gene transcription by autotrophic microorganisms is repressed by feedback inhibition from soil organic carbon (Lianget al.2017,2019;Wanget al.2020;Craiget al.2021;Zhanget al.2021). Our study explored the relationship between soil carbon saturation and the abundance and diversity ofcbbL-carrying bacteria and showed a significant exponential relationship between SCSD and the abundance ofcbbL-carrying bacteria,but no relationship between SCSD and the diversity ofcbbLcarrying bacteria,suggesting that low SCSD limits the abundance ofcbbL-carrying bacteria under NT,but has no effect on their diversity.
We therefore discuss the possible mechanisms by which low SCSD inhibits the abundance ofcbbLcarrying bacteria. There are specific small molecule organic metabolites that have been reported to inhibit the CBB cycle (Dangel and Tabita 2015;Gruberet al.2017),such as phoglycolate,a known inhibitor of triosephosphate isomerase enzyme in the Calvin cycle,and the production of phoglycolate during aerobic autotrophic growth is inevitable due to the oxygenation catalysis of RuBisCO (Shivelyet al.1998).Some of the non-substrate small molecules (such as phosphoglycolate) produced by the CBB cycle and the small waste metabolites (usually acids) produced during dissimilation have structures similar to those of the inhibitors described above (Zark and Dittmar 2018),so those small molecules may have stronger feedback inhibition on the growth of autotrophic microbial cells.In general,these intracellular free organic carbons(IFOC),such as non-substrate small molecules and the small molecule waste metabolites are gradually released outside the cell to form EFOC,which are important precursors for the formation of mineralassociated organic carbons (Wanget al.2018;Yeet al.2020;Craiget al.2021). However,when soil has a low carbon saturation deficit,there is an accumulation of EFOC,which inhibits the release of IFOC,thus inhibiting the transcription of CBB genes and reducing the number of autotrophic bacteria (Zhanget al.2021).Consistent with previous findings,an increase in EFOC or exogenous organic carbon decreases CBB gene transcription,which in turn decreases the number of autotrophic bacteria (Wanget al.2018,2020). Our results indicate that the inhibition ofcbbL-carrying bacteria abundance by soil carbon saturation is not linear,but exponential. It indicates that the smaller the SCSD the greater the suppressive effect on the population ofcbbL-carrying bacteria. This may be because the process of carbon saturation is also not linear,but hierarchical (Stewartet al.2009). Our study explored the effects of soil carbon saturation on the abundance ofcbbL-carrying bacteria in farmland with different tillage systems,and provided evidence for the existence of a relationship between biological and physicochemical carbon sequestration mechanisms in farmland systems.
5.Conclusion
This study examined the effect of soil carbon levels on autotrophic CO2-fixing bacteria under long-term conservation tillage practices. Long-term NT reduced SCSD,abundance and diversity ofcbbL-carrying bacteria,altered the community structure ofcbbLcarrying bacteria,and increased the relative abundance of the genusThiodictyon. Low SCSD limits the abundance ofcbbL-carrying bacteria,but has no effect on their diversity. Our study provides evidence for the existence of a relationship between biological and physicochemical carbon sequestration mechanisms in farmland systems. Further research should be conducted to examine feedback from the biological carbon sequestration pathway to the physicochemical carbon sequestration pathway and inhibition of the biological carbon sequestration pathway by the physicochemical carbon sequestration pathway. Further research is also needed on the carbon fixation yield by autotrophic CO2-fixing bacteria and the capacity of the soil carbon pool under farm ecosystems in northern China.
Acknowledgements
We thank all those who helped with field and laboratory work at the Institute of Environment and Sustainable Development in Agriculture,Chinese Academy of Agricultural Sciences. We thank the anonymous reviewers for their careful reading of our manuscript and their many insightful comments and suggestions. This work was supported by the National Natural Science Foundation of China (31171512 and 42007312),the Key R&D Program of Hainan Province,China (ZDYF2020084),the Natural Science Foundation of Shandong Province,China (ZR2020QD117),the Research Fund for Introduced High-level Talents of Qingdao Agricultural University,China (11201103) and the Central Public-interest Scientific Institution Basal Research Fund,China (BSRF202001).
Declaration of competing interest
The authors declare that they have no conflict of interest.
 Journal of Integrative Agriculture2022年8期
Journal of Integrative Agriculture2022年8期
- Journal of Integrative Agriculture的其它文章
- Historical trends in maize morphology from the 1950s to the 2010s in China
- The rhizosphere microbial complex in plant health:A review of interaction dynamics
- Allele mining of wheat ABA receptor at TaPYL4 suggests neofunctionalization among the wheat homoeologs
- Characterization of chromosome segment substitution lines reveals candidate genes associated with the nodule number in soybean
- Transcriptional profling between yellow-and black-seeded Brassica napus reveals molecular modulations on flavonoid and fatty acid content
- An economic and viable approach to improve wheat quality in Qinghai–Tibetan Plateau,China
