运动对肥胖绝经后妇女血脂及体成分干预效果的Meta分析
梁 敏,付 玉,段意梅,李顺昌
运动对肥胖绝经后妇女血脂及体成分干预效果的Meta分析
梁 敏1,付 玉2,段意梅2,李顺昌1
1.成都体育学院运动医学与健康研究所,四川 成都,610041;2.成都体育学院运动医学与健康学院,四川 成都,610041。
评价运动对肥胖绝经后妇女血脂和体成分的干预效果。计算机检索PubMed、EMbase、WanFang Data 和CNKI等数据库,搜集运动干预肥胖绝经后妇女代谢和体成分的相关RCT,检索时限设定为建库至2020年3月,由2名研究者按纳入和排除标准筛选文献并提取有效数据,进行质量评价。采用RevMan 5.3软件对最终纳入的文献数据进行Meta分析。最终纳入13个RCT,包括1042例受试者。Meta分析结果显示,甘油三酯[SMD=-0.19,95%CI(-0.45,0.08),P=0.17]无明显改善作用,但运动后肥胖绝经后妇女高密度脂蛋白[SMD=0.75,95%CI(0.20,1.30),P=0.008]明显升高,低密度脂蛋白[SMD=-0.45,95%CI(-0.79,-0.12),P=0.008]显著下降;体成分方面,运动在改善肥胖绝经后妇女体脂百分比[SMD=-0.82,95%CI(-1.26,-0.38),P=0.0003]和BMI[SMD=-0.61,95%CI(-1.05,-0.16),P=0.008]上具有明显优势,而腰臀比[SMD=-1.51,95%CI(-3.41,0.39),P=0.12]的变化无显著差异。不同运动形式分析得出:与单纯运动相比,运动加饮食调理对BMI[SMD=0.54,95%CI(0.29,0.79),P<0.0001]的改善效果更加明显;而与单纯有氧运动相比,有氧联合抗阻运动对体脂百分比[SMD=0.59,95%CI(-0.46,1.63),P=0.27]和甘油三酯[SMD=0.26,95%CI(-0.33,0.86),P=0.38]的影响无明显差异。运动干预可明显改善肥胖绝经后妇女的体成分和部分血脂相关指标,且运动加饮食调理改善效果更加明显。但受纳入研究数量和质量的限制,上述结论尚待更多高质量研究或进行亚组分析予以深入拓展。
运动;肥胖;绝经后妇女;血脂;体成分;Meta分析
近几十年来,肥胖病史无前例地增长,对人类健康的威胁日益加剧。截止2014年,全球超过19亿成年人身体超重[1][2],肥胖的高危人群是绝经后妇女[3];根据2015年的全球数据显示,60-64岁年龄段的女性肥胖超过男性[4]。因而,超重和肥胖妇女可能需要更多的临床护理和体重干预[5]。运动可以提升能量消耗,减少体脂含量,增加肌肉质量,改善机体内分泌稳态[6]。可见,中老年妇女持续进行体育锻炼是非常有必要的。研究发现,超重和肥胖妇女长期进行体育锻炼可明显增强体质,改善心理状态[7],进而降低罹患绝经代谢综合征的风险[8][9]。但对关于长期体育锻炼对肥胖绝经后人群的影响的共识尚不清楚,且无系统评价运动与绝经肥胖妇女的关系。本研究采用循证医学方法,对国内外有关运动干预肥胖绝经后妇女的随机对照试验(randomized con-trolled trials,RCTs)进行系统综述和Meta分析,评价运动对肥胖绝经后妇女的血脂和体成分的干预疗效,以期为运动在治疗肥胖绝经后人群的临床应用提供理论依据和实践参考。
1 资料和方法
1.1 纳入与排除标准
1.1.1 研究类型 随机对照试验(randomized controlled trial RCT);语言为中文或英文。
1.1.2 研究对象 符合肥胖绝经妇女判定要求并进行运动干预,其种族、国籍不限。
1.1.3 纳入标准 根据WHO判定BMI≥23 kg / m2为超重,≥25 kg / m2为肥胖,已绝经妇女≥半年;经过询问病史和体格检查排除有急性疾病;试验组运动干预或运动加饮食干预,对照组不干预或普通饮食干预。
1.1.4 排除标准 (1)非中、英文文献;(2)重复发表的文献;(3)结局指标数据全缺失;(4)服用减肥药或其他激素的受试者;(5)具有不良生活习惯,运动系统、心血管系统等不适宜参加运动的各种急性慢性病;(6)结局指标单位不同。
1.1.5 结局指标 代谢变量指标:(1)甘油三酯(TG);(2)高密度脂蛋白(HDL-C);(3)低密度脂蛋白(LDL-C);体成分指标:(4)BMI;(5)腰臀比(WHR);(6)体脂百分比(Fat%)。
1.2 文献检索策略
检索PubMed、EMbase、WanFang Data 和CNKI 数据库,搜集运动干预肥胖绝经后妇女代谢和体成分的相关RCT,检索时限设定为建库至2020年3月,同时追溯检索纳入文献的参考文献。中文检索词包括:运动,肥胖(超重),绝经妇女,糖脂代谢,体成分,随机对照,临床研究等;英文检索词包括:exercise,exercise and overweight,postmenopausal women,glycolipid metabolism,Body components,randomized controlled trials,clinical trial等。
1.3 资料提取及质量评价
由2名研究者独立筛选文献、提取资料并交叉核对。如有分歧,则通过讨论或与第三方协商解决。文献筛选时首先阅读文题,在排除明显不相关的文献后,进一步阅读摘要和全文以确定是否纳入。如有需要,通过邮件、电话联系原始研究作者获取未确定但对本研究非常重要的信息。资料提取内容包括:(1)纳入研究的基本信息:研究题目、第一作者、发表时间等;(2)研究对象的基线特征和干预措施;(3)偏倚风险评价的关键要素;(4)所关注的结局指标和结果测量数据。
1.4 统计学分析
采用国际循证医学协作网Cochrane 提供的RevMan 5.3软件进行Meta分析。统计学方法参照Cochrane系统评价手册[10],计量资料以标准化均数差(standardized mean difference,SMD(或均数差(weighted mean difference,WMD)为效应指标,计数资料以相对危险度(risk ratio,RR)为效应指标,效应量均给出其点估计值和95%可信区间(confidence interval,CI);首先进行异质性检验,结合I2定量对纳入研究的同质性进行判定;若各纳入研究间同质性较好(P > 0.05或I2< 50%),则采用固定效应模型进行Meta分析;反之,采用随机效应模型进行Meta分析。以P < 0.05为差异有统计学意义。
2 结 果
2.1 文献筛选流程及结果
初检共获得相关文献783篇,经逐层筛选后,最终纳入13个RCTs[11-23],包括1042例试验数量。文献筛选流程及结果见图1。

图1 文献检索流程
2.2 纳入文献的质量评价(或者偏倚风险分析)
纳入文献信息对照Cochrane系统评价标准分别从随机分组产生、隐蔽分组、双盲实验、效应指标盲检、实验数据不完整、实验结果的选择性报告、其他偏倚风险7个评价指标对纳入文献偏倚风险进行综合评价。其中黄彩华、Brochu M[16]、Trabka[23]、Ryan A S[12]、Serra M C[17]等的研究因试验前提前告知参与者试验方案,对结局指标产生影响的风险较高;其中颜廷[15]、顾国新[11]、Roussel M[18]、Park[22]等文献质量较好;对图2和图3总体分析,纳入分析文献存在一定偏倚性,文献整体质量处与中等偏上。
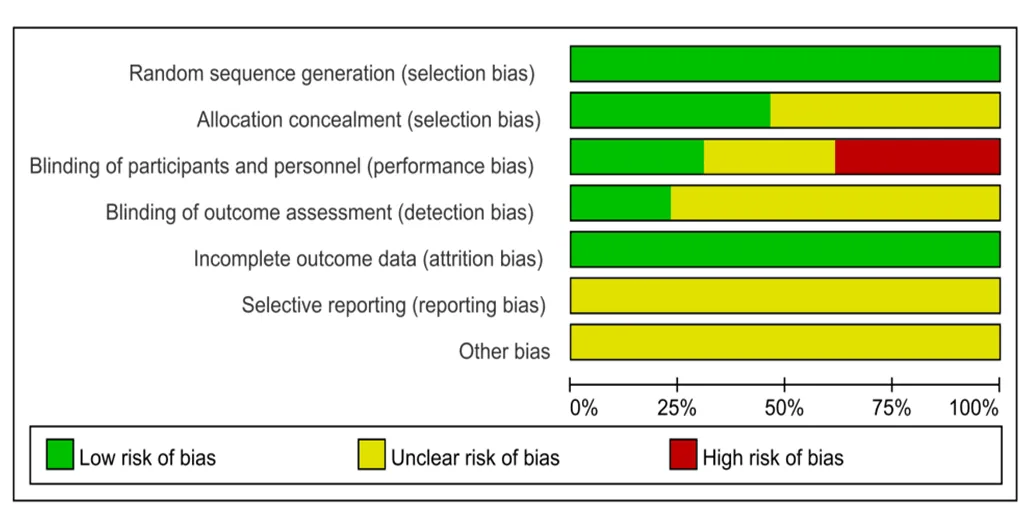
图2 纳入文献偏倚评价图
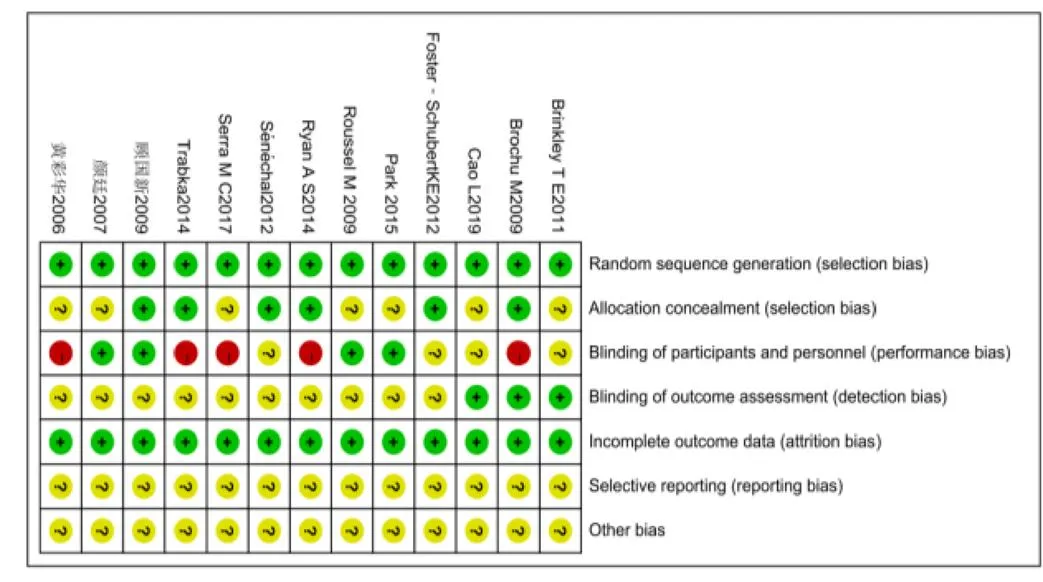
图3 纳入文献偏倚总结
2.3 纳入研究的基本特征,见表1。

表1 纳入研究的基本特征
注:①甘油三酯(TG)、②高密度脂蛋白(HDL-C)、③低密度脂蛋白(LDL-C);体成分指标:④BMI、⑤腰臀比(WHR)、⑥体脂百分比(Fat%)T1运动组、T2运动+饮食、T3有氧+抗阻
2.4 Meta分析结果
2.4.1 运动对肥胖绝经后妇女糖血脂干预疗效的Meta分析控制甘油三酯方面,共纳入6个RCT[11,14,16,21,23,19],包含235例受试者。固定效应模型Meta分析结果显示:对比常规生活,运动干预并未表现出明显的统计学差异[SMD=-0.19,95%CI(-0.45,0.08),=0.17](图4)。
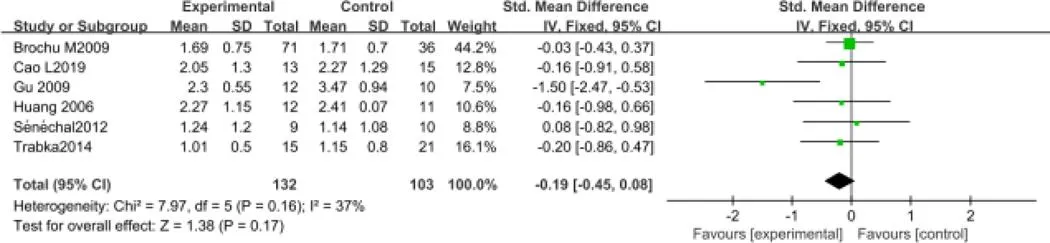
图4 运动对肥胖绝经后妇女甘油三酯干预疗效的Meta分析
控制高密度脂蛋白方面,共纳入6个RCT[11,14,17,19,21,23],包含530例受试者。随机效应模型Meta分析结果显示:对比常规生活,运动干预表现出明显的统计学差异[SMD=0.75,95%CI(0.20,1.30),P=0.008](图5)。

图5 运动对肥胖绝经后妇女高密度脂蛋白干预疗效的Meta分析
控制低密度脂蛋白方面,共纳入6个RCT[11,14,18,19,23],包含530 例受试者。随机效应模型Meta分析结果显示:对比常规生活,运动干预表现出明显的统计学差异[SMD=-0.45,95%CI(-0.79,-0.12),P=0.008](图6)。
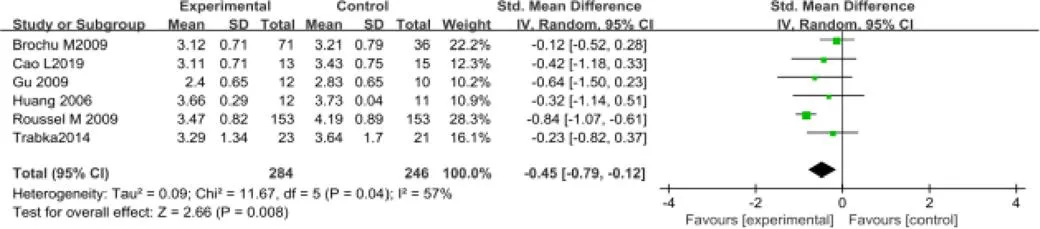
图6 运动对肥胖绝经后妇女低密度脂蛋白干预疗效的Meta分析
2.4.2 运动对肥胖绝经后妇女体成分干预效果的Meta分析控制体脂百分比方面,共纳入9个RCT[12-15,17,19-22],包含635例受试者。随机效应模型Meta分析结果显示:对比常规生活,运动干预表现出明显的统计学差异[SMD=-0.82,95%CI(-1.26,-0.38),P=0.0003](图7)。

图7 运动对肥胖绝经后妇女体脂百分比干预疗效的Meta分析
控制BMI方面,共纳入9个RCT[12-15,17-18,19-21],包含923例受试者。随机效应模型Meta 分析结果显示:对比常规生活,运动干预表现出明显的统计学差异[SMD=-0.61,95%CI(-1.05,-0.16),P=0.008](图8)。

图8 运动对肥胖绝经后妇女BMI干预疗效的Meta分析
控制腰臀比方面,共纳入4个RCT[11,13,17,19],包含146例受试者。随机效应模型Meta分析结果显示:对比常规生活,运动干预并未表现出明显的统计学差异[SMD=-1.51,95%CI(-3.41,0.39),=0.12](图9)。
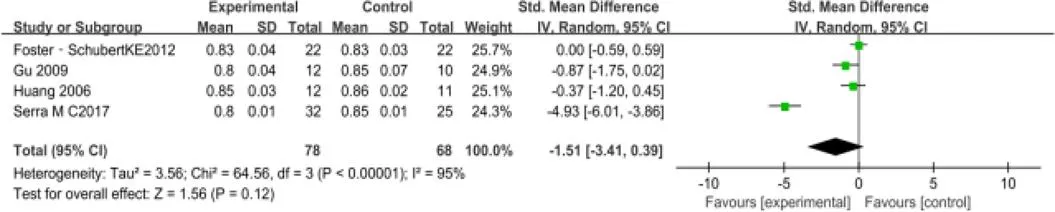
图9 运动对肥胖绝经后妇女腰臀比干预疗效的Meta分析
2.4.3 不同运动形式对肥胖绝经后妇女干预疗效的Meta分析 控制BMI方面,共纳入2个RCT[11,13],包含358例受试者。固定效应模型Meta分析结果显示:对比运动组,运动+饮食调理组表现出明显的统计学差异[SMD=0.54,95%CI(0.29,0.79),P<0.0001](表2)。
控制体脂百分比方面,共纳入3个RCT[15,19,21],包含90例受试者。固定效应模型Meta分析结果显示:有氧运动与有氧+抗阻组相比未表现出明显的统计学差异[SMD=0.59,95%CI(-0.46,1.63),P=0.27](表2)。
控制甘油三酯方面,共纳入2个RCT[19,21],包含44例受试者。固定效应模型Meta分析结果显示:有氧运动与有氧+抗阻组相比未表现出明显的统计学差异[SMD=0.26,95%CI(-0.33,0.86),P=0.38](表2)。

表2 不同运动形式对肥胖绝经后妇女干预疗效的Meta分析
3 讨 论
过度肥胖会增加患脑血管疾病(CVD)、糖尿病、胰岛素抵抗和代谢综合征等风险,这为体育锻炼对身体健康的积极益处提供了有利的支持,此外肥胖女性进行体育锻炼可减少乳腺癌风险相关的生物标志物,即使在运动后没有明显的体重的减轻[24-26]。由于女性在40岁后有氧运动加速下降,更年期伴随着身体成分的变化会影响女性绝经后的状态,特别是肥胖中年妇女,所以进行运动训练对健康是非常必要的[27]。有研究评估了运动或不运动对绝经后女性腹部脂肪的影响,证明运动可减少皮下和腹部内脂肪,但运动加饮食调理可能更有效[28],与Serra M C[17]得出的结论相同,当运动与少量热量摄入相结合时,能更有效改善肥胖状态,但具体干预疗效我们不得而知。Ortmeyer H K等[29]试验对糖耐量受损肥胖绝经后女性进行了6个月的有氧运动训练结果发现运动能增强脂肪酸代谢能力。Wiklund P等[30]比较了6周有氧运动和饮食对绝经妇女的血清代谢组学和心血管代谢危险因素的影响发现,有氧运动可以改善葡萄糖和脂质代谢,但短期的效果不能产生可衡量的健康益处,所以对于运动锻炼要长期有效的进行。运动也可对脂联素和瘦素产生有益的影响[31]。有研究发现,12周抗阻运动训练对体成分的影响与对照组相比无明显变化[32],可能抗阻运动在改善肥胖绝经后妇女体成分方面不如有氧运动,但还需要进行验证。本研究大部分选用有氧运动作为主要运动形式。此外冲刺间隔训练(SIT)、高强度间歇训练(HIIT)和联合训练(CT)等[33-35]运动开始在绝经后肥胖女性中进行试验,为找到合适此类人群锻炼进行不断的尝试,不同的运动方式的形式是否会影响肥胖绝经后妇女相关代谢标志物及身体的变化我们不得而知[36]。
本文Meta分析结果显示,在代谢方面,运动对肥胖绝经后妇女的高密度脂蛋白、低密度脂蛋白具有明显的改善,但空腹血糖、甘油三酯没有明显变化,提示运动可能对改善脂代谢的作用要显著于糖代谢,这可能与肥胖主要造成的脂肪累积含量有关,而运动能有效的进行热量的消耗,所以在糖代谢方面的改善还需要更多地试验支撑;在体成分方面,运动能有效改善肥胖绝经后妇女的体脂百分比和BMI指数,说明身体状态的改善与进行运动相关;由于纳入的文献对不同的运动方式的研究较少,所以不同运动形式分析只能简单的说明运动同时配合饮食调理能更加显著性的改善肥胖绝经后妇女的身体状况,对于有氧抗阻的联合运动并没有显示出更好地益处。以上结论仍需更多的尚需开展更多高质量、多中心的随机对照试验研究加以支持验证,未来的研究可以针对不同的运动方式,运动频率等进行探讨,为肥胖绝经后妇女的运动最佳方案提出对策与建议。
本研究存在以下局限:(1)部分纳入研究的偏倚风险较大,可能存在测量、实施等偏倚;(2)受研究数量限制,无法进行亚组分析,没有进行更深入的分析;(3)干预方式及形式和强度参数不完全一致,也可能导致临床异质性;(4)受研究内容显示,血脂的指标纳入少。
4 结 论
综上所述,运动干预在改善肥胖绝经后妇女的血脂部分指标和体成分方面具有明显的效果,其中运动加饮食调理可能存在明显的优势。但受纳入研究数量和质量的限制,上述结论尚待更多高质量研究或进行亚组分析予以深入拓展。
[1] Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, et al. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention[J]. CA Cancer J Clin, 2017, 67(05): 378~397.
[2] World Health Organization. Obesity and overweight fact sheet. 2017.
[3] Lambrinoudaki I, Brincat M, Erel CT, Gambacciani M, et al. EMAS position statement: managing obese postmenopausal women[J]. Maturitas, 2010(66): 323~326.
[4] Chooi Y.C, Ding C, Magkos F.. The epidemiology of obesity[J]. Metabolism Clinical and Experimental, 2019(92): 6~10.
[5] Shifren J. L., Gass M. L. S. The North American menopause society recommendations for clinical care of midlife women[J]. Journal of The North American Menopause Society, 2014, 21(10):1~25.
[6] LaMonte M J, Wactawski-Wende J, Larson J C, et al. Association of physical activity and fracture risk among postmenopausal women[J]. JAMA network open, 2019, 2(10): 1914084~1914084.
[7] Peterson J. A., Ward-Smith P. Choose to move for positive living: physical activity program for obese women. Holistic Nursing Practice. 2012, 26(03):120~128.
[8] Sowers M, Zheng H, Tomey K, et al. Changes in body composition in women oversix years at midlife: ovarian and chronological aging[J]. J Clin Endocrinol Metab, 2007, 92(03): 895~901.
[9] Stachowiak G, Pertynski T, Pertynska-Marczewska M. Metabolic disorders inmenopause. Prz Menopauzalny. 2015, 14(01): 59~64.
[10] Higgins JP, Altman DG, Gøtzsche PC, et al.The Cochrane Collaboration's tool for assessing risk of bias in randomised trials[J]. BMJ, 2011, 343(5928): 1~9.
[11] 顾国新. 有氧运动与饮食控制对绝经肥胖妇女leptin抵抗状态的影响研究[D].苏州大学,2009.
[12] Ryan A S, Ge S, Blumenthal J B, et al. Aerobic exercise and weight loss reduce vascular markers of inflammation and improve insulin sensitivity in obese women[J]. Journal of the American Geriatrics Society, 2014, 62(04): 607~614.
[13] Foster‐Schubert K E, Alfano C M, Duggan C R, et al. Effect of diet and exercise, alone or combined, on weight and body composition in overweight‐to‐obese postmenopausal women[J]. Obesity, 2012, 20(08): 1628~1638.
[14] Cao L, Jiang Y, Li Q, et al. Exercise Training at Maximal Fat Oxidation Intensity for Overweight or Obese Older Women: A Randomized Study[J]. Journal of Sports Science and Medicine, 2019, 18(03): 413~418.
[15] 颜 廷.不同运动方式对绝经后肥胖妇女身体成分影响的研究[J].临沂师范学院学报,2007(06):85~88.
[16] Brochu M, Malita M F, Messier V, et al. Resistance training does not contribute to improving the metabolic profile after a 6-month weight loss program in overweight and obese postmenopausal women[J]. The Journal of Clinical Endocrinology & Metabolism, 2009, 94(09): 3226~3233.
[17] Serra M C, Blumenthal J B, Addison O R, et al. Effects of weight loss with and without exercise on regional body fat distribution in postmenopausal women[J]. Annals of Nutrition and Metabolism, 2017, 70(04): 312~320.
[18] Roussel M, Garnier S, Lemoine S, et al. Influence of a walking program on the metabolic risk profile of obese postmenopausal women[J]. Menopause, 2009, 16(03): 566~575.
[19] 黄彩华. 运动对肥胖绝经妇女血清脂联素、瘦素水平的影响[D].福建师范大学,2006.
[20] Brinkley T E, Wang X, Kume N, et al. Caloric restriction, aerobic exercise training and soluble lectin-like oxidized LDL receptor-1 levels in overweight and obese post-menopausal women[J]. International journal of obesity, 2011, 35(06): 793.
[21] Sénéchal, Martin, et al. The effects of lifestyle interventions in dynapenic-obese postmenopausal women[J]. Menopause, 2012, 19(09): 1015~1021.
[22] Park, Sung-Mo, Yi-Sub Kwak, et al. The effects of combined exercise on health-related fitness, endotoxin, and immune function of postmenopausal women with abdominal obesity[J]. Journal of immunology research, 2015.
[23] Trabka, Bartosz, et al. Effect of a MAST exercise program on anthropometric parameters, physical fitness, and serum lipid levels in obese postmenopausal women[J]. Journal of human kinetics, 2013, 42: 149~155.
[24] Ross R, Freeman J, Hudson R, et al. Abdominal obesity, muscle composition, and insulin resistance in premenopausal women[J]. J Clin Endocrinol Metab, 2002, 87: 5044~5051.
[25] Arnold M, Pandeya N, Byrnes G, et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study[J]. Lancet Oncol, 2015, 16: 36~46.
[26] Krishnan K, Bassett JK, MacInnis RJ, et al. Associations between weight in early adulthood, change in weight, and breast cancer risk in postmenopausal women[J]. Cancer Epidemiol Biomarkers Prev, 2013, 22: 1409~1416.
[27] Earnest CP, Blair SN, Church TS. Age attenuated response to aerobic conditioning in postmenopausal women[J]. Eur J Appl Physiol, 2010, 110(01):75~82.
[28] Van Gemert W A, Peeters P H, May A M, et al. Effect of diet with or without exercise on abdominal fat in postmenopausal women–a randomised trial[J]. BMC public health, 2019, 19(01): 174.
[29] Ortmeyer H K, Goldberg A P, Ryan A S. Exercise with weight loss improves adipose tissue and skeletal muscle markers of fatty acid metabolism in postmenopausal women[J]. Obesity, 2017, 25(07): 1246~1253.
[30] Wiklund P, Alen M, Munukka E, et al. Metabolic response to 6-week aerobic exercise training and dieting in previously sedentary overweight and obese pre-menopausal women: a randomized trial[J]. Journal of Sport and Health Science, 2014, 3(03): 217~224.
[31] Abbenhardt C, McTiernan A, Alfano C M, et al. Effects of individual and combined dietary weight loss and exercise interventions in postmenopausal women on adiponectin and leptin levels[J]. Journal of internal medicine, 2013, 274(02): 163~175.
[32] Wong A, Figueroa A. The Effects of Low-Intensity Resistance Exercise on Cardiac Autonomic Function and Muscle Strength in Obese Postmenopausal Women[J]. Journal of aging and physical activity, 2019, 27(06): 855~860.
[33] Boutcher, YATI N, et al. The Effect of Sprint Interval Training on Body Composition of Postmenopausal Women[J]. Medicine and science in sports and exercise51.7(2019): 1413~1419.
[34] Gilbertson N M, Eichner N Z M, Heiston E M, et al. A low-calorie diet with or without interval exercise training improves adiposopathy in obese women[J]. Applied Physiology, Nutrition, and Metabolism, 2019, 44(10): 1057~1064.
[35] Nunes P R P, Martins F M, Souza A P, et al. Comparative effects of high-intensity interval training with combined training on physical function markers in obese postmenopausal women: a randomized controlled trial[J]. Menopause, 2019, 26(11): 1242~1249.
[36] Zaki M E. Effects of whole body vibration and resistance training on bone mineral density and anthropometry in obese postmenopausal women[J]. Journal of osteoporosis, 2014, 2014.
Effect of Exercise on Glucose and Lipid Metabolism and body Composition in obese Postmenopausal Women :A Meta-analysis
LIANG Min1, FU Yu2, DUAN Yimei2, et al
1.Institute of Sports Medicine and Health Chengdu Sport University, Chengdu Sichuan, 610041, China;2.College of Sports Medicine and Health, Chengdu Sport University, Chengdu Sichuan, 610041, China.
To evaluate the effect of exercise on blood lipids and body composition of obese postmenopausal women.The computer searched PubMed, EMbase, WanFang Data, and CNKI databases to collect exercise-related RCT related to metabolism and body composition in obese postmenopausal women. The search period was set to build the database until March 2020, and 2 researchers included and excluded The standard screens the literature and extracts valid data for quality evaluation. Meta-analysis was performed on the finally included literature data using RevMan 5.3 software.Thirteen RCTs were included, including 1042 subjects. Meta analysis results show that triglyceride [SMD = -0.19, 95% CI(-0.45, 0.08), P = 0.17] has no significant improvement, but high-density lipoprotein [SMD = 0.75, 95] % CI(0.20, 1.30), P = 0.008] increased significantly, low-density lipoprotein [SMD = -0.45, 95% CI(-0.79, -0.12), P = 0.008] decreased significantly; in terms of body composition, exercise in Improve the percentage of body fat in obese postmenopausal women [SMD = -0.82, 95% CI(-1.26, -0.38), P = 0.0003] and BMI [SMD = -0.61, 95% CI(-1.05, -0.16), P = 0.008] has obvious advantages, but the waist-hip ratio [SMD = -1.51, 95% CI(-3.41, 0.39), P = 0.12] has no significant difference. Analysis of different forms of exercise shows that compared with simple exercise, exercise plus diet conditioning has a more significant improvement effect on BMI [SMD = 0.54, 95% CI(0.29, 0.79), P <0.0001]; compared with pure aerobic exercise Ratio, aerobic combined resistance exercise on body fat percentage [SMD = 0.59, 95% CI(-0.46, 1.63), P = 0.27] and triglycerides [SMD = 0.26, 95% CI(-0.33, 0.86), P = 0.38].Exercise intervention can significantly improve the body composition and some lipid-related indexes of obese postmenopausal women, and the effect of exercise plus diet conditioning is more obvious. However, limited by the number and quality of included studies, the above conclusions are yet to be verified and supported by more high-quality studies or subgroup analyses.
Exercise; Obesity; Postmenopausal women; Blood fat; Body composition; Meta analysis
1007―6891(2022)04―0041―06
10.13932/j.cnki.sctykx.2022.04.08
2020-06-08
2021-07-21
四川省科技计划项目(项目编号:2016J13-019)。
G804.21
A

