Ursodeoxycholic acid prevention on cholestasis associated with total parenteral nutrition in preterm infants: a randomized trial
Si-Ying Liu · Li-Wen Chang ·Jing Wang · Min Xie · Lei-Lei Chen · Wei Liu
Abstract
Keywords Cholestasis · Parenteral nutrition · Preterm infant · Ursodeoxycholic acid
lntroduction
Parenteral nutrition (PN) provides life-saving nutritional support for preterm infants during intestinal growth and adaptation [ 1– 4]. However, a known complication of longterm PN is PN-associated liver disease (PNALD), among which cholestasis associated with total parenteral nutrition(PNAC) is the most common [ 5– 7]. PNAC is characterized by a broad spectrum of signs and symptoms, ranging from conjugated hyperbilirubinemia in a newborn, an enlargement of the liver, a high-level serum alanine aminotransferase(ALT), and a high-level serum aspartate aminotransferase(AST) to cirrhosis and liver failure [ 3, 8– 10]. The incidence of PNAC is relatively high and is estimated up to 60% of the neonatal intensive care unit (NICU) infants receiving longterm PN [ 5, 6, 11– 13]. Major risk factors of PNAC include prematurity, low birth weight, early and prolonged exposure to PN, and early-onset sepsis [ 1, 5, 12– 14]. Based on the current recommendations, PNAC can be alleviated by stopping PN immediately or by using ursodeoxycholic acid (UDCA)and other drugs [ 15– 17]. However, once cholestasis occurs,there will inevitably be some adverse outcomes in premature infants, such as weight loss or even persistent liver disease or hepatic failure [ 4, 18]. Considering that long-term PN, either total or partial, is inevitable for preterm infants, alternative management strategies for PNAC are required.
Ursodeoxycholic acid (UDCA) is present in human bile at a low concentration, so it has advantages of safety and fewer adverse reactions. It has been proposed as a treatment option for PNAC in preterm infants, children and adults [ 15,19]. Traditional reports mainly focused on evaluating the impacts of UDCA on the treatment of PNAC through retrospective case data [ 19, 20]. However, the effects of UDCA prophylaxis on PNAC in preterm infants had not been studied systematically.
Therefore, we focused on the prevention of cholestasis by oral UDCA as early as possible. This study aimed to investigate the prophylactic effect of preventive oral UDCA on PNAC in preterm infants. The collected data reflected the severity of neonatal cholestasis and liver injury and the clinical outcomes. We believe that these data provide important information about the potential role of UDCA on improving the prognosis of cholestasis in premature infants.Accordingly, our work will provide a solution to the widely concerned clinical problem of PNAC in NICU infants. This study had included 102 premature infants admitted to Tongji Hospital, Wuhan, China, from January 2021 to July 2021.
Methods
Study design
We treated UDCA prophylaxis and no prophylaxis as management strategies for PNAC in this prospective, randomized, open-label, proof-of-concept trial. Preterm infants with UDCA prophylaxis were enrolled in the UDCA group, and other infants without UDCA prophylaxis were included in the control group.
Study participants
A total of 124 low-birth-weight preterm neonates were screened for their eligibility. All the infants fulfilled the following criteria in Table 1. The study was approved by the Ethics Committee of Tongji Hospital (approval number: T J-IRB20210828, trial registration number:NCT05043194). All parents of the included infants were informed about the possible effects of UDCA and the course of PNAC. Written informed consents were obtained from parents.
Randomization and masking
All preterm infants in the study were assigned to the smallest vacancy number from 1 to 70 when they entered the NICU. This number would not be released until a preterm infant was discharged. During the experiment, we took the odd-numbered newborns as the control group and the even-numbered ones as the UDCA group. The intervention started at the first week after birth when most preterm infants had started microfeeding.
Table 1 The design of study participants is cited
Inclusion criteria(a) admission to the hospital within 24 h after birth(b) gestational age: < 34 wk(c) birth weight: < 1800 g(d) total parenteral nutrition (TPN) treatment from the first day of life(e) TPN last for more than 2 wk Exclusion criteria(a) major congenital abnormality, chromosomal abnormality, and genetic metabolic disease(b) congenital intrauterine infection(c) structural liver abnormality(d) surgical treatment during hospitalization(e) severe symptoms of digestive system disease before TPN(f) cholestasis at the time of admission to the hospital(g) incompletion or withdrawal of treatment during hospitalization
Feeding regimen
The dosage and usage of PN solution referred to the 2014 European guidelines (Table 2) [ 21]. The percentage of the medium–long-chain fat emulsion in the PN solution is 20%,including 6% soy oil, 6% medium chain triglycerides, 5%olive oil, and 3% fish oil. The percentage of amino acids essential for preterm infants is 6%. PN also involves vitamins, glucose, electrolytes, and trace elements. If there were no unsuitable conditions for enteral nutrition, preterm infants would be fed with 1–3 mL breast milk or infant formulas per meal within 12–24 hours after birth, q3 hours–q6 hours, which was defined as microfeeding. The forms of enteral nutrition were random. If the mother of an infant had no contraindications to breastfeeding, breast milk was given to the infant; otherwise, an infant formula was given.
Table 2 Feeding regimen of preterm infants
Variables Initial dose Increase rate Maximum dose Application Amino acids (g/kg/d) 0.5–2.0 1.0 3.5–4.0 24 h after birth Fat emulsion (g/kg/d) 0.5–1.0 0.5–1.0 3.0 24 h after birth Glucose (mg/kg/min) 4.0–6.0 1.0–2.0 11.0–14.0 Soon Microfeeding (mL/kg/d) 1.0–3.0 10–20 150–180 12 to 24 h after birth
Treatment specifications
Premature infants assigned to the prophylaxis group were given UDCA from the 7th day after birth when enteral nutrition had been used and cholestasis had not yet occurred.Prophylaxis consisted of commercially available UDCA capsules (Ursofalk, 250 mg/capsule), starting with oral administration of pharmacologic dose of 20–25 mg/kg/day, twice daily [ 22– 24]. UDCA was suspended in water and shaken well, as is necessary, before being given to premature infants via nasogastric tube or infants' feeding bottle. UDCA was discontinued if cholestasis did not occur during the hospitalization, UDCA was discontinued after 1 week from stopping parenteral nutrition; If cholestasis occurred, UDCA would not discontinue until the cholestasis was cured. Likewise, the same dose of UDCA was taken by the infants in the control group if they developed cholestasis. The control group had not been treated with any kind of placebo.
Biochemical examinations and relevant diagnostic criteria
The primary endpoints were the average values of indicators in the two groups: the peak value of total bilirubin (TB), the peak value of direct bilirubin (DB), ALT, γ-glutamine transferase (γ-GT), alkaline phosphatase (ALP), total cholesterol,and total bile acids. They reflected the severity of reactive cholestasis (direct bilirubin > 34 μmol/L after 14 days of PN when other liver disorders were excluded [ 15, 25]). Secondary endpoints included: (1) ALT, AST, the peak value of TB,and the peak value of DB. They reflected the severity of liver injury in the two groups through comparative analysis the changes of these indicators over time; (2) Neonatal cholestasis and its related complications: late-onset sepsis (defined by positive blood culture and treatment with antibiotics for ≥ 15 days) [ 26], necrotizing enterocolitis (NEC) ≥ stage 2, according to the Bell’s criteria [ 27], feeding intolerance(FI), and severe intraventricular hemorrhage (IVH) ≥ grade 3 [ 28]. The incidence of them was compared between the two groups, and (3) Length of stay and other clinical outcomes: weight gain, length of stay, number of days required to achieve complete enteral feeding, stool color, and so on.The basic demographic information also was collected,including gestational age, birth weight, sex, birth asphyxia,and breastfeeding.
Statistical analysis
Statistical analysis in the two groups was performed with the SPSS 23.0 statistical package program for Mac. All continuous data were checked byt
-test or non-parametric rank-sum test, as appropriate. The Chi-squared (χ 2 ) test was used for the analysis of enumeration data of two groups. AP
value < 0.05 was considered as a statistical difference.The continuous variables of birth weight, duration of feeding, duration of hospitalization, and daily weight gain met normal distribution, so thet
-test was used to compare the means of two sets of data expressed as the mean ± standard deviation. The other continuous baseline characteristics were compared by non-parametric rank-sum test and expressed as median (interquartile range). The Chi-squared(χ 2 ) test was used for the comparison of sex data of two groups.Differences in biochemical examinations were also assessed byt
-test or non-parametric rank-sum test, as appropriate, and differences of incidence of neonatal cholestasis and its associated complication were examined using χ 2 test.The sample size was calculated based on a preliminary experiment. UDCA prophylaxis would significantly improve DB compared to no prophylaxis (average improvement in DB 0.352 mg/dL; 6 μmol/L) with a standard deviation of 0.235 mg/dL (4 μmol/L). When N of each group was 38, the power was 80%, and the confidence was 95%.
Results
Baseline characteristics of the preterm infants
A total of 124 preterm infants were screened in trial, and 102 of them were eligible and were randomized in the study(Fig. 1). The infants were randomly assigned to two groups with 42 in the prevention group and 60 in the control group.There were 47 males and 55 females, with an average gestational age of 31.03 ± 2.36 weeks and an average birth weight of 1391.3 ± 327.7 g. All infants in the prevention group started taking UDCA one week after birth. During NICU stay, no infant got cholestasis in 42 patients in the prevention group,while 7 of 60 patients in the control group got cholestasis.The baseline information of the study patients, including gestational age, birth weight, sex, birth asphyxia, and breastfeeding, was summarized in Table 3. There were no apparent differences in gestational age, birth weight, sex,birth asphyxia, and breastfeeding (P
> 0.05). In addition, no cholestasis was found in either group before intervention.No side effects, such as diarrhea and vomiting, were found in this study.Comparison of the severity of neonatal cholestasis in the preterm infants with or without UDCA prevention
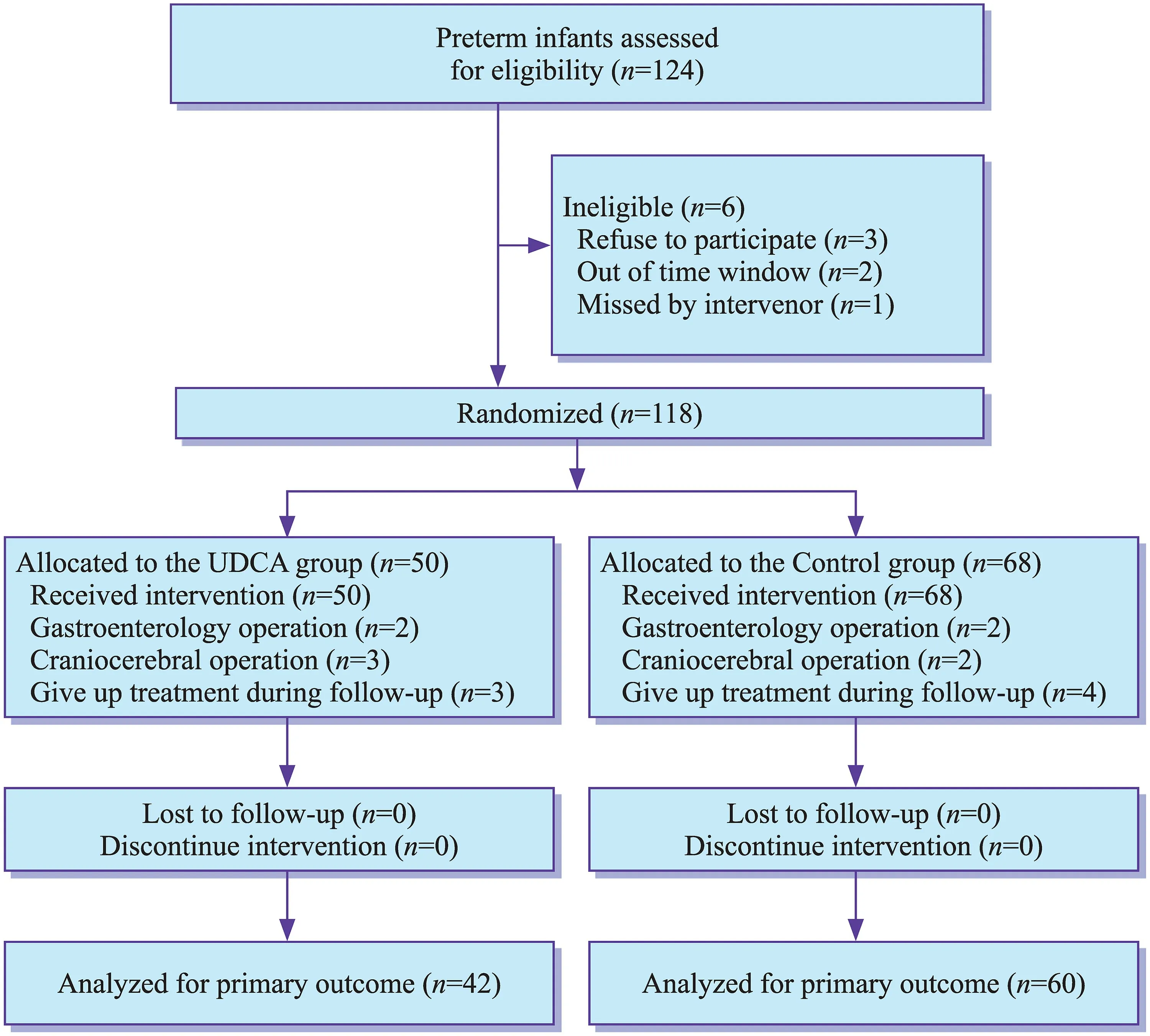
Fig. 1 Flow diagram of patients(preterm infants in Tongji Hospital from January 2021 to July 2021) assigned to the ursodeoxycholic acid (UDCA)and control groups. Cholestasis was defined as direct bilirubin > 34 μmol/L after 14 days of parenteral nutrition when other liver disorders were excluded[ 15, 25]
Table 3 Baseline characteristics analysis in the study for the ursodeoxycholic acid (UDCA) and control groups
Baseline characteristics UDCA group( n = 42)Control group( n = 60)P Gestational age (wks), median (interquartile range) 30.22 (29.40–32.79) 30.86 (29.33–32.72) 0.981 Birth body weight (g), mean ± SD 2155 ± 284 2237 ± 256 0.138 Male, n (%) 21 (50.0) 34 (56.7) 0.506 Birth asphyxia, n (%) 13 (31.0) 15 (25.0) 0.507 Breastfeeding, n (%) 15 (35.7) 23 (38.3) 0.788
The serum biochemical results of the two groups are shown in Table 4. Differences in serum TB level and DB level between study groups did not reach significance before intervention. However, after intervention, the peak of serum TB (101.1 ± 34 vs. 116.5 ± 28.7 μmol/L,P
< 0.05) and DB(13.0 (12–16) vs. 15.2 (12.5–19.6) μmol/L,P
< 0.05) were each distinctly different between prevention and control groups. Moreover, the increased rate of DB level in patients treated with UDCA prophylaxis decreased after intervention (P
< 0.05, Fig. 2 a). The average of peak direct bilirubin in Table 4 did not match the average of direct bilirubin in Fig. 2 a in the course of the disease because the peak direct bilirubin for each premature infant appeared at different times. The γ-GT activity level of the control group continued to increase since the third week. It was markedly higher than that of the prevention group at the 5th and 6th weeks(P
< 0.05) in Fig. 2 b. We also studied the effect of UDCA prophylaxis on the severity of neonatal cholestasis. The effect was evaluated based on a series of biochemical tests(Table 4). The severity of neonatal cholestasis in the UDCA group was significantly lower than that of the control group.It was manifested by the apparent lower value for the peak level of TB (P
< 0.05), the peak level of DB (P
< 0.05), γ-GT level (42.1 ± 19.1 vs. 127 ± 60.9 U/L,P
< 0.05), and the total cholesterol level (1.88 (1.65–2.04) vs. 2.51 (1.99–2.91)mmol/L,P
< 0.05) in the UDCA vs. control groups. The differences between study groups did not reach significance(P
> 0.05) in the levels of ALT, ALP, and total bile acids.The comparison of the three indicators in two groups were ALT [6 (5–8) vs. 6 (5–8) U/L], ALP [361 (274–416) vs. 343(249.5–372.5) U/L], and total bile acids [73.3 (23.0–88.3)vs. 24.9 (12.5–97.6) μmol/L], respectively.
Table 4 Biochemical examinations analysis in the study with or without ursodeoxycholic acid (UDCA) prevention
† Significant values. The direct bilirubin and total bilirubin were analyzed with the mean value of the peak value of the preterm infants
Biochemical examinations UDCA group Control group P(n = 42)(n = 60)Peak total bilirubin (μmol/L) 102.7 ± 34 116.1 ± 29.1 0.036 †Peak direct bilirubin (μmol/L) 13.0 (12–16) 15.2 (12.5–19.6) 0.022 †ALT (U/L) 6 (5–8) 6 (5–8) 0.565 Total bile acids (μmol/L) 73.3 (23.0–88.3) 24.9 (12.5–97.6) 0.370 γ-Glutamine transferase (U/L) 42.1 ± 19.1 127 ± 60.9 < 0.001 †Alkaline phosphatase (U/L) 361 (274–416) 343 (249.5–372.5) 0.113 Total cholesterol (mmol/L) 1.88 (1.65–2.04) 2.51 (1.99–2.91) 0.020 †ALT (U/L)Baseline 5 (5–6) 5 (5–7.75) 0.056 4th wk 6 (5–8) 6 (5–8) 0.565 6th wk 8 (5.8–10) 7.5 (6.3–10.5) 0.713 AST (U/L)Baseline 18 (15–23) 19 (16.3–23.8) 0.271 4th wk 20 (18–31) 20 (16.5–24) 0.608 6th wk 19 (17.5–23) 20 (17–24) 0.448 Peak total bilirubin (μmol/L)Baseline 105.9 ± 34.7 101.7 ± 31.8 0.595 4th wk 102.7 ± 33.9 116.1 ± 29.2 0.038 †6th wk 101.1 ± 34 116.5 ± 28.7 0.036 †Peak direct bilirubin (μmol/L)Baseline 6.49 ± 2.48 6.25 ± 2.30 0.628 4th wk 13.3 ± 3.6 23.5 ± 22.6 0.044 †6th wk 13.0 (12–16) 15.2 (12.5–19.6) 0.022 †
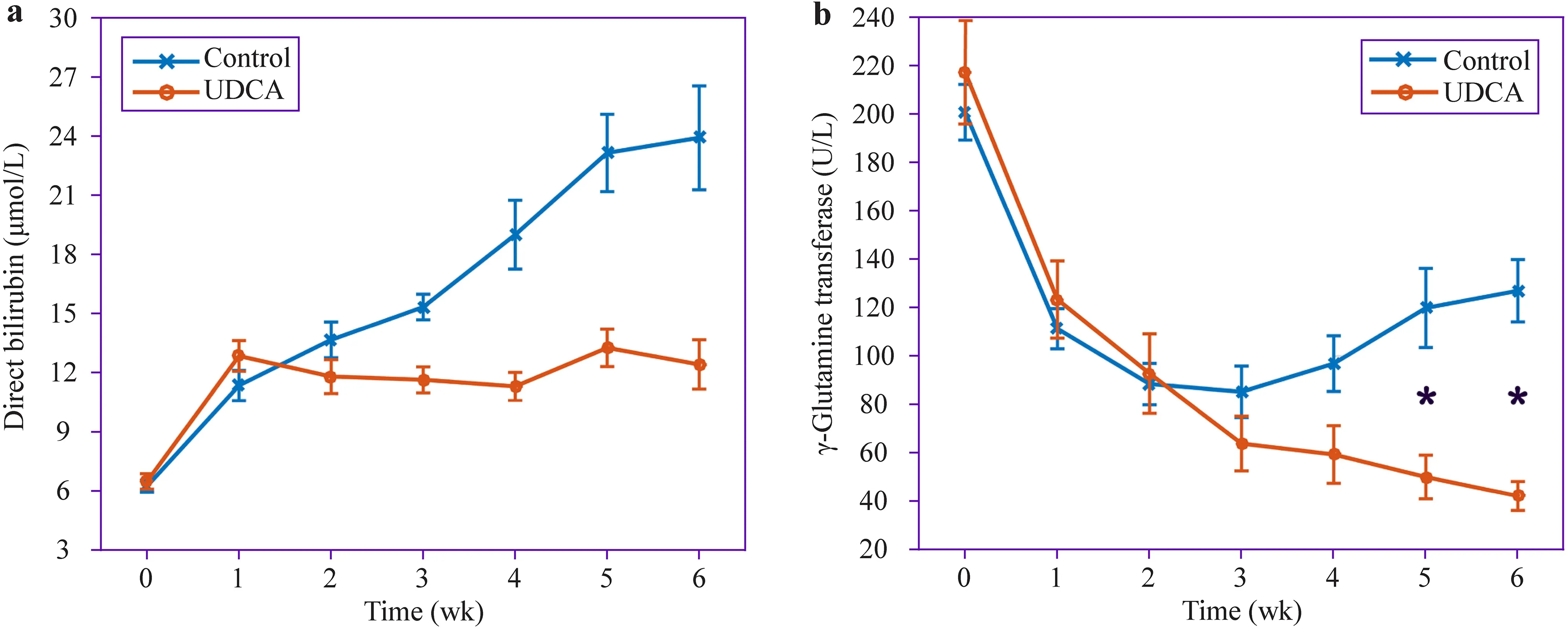
Fig. 2 a The direct bilirubin variation of the preterm infants in the course of the disease. b The γ-Glutamine transferase variation of the preterm infants in the course of the disease. * P < 0.05
Comparison of the liver function in the preterm infants with or without UDCA prevention
Cholestasis can cause corresponding liver injury. We also compared liver function between the UDCA prevention group and the non-prevention group in Table 4. The differences between the UDCA and control groups did not reach significance in liver enzyme elevations based on laboratory liver function tests.
Comparison of the clinical outcomes and the proportion of neonatal cholestasis and its associated complications in the preterm infants with or without UDCA prevention
Because the duration of PN exposure, the duration to complete enteral nutrition, duration of starting microfeeding, and the passage time of meconium were related to the development of PNAC, we compared the four clinical characteristics in two groups in Table 5. No statistical differences were found between the two groups. To demonstrate the benefits of PNAC prognosis, we compared the length of hospital stay and average daily weight gain of the two groups. The length of hospital stay in the prevention group was shorter than that of the control group (34 ± 16 vs. 40 ± 21 days, respectively)(Table 5).
To determine the potential preventive effects of UDCA on neonatal PNAC complications, we compared the incidence of neonatal cholestasis and its related complications between the two groups. Compared with the control group,the proportion of neonatal cholestasis in the UDCA group was significantly lower (0.0% vs. 11.7%,P
< 0.05, Table 5).Admittedly, a Kaplan–Meier graph would presumably show upward trend of the control group; however, there is no cholestasis in the UDCA group. Thus, the obvious difference between the two groups could be obtained directly. Additional analysis of neonatal cholestasis-related complications showed that the proportion of cases with FI (14.3% vs.41.7%,P
< 0.05) in the UDCA group was reduced markedly.Discussion
We described the randomized clinical trial on UDCA prophylaxis in preterm infants admitted to the NICU at risk for PNAC. The DB and TB levels in the patients who received early UDCA prophylaxis were markedly decreased. The incidence of cholestasis in preterm neonates with early UDCA supplementation was significantly lower.
The immature development of organ systems of premature infants makes them more prone to cholestasis. PNAC is the most common complication of long-term intravenous nutrition [ 13]. A portion of liver dysfunction might be reversible if PN is reduced or stopped [ 29]. However,suspending TPN can lead to stopping gaining weight or even to weight loss in preterm infants, which results in an increase in the duration of hospitalization [ 18]. Furthermore, cholestasis has a long-lasting effect on prospective weight gain in infants. Cholestasis can increase the incidence of some comorbidities (such as, NEC and septicemia) [ 30]. All of these will bring an enormous challenge to the treatment of premature infants.
UDCA is a natural bile acid that has been proposed as a treatment option for cholestasis related to parenteral nutrition in adults, children, and premature infants [ 15, 22].Heubi et al. [ 31] reported that the tauroursodeoxycholic acid (TUDCA) was ineffective against PNAC in preterm infants receiving PN. However, in recent studies, UDCA effectively treated PNAC in children with low birth weight,short bowel syndrome (SBS), and intestinal failure (IF)[ 22, 24, 32]. Nonetheless, the effects of UDCA prophylaxis supplementation on neonatal cholestasis in preterm infants have not been studied systematically. A larger sample of 102 preterm infants than other published papers showed that UDCA prophylaxis is beneficial in preventing PNAC in NICU infants receiving prolonged PN.
Table 5 Clinical outcomes analysis in the study with or without ursodeoxycholic acid (UDCA) prevention
necrotizing enterocolitis of newborn; feeding intolerance; intraventricular hemorrhage. † Significant values
Clinical characteristics UDCA group( n = 42)Control gruop( n = 60)P Duration of PN (d), median (interquartile range) 25 (15–32.5) 24 (16–41) 0.716 Duration of meconium passage (d), median (interquartile range) 6.5 (4–9) 7 (4–10) 0.231 Duration of starting microfeeding (h), median (interquartile range) 66.25 (40.75–82.5) 48 (28.5–88) 0.235 Duration of hospitalization (d), mean ± SD 34 ± 16 40 ± 21 0.170 Daily weight gain (g), mean ± SD 21.613 ± 6.4 21.607 ± 6.0 0.996 Neonatal cholestasis, n (%) 0 (0.0) 7 (11.7) 0.022 †Hospital acquired sepsis, n (%) 3 (7.1) 4 (6.7) 0.925 NEC, n (%) 1 (2.4) 2 (3.3) 0.779 FI, n (%) 6 (14.3) 25 (41.7) 0.003 †IVH, n (%) 12 (28.6) 18 (30.0) 0.876
The action of UDCA on PNAC probably depends on a relative substitution of the hydrophilic, cytoprotective, and non-toxic UDCA for lipophilic, detergent-like, and toxic bile acids, on an improvement in the secretory capacity of the hepatocytes, and on an improvement in the processes of immune-regulation [ 15, 19]. UDCA was proved to be safe and well tolerated in preterm infants with a dosage of 5–30 mg/kg/day, even up to 50 mg/kg/day [ 15, 19, 20].Hence, a UDCA dose at 20–25 mg/kg/day in this study was safe. This higher dose was more effective than the standard dose [ 33]. It could be explained by the increased reaction to UDCA in this study relative to other studies. As for side effects of treatment with UDCA, the most common one is loose stools or diarrhea, occurring in 1%–10%. If diarrhea occurs, the dose of UDCA would need to be reduced or stopped while and giving support treatment in the meantime.The infants in the two groups completed the trial without any laboratory and clinical assessment abnormalities.
In this randomized controlled trial, the incidence of cholestasis and its associated FI in preterm neonates with early UDCA supplementation was significantly lower. The lower incidence of FI might combine with UDCA to increase the secretion levels of liver cells. Furthermore, comparing the rate of change in the biochemical data between both groups, the DB level markedly decreased in the patients who received early UDCA therapy making DB level a leading detection indicator of cholestasis [ 26, 34].
Because the γ-GT activity is regarded as the earliest sensitive parameter of cholestasis, it has become a widely used marker in detecting PNAC and in assessing the efficacy of UDCA treatment [ 15 ]. One of the findings in our study was the observation of a constant reduction in γ-GT activity level over time in the UDCA group, which was consistent with former studies. We also found other beneficial effects of UDCA supplementation in preterm infants. In the control group, the serum level of total cholesterol was significantly higher than that of the prevention group, which was more common in diseases, such as biliary obstruction [ 35].
However, other biochemical outcomes of liver function,such as ALT, AST, and ALP, did not respond to UDCA.Liver enzymes were released into the bloodstream from damaged cells when factors damaged the membrane of the liver cell. Thus, blood concentrations of liver enzymes had been used as indicators of hepatocyte injury [ 15]. However,transaminase did not obviously change associated with the histopathologic development of PNAC. Maybe the statistical differences of transaminase and ALP would spring up if further analysis of biochemical outcomes of liver function was obtained. The tauroursodeoxycholic acid and glycoursodeoxycholic acid in a bile acids profile are produced and bile acids enterohepatic circulation is accelerated when UDCA was taken orally [ 36]. Therefore, the level of total bile acids in the UDCA group was higher than that in the control group.
Finally, this study also explored the effect of UDCA prophylaxis on the adverse outcomes of PNAC. The differences did not reach significance between study groups in these clinical outcomes: time to complete enteral nutrition,meconium passage time, average daily weight gain, hospitalization days, and the incidence of septicemia, NEC, and IVH. If further investigation in a large sample was carried out, the clinical outcomes between the two groups would show apparent differences.
Consequently, the present study had the following findings: (1) UDCA prevention markedly reduced the risk for neonatal cholestasis and its related complications in preterm infants (Table 5); (2) UDCA prevention was prominently associated with less severity of neonatal cholestasis and better improvement of some of the liver function indicators in the preterm infants (Table 4), and (3) UDCA prevention had some improvements on the duration of hospitalization, the duration of meconium passage, and other outcomes in the preterm infants just from the data (Table 5), although these differences were not significant. In general, these findings suggested that UDCA prevention had apparent beneficial impacts on the management of cholestasis in preterm infants.
Nonetheless, the study had some limitations. Firstly, additional investigations involving multicenter and a larger number of infants should be carried out to observe the underlying problems. Secondly, preterm babies should be divided into different groups for comparison and observation according to the international weight classification.
The gastrointestinal tract of a premature infant is inherently fragile and immature. We do hope that UDCA intravenous formulation becomes available so that it can be used earlier in premature infants. An intravenous formulation of UDCA may provide a more convenient way to prevent neonatal cholestasis and may be introduced into UDCA therapy immediately. FGF19 is a hormone secreted by the gut in response to bile acids stimulation of the FXR gene in the ileum. FGF19 plays an important role in regulating bile acids homeostasis. The measurement of FGF19 would provide important information about bile flow into the gut and the ability to activate gut FXR in preterm infants.
In conclusion, this randomized, unmasked, controlled trial illustrated that early prevention with UDCA appeared to be very successful in preventing PNAC and in reducing the severity of PNAC. UDCA also protected liver function and reduced the occurrence of other clinical complications. Moreover, UDCA improved the prognosis of premature infants. We hope that additional investigations involving multicenter, classification, and a large sample are carried out, which will show more accurate results and be more promising to confirm the efficacy of UDCA in the prevention of cholestasis.
Author contributions
SL: conceptualization, data curation, writing–original draft, writing–review and editing, and software. WL: conceptualization, writing–review and editing, and supervision. LC and JW: investigation and project administration. LC and MX: software and supervision and validation. All authors have drafted the article or revised it critically for important intellectual content. All authors have read and agreed to the published version of the manuscript.Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.Data availability
The datasets generated and analyzed during this study are available from the corresponding author on reasonable request.Declarations
Ethical approval
All procedures performed in studies involving human participants were conducted according to the guidelines of the 1994 Declaration of Helsinki, and approved by the Ethics Committee of Tongji Hospital (TJ-IRB20210828).Conflict of interest
No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.Informed consent
Informed consents have been obtained from all individual participant’s parent or legal guardian included in the study. World Journal of Pediatrics2022年2期
World Journal of Pediatrics2022年2期
- World Journal of Pediatrics的其它文章
- Duplex kidneys and the risk of urinary tract infection in children
- Surgical management in pediatric neuroblastoma diagnosis and treatment: a 20-year, single-center experience
- Clinical practice guidelines in multisystem inflammatory syndrome(MIS-C) related to COVID-19: a critical review and recommendations
- A review of the risk factors associated with juvenile-onset recurrent respiratory papillomatosis: genetic, immune and clinical aspects
- Tribute to reviewers (January 1, 2021 to December 31, 2021)
- Epidemiology and region-specific risk factors for low Apgar scores in China: a nationwide study
