Characterizing the patient experience during neoadjuvant therapy for pancreatic ductal adenocarcinoma:A qualitative study
INTRODUCTION
The delivery of chemotherapy and/or radiation therapy prior to surgery,known as neoadjuvant therapy(NT),is increasingly utilized for patients with pancreatic ductal adenocarcinoma(PDAC)[1,2].Since a significant proportion of patients are unable to receive all intended adjuvant therapies following major pancreatectomy,NT ensures the receipt of some systemic therapy and leads to improved rates of multimodality therapy.NT also improves margin-negative resection rates,enhances patient selection by ensuring the absence of rapid tumor progression prior to surgery,enables an
test of the efficacy of chemotherapy,and based on emerging evidence from randomized controlled trials,may lead to improved overall survival[3-7].Based on these advantages,NT is now the recommended approach for borderline resectable(BR)and locally advanced(LA)cancers and an increasingly utilized option for potentially resectable(PR)disease according to national guidelines[3,8,9].
Despite its increasing use in PDAC and other cancer types[2],little is known about the patient experience during NT.Indeed,the neoadjuvant time period might be particularly distressing for patients who must cope with not only the toxicity of treatment,but also side effects from the tumor itself which remains
.Furthermore,little is known about the psychosocial impact of NT particularly given many patients’ inherent preference for “just getting the cancer out,” as well as the uncertainty of future surgery[10].A recent systematic review found scarce data on quality of life(QOL)during NT for PDAC and no existing literature on other aspects of the patient experience[11].In contrast to immediate surgery,NT is also inherently multi-disciplinary in nature.As such,there may be barriers to effective care initiation and coordination that impede the completion of all scheduled therapy and the receipt of surgical resection[12].
Mrs. Katrinka was not the type of ordinary mother who lets a 60 foot tall crane sit around in her back yard. No, sirree. Not that type of ordinary mother at all.
This gave them the alarm; they drew their sabres, and went to the door, which opened on their Captain s saying: Open, Sesame! Cassim, who had heard the trampling15 of their horses feet, resolved to sell his life dearly, so when the door opened he leaped out and threw the Captain down
While not previously studied in PDAC,in practice,multiple barriers to the use of NT are often expressed by patients.For example,some patients may have financial concerns secondary to missing work by “delaying” surgery.Others worry about arranging and/or affording transportation for NT due to long travel distances.Additionally,in our study,most,if not all patients,experienced physical and emotional symptoms during NT.Furthermore,the development of toxicities during the course of NT may prove to be a potential barrier that may worsen a patient’s ability to subsequently undergo an operation.A meta-analysis of 38 studies of which 1738 patients received NT found approximately 64% of patients experienced at least grade III toxicity[35].In fact,this number may be magnified at community hospitals which may not have the same resources as tertiary referral centers to manage toxicities and progress patients through therapy[36-38].
MATERIALS AND METHODS
Study design and population
Patients with PDAC undergoing NT prior to planned surgery in the future were recruited to participate in this qualitative study.All treatment decisions at our institution are made at a pancreatic cancer specialty specific multidisciplinary clinic and made on an individualized basis.Participants were identified by prospectively screening ambulatory clinics at The Ohio State University Wexner Medical Center and James Comprehensive Cancer Center.Inclusion criteria included receiving at least two cycles of chemotherapy in a neoadjuvant intent,still eligible for surgical resection,and English language speaking,without restrictions on age,race,or disability.Eligible patients were contacted by phone,informed consent was obtained,and an interview was scheduled at the participant’s convenience.Due to the COVID-19 pandemic,interviews were conducted by phone between August 2020 and October 2021.
Interview guide and process
The interview script was developed using evidence synthesis,stakeholder engagement,and expert opinion.The content of the interviews focused on patient treatment preferences,perspectives on the decision-making process,and all aspects of the patient experience during NT;recommendations on opportunities to improve the delivery of NT were also sought.Questions were open-ended,prompting additional questions depending on the responses of the interviewees(Supplementary material).This type of interview method was selected due to the descriptive nature of the research.Semi-structured interviews allow researchers to discuss topics of interest more in detail by elaborating on emerging themes and asking probing questions.A nominal gift card was given to participants for their participation.This study was approved by the Institutional Review Board of The Ohio State University(IRB# 2019C0155).
Statistical analysis
There are several limitations to our study.Although our study reached theme saturation,the relatively small sample size and single institution design means that the findings may not be generalizable to all patients with PDAC who are receiving NT.Additionally,our study includes patients with PR,BR and LA disease where larger sample sizes are required to investigate if the patient experience differs according to anatomic stage(
,patients with LA disease may have lower expectations of undergoing resection and/or greater burden of cancer-related symptoms than patients with PR disease.)Finally,it is unclear if the patient experience is temporal-dependent and since interviews were performed at a single time during NT,future research may focus on longitudinal evaluations of the patient experience.
RESULTS
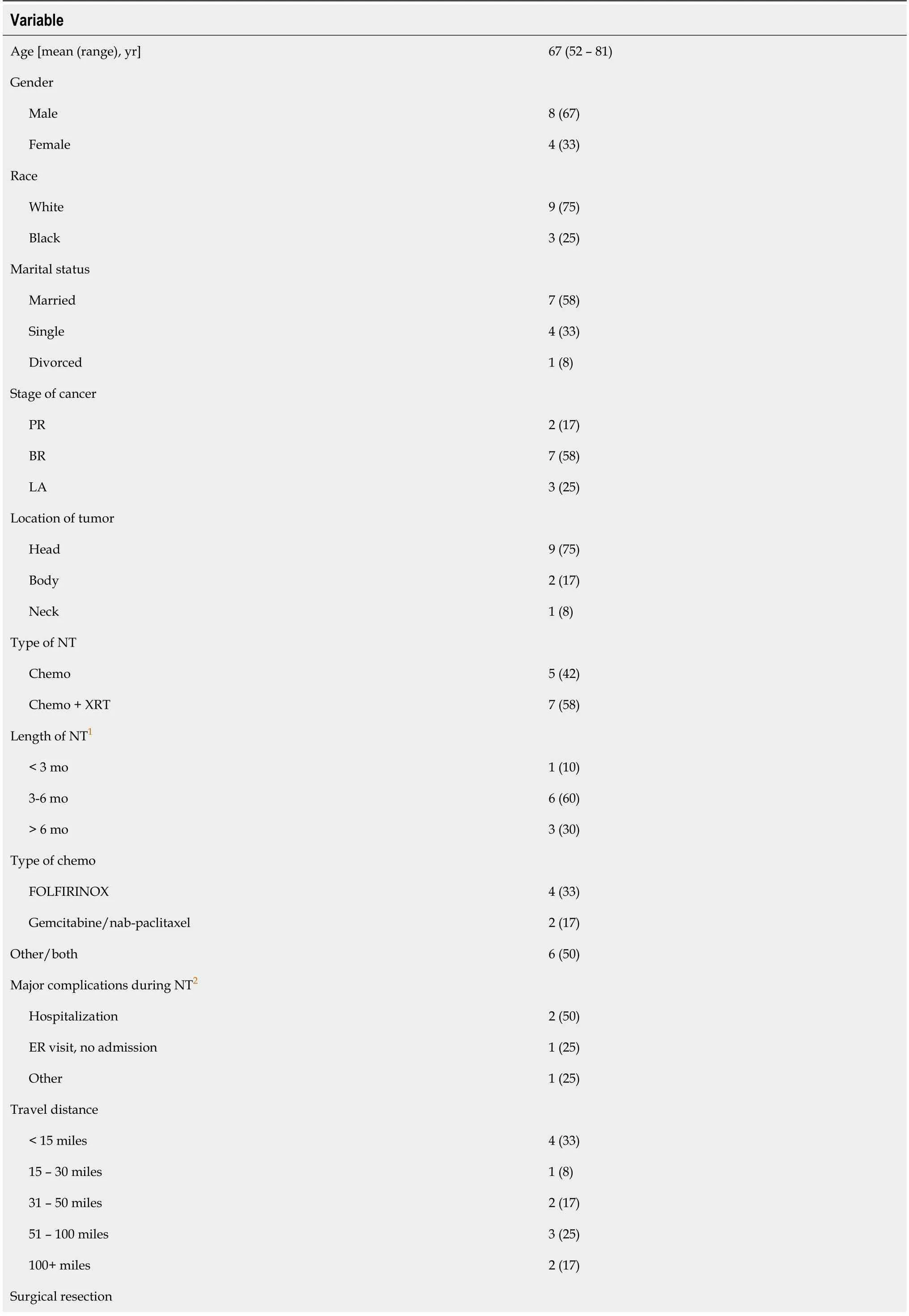
Participant characteristics
A total of 12 patients participated in the interviews.On average,patients were 67 years old,ranging from 52 to 81 years.Patients with BR(
= 7,58%),PR(
= 2,17%),and LA(
= 3,25%)cancers participated in the study.A majority of patients(
= 7,58%)received chemotherapy and radiation therapy before their planned surgery while others(
= 5,42%)received just chemotherapy.At most recent follow-up,most patients(
= 10,83%)had completed NT with 8 patients(67%)undergoing surgical resection of their tumor.Complete participant characteristics are reported in Table 1.
The study was reviewed and approved by the Institutional Review Board of The Ohio State University.

Patient perspectives on neoadjuvant therapy
Among the 12 patients who participated in the interviews,the vast majority(
= 11,92%)were not familiar with the concept of NT at the time of initial consultation.All subjects reported that NT was the doctor’s recommendation and most(
= 8,67%)explained that NT was presented to them as the only option.All interviewees indicated that improving resectability was the main rationale for choosing NT.While some(
= 6,50%)patients indicated that before meeting with their physicians they did not have a preference for a specific treatment plan,others(
= 4,33%)expressed that they had hoped to avoid chemotherapy and undergo upfront surgery.All patients indicated that their main source of information were members of their health care team while other sources of information discussed included the internet(
= 4,33%),family and friends(
= 3,25%),and educational materials(
= 1,8%).While most patients(
= 9,75%)discussed their prognosis in a hopeful manner,some(
= 5,42%)acknowledged the poor prognosis generally associated with PDAC and others(
= 3,25%)expressed uncertainty surrounding the prognosis.
Patient experience during neoadjuvant therapy
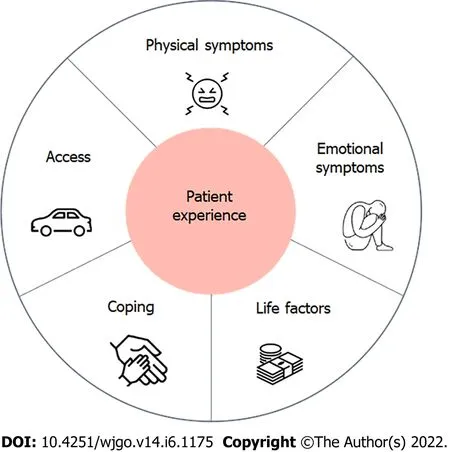
Five subthemes of patient experiences during NT emerged: physical symptoms,emotional symptoms,coping mechanisms,access to care,and life factors(Figure 1 and Table 2).All participants reported elements of each of the five subthemes.

A few patients(
= 3,25%)discussed that they did not experience any major side effects and they were tolerating their therapy well(“I have never had any symptoms.No throwing up,no nothing.”).However,most patients reported experiencing major side effects from their treatment.Many patients reported feeling weak(
= 6,50%).One patient stated: “I’ll say at night,I am going to do this,that and the other and the next day comes and my body says ‘no,we’re not going to do that’.” Others mentioned challenges around weight loss,loss of appetite,and the taste of food(
= 5,42%),as well as a general feeling of sickness(
= 4,33%)(“After getting chemo for the next 5 days I’m sick as a dog.”).
In addition to shock experienced during their diagnosis,patients reported varying rates of fear and depression(“… it scared me.It depressed me.”).A few patients(
= 3,25%)shared concerns for their family and friends’ well-being,regarding uncertainty about next steps in treatment,and about their overall prognosis.One patient stated: “I love my wife and I want to be around for her.It’s hard.” Some(
= 3,25%)also shared not wanting to think about and dwell too much on their diagnosis and treatment approach,as well as the need for not too much information,as it leads to unnecessary anxiety.
The main coping and support mechanism cited by most patients(
= 10,83%)was support from family members.Tangible aspects of support included family members and friends offering rides to appointments,discussing different treatment options,helping with coordinating care and reaching out to the medical team,as well as helping with chores around the house.Patients putting their trust in their religious faith was another coping and support mechanism mentioned by some(
= 5,42%).One patient stated: “I’m a religious person,so that’s enough said.” Several patients(
= 4,33%)also mentioned receiving support from different members of the medical team(“So,there is always someone here to answer my questions,which also feels good and gives you comfort.”).
For most,access and coordination was an important but feasible aspect of NT.This included minimal obstacles associated with traveling to medical appointments(
= 8,67%),scheduling appointments(
= 6,50%),contacting doctors(
= 6,50%),getting answers to questions(
= 4,33%),getting insurance to cover treatments(
= 3,25%),or seeing a doctors and getting referrals(
= 2,17%).While in general patients did not experience major complications accessing and coordinating care,a minority of patients reported some barriers.A few(
= 3,25%)highlighted that traveling to appointments was burdensome.One patient explained: “Every time we have to have something done,it’s two hours out of our day,about 2.5 h out of our day just driving to the place.But we made that choice knowing that was the case for the care,the treatment and we’ve been proceeding.”
Finally,all patients described the need to integrate their treatment and condition with their normal life circumstances.Several patients discussed the impact of NT on other aspects of their life.NT impacting a patient’s work and financial situation were the most commonly cited sub-themes.Many patients discussed that missing work was not a major challenge they were faced with(
= 5,42%),and that they did not experience major financial concerns(
= 6,50%).Yet,some(
= 3,25%)expressed concern around not being able to work and the burden it placed on them financially(
= 4,33%).One patient stated: “I’ve been off since all this happened(…)drives me kinda nuts.I used to work all the time.But I got no energy now.” Another patient explained: “I had to withdraw money to get me through this that was an extra expense,as I am a senior.And I’m on a fixed income.So,I hadn’t counted on that.” Other life aspects mentioned were patients having to deal with other health problems(
= 1,8%)at the same time they are on NT and needing help with daily activities(
= 2,17%).
Recommendations for improving the experience of neoadjuvant therapy
The most commonly cited recommendation for improving the experience of NT was to provide better education and more information on NT(
= 7,58%).Patients highlighted the need for more information on: the rationale behind choosing NT prior to surgery,the anticipated surgery and its likelihood of occurring after NT,as well as general information prior to starting NT treatment.Patients also discussed that more discussions with physicians could potentially be helpful,but also,highlighted the need for information tailoring(not too much
not too little).The need for seeing different members of the healthcare team,including ancillary services was also frequently cited as a recommendation for improving the experience of NT(
= 5,42%).Patients discussed the importance of seeing psychologists,palliative care doctors,case workers,physical therapists,and nutritionists.Better coordination and communication(
= 2,17%)and better treatments(
= 2,17%)were also offered as potential recommendations.
DISCUSSION
Pancreatic ductal adenocarcinoma is a highly aggressive malignancy,often thought of as a systemic disease at the time of diagnosis,that requires multimodal therapy with a combination of surgery and chemotherapy in order to achieve meaningful long-term survival[14-17].NT is being increasingly utilized in patients with localized PDAC[1,3,18].Previous research on NT for PDAC has focused on its safety,efficacy,and cost-effectiveness with little data on patient-centered preferences or experiences during NT.In this qualitative study of patients actively receiving NT for PDAC,we found several important observations.First,patients are generally unfamiliar with the concept of NT prior to meeting with an oncologist.While many have an inherent preference for upfront surgery,most understand their providers’ recommendation for NT as an attempt to improve resectability(or likelihood of achieving margin-negative resection).Second,patients have unique experiences and care needs during NT that providers should be aware of in order to optimize patient-centered outcomes.A patient-centered approach that supports physical and emotional symptoms and recognizes the importance of life integration is required.Third,specific recommendations for improving the experience of NT prior to surgery were identified.
“She is fat and pretty, and she has been fed with the kernels1 of nuts,” said the old robber-woman, who had a long beard and eyebrows2 that hung over her eyes. “She is as good as a little lamb; how nice she will taste!” and as she said this, she drew forth3 a shining knife, that glittered horribly. “Oh!” screamed the old woman the same moment; for her own daughter, who held her back, had bitten her in the ear. She was a wild and naughty girl, and the mother called her an ugly thing, and had not time to kill Gerda.
Interestingly,although patients in our study were actively receiving NT,most were relatively unfamiliar with the concept of NT.All patients expressed that NT was the recommendation of their doctor.While a few patients expressed their desire for a surgery-first approach or to avoid chemotherapy altogether,nevertheless,all patients eventually came to understand and agree with the rationale for NT.This may highlight a disconnect between patients and providers in that systemic chemotherapy is part of the treatment of all patients with pancreatic cancer as even patients with localized cancers who have undergone resection are likely to experience disease recurrence[19,20].Similar results were found in patients with breast cancer.A study in women with breast cancer who underwent NT found a majority of women understood that chemotherapy was given prior to surgery in order to shrink the tumor but did not grasp the concept that chemotherapy is utilized to treat systemic disease beyond simply local tumor control[21].
Missing from prior studies has been an evaluation of patient-centered preferences and outcomes regarding the use of NT for PDAC.Cancer-related treatment decisions are complex and require consideration of multiple factors;such decisions are often made in the context of shared decision making(SDM),a model in which informed and engaged patients make health-care decisions in conjunction with their providers[22].The degree to which patients are involved in the SDM process of choosing NT or immediate surgery is unclear.Most patients with cancer desire an active role in making decisions about their care[23]and such patient-centered decision making has been shown to improve patients’ understanding of their treatment options,satisfaction with their health care,and overall quality of life(QOL)[24-26].Previous research in breast and rectal cancer suggest patient-centered approaches to SDM regarding NT are lacking in clinical practice[27-29].Indeed,SDM is under-utilized by surgeons in general[30].Additionally,it is well known that strong emotions and fears may influence treatment decision making[31].Specifically,emotions may cause behavior or decisions to diverge from more rational or practical decision making consistent with one’s values[32].For example,patients state their desire to “just get the cancer out” even if this emotional response does not align with one’s values,priorities,or optimal treatment strategy.We found most patients believed NT was their only treatment option moving forward.This is not surprising as a majority of the patients in our study had either BR or LA disease which is currently the preferred treatment strategy based on recent randomized controlled trials[33,34].Another study has found that most patients believed there was no other treatment option and thus accepted NT[21].Understanding patient preferences,values,and expectations regarding NT will improve SDM which will lead to not only delivering patient-centered care but also the opportunity to overcome barriers to patient acceptance of NT.
46.Both reigned over the kingdom in peace and happiness: Thus they are married and live happily ever after in true fairy tale fashion. Note also that they cannot live happily ever after until the villain has been destroyed and removed from their lives.Return to place in story.
Therefore,the purpose of this qualitative study was to characterize the patient experience during NT for PDAC.Specifically,we sought to understand patient treatment preferences,information needs,the physical and psychosocial impact of treatment,and barriers to successful initiation and delivery of NT.A better understanding of all aspects of the patient experience during NT may identify opportunities to design interventions aimed at improving QOL during NT,facilitating completion of NT and receipt of surgery,and ultimately optimizing the long-term outcomes of patients with PDAC.
For customer service answering the telephone is very important, our ringing normally6 comes from the information desk, tills, customers and others. Mostly they ask for prices, advice or looking for some staff to use. At the beginning, if I was near the telephone I liked to pick up and answer it, after a few times I was afraid, because I couldn’t completely7 understood what’s said by the customer at the phone.
In conclusion,this is the first qualitative study to characterize the experience of patient’s receiving NT for localized PDAC.Our findings clarify the lack of familiarity with the concept of NT prior to initiating treatment,the unique care needs of patients receiving NT,and recommendations to improve the delivery of cancer care in the neoadjuvant setting.These data provide a framework to allow for a better understanding of the patient experience during NT and highlight opportunities for patient-centered interventions aimed at improving quality and quantity of life outcomes of those with PDAC.
The findings from our study provide a framework to allow for a better understanding of the patient experience during NT and highlight opportunities for inter-disciplinary interventions to improve patient-centered outcomes of those with PDAC.Indeed,many patients who receive NT fail to either complete NT or to undergo subsequent pancreatectomy with common reasons including disease progression or worsening performance status due to toxicity[33,39,40].Furthermore,since failing to complete therapy or undergo surgical resection is associated with a worse prognosis,having a patientcentered approach to understand potential barriers to completion is essential.As we have demonstrated in this study,patients experience both physical and emotional symptoms during treatment and require a team approach with the help of ancillary services to help complete therapy.Involvement of patient navigators,social workers,nutritionists,and physical therapists to address patient concerns and symptoms may aid to improve the high attrition rate in patients receiving NT.Previous research has highlighted patient dissatisfaction with the lack of access to counseling services,support groups,and educational tools[41].
All interviews occurred by phone,were audio recorded,and then manually transcribed verbatim by the researchers.Transcripts were then uploaded to NVivo12(QSR International,Australia)for data extraction,synthesis,and analysis purposes.Data extraction followed an integrated approach,including both an inductive and deductive coding methodology[13].The following preliminary codes were developed before a more in-depth,inductive coding process took place:
and
.Two researchers independently coded the transcripts for sub-themes in an iterative fashion until thematic saturation was achieved[13].Interviews were then re-reviewed and coded using the final codebook.When coding from both independent researchers was not concordant,these instances were reviewed with a third researcher at team meetings.These sections and codes were discussed until a consensus was reached.Demographic data from participants were summarized and illustrative quotes in each theme were selected.
Dad had tears in his eyes, too -- tears of joy. His face shone with the light of a thousand galaxies8 and I saw in his eyes the eyes of Santa Claus. The real Santa Claus. The one who spent time choosing special things I wanted for all the Christmases past since the time I had come to live on this planet. The Santa who ate my carefully decorated cookies and drank the warm milk. The Santa who probably ate the carrot I left for Rudolph. The Santa who -- despite his utter lack of mechanical skills -- put together bicycles, wagons9 and otehr miscellaneous items during the wee hours of Christmas mornings.
CONCLUSION
We found that patients with PDAC receiving NT must balance their cancer treatment with other aspects of their lives such as family responsibilities and work in addition to coping with the physical and emotional symptoms that accompany their new diagnosis and treatment(Figure 1).These findings are similar to a previous qualitative study of patients with breast cancer receiving NT.Beaver
[21]reported five themes among women receiving NT: Coping with the rapid transition from “well” to “ill”,information needs and decision making,needing support and empathy,impact on family,and creating a new “normal”.These findings suggest similar experiences among patients receiving chemotherapy prior to surgery regardless of cancer type.While patients with PDAC certainly have unique challenges such as biliary obstruction,malnutrition,gastric outlet obstruction,as well as cancer-related pain,additional research is needed on supporting the general care needs directly influenced by the neoadjuvant aspects of treatment.
ARTICLE HIGHLIGHTS
Research background
Neoadjuvant therapy(NT)has increasingly been utilized for patients with localized pancreatic ductal adenocarcinoma(PDAC).It is the recommended approach for borderline resectable(BR)and locally advanced(LA)and it has also increasingly been utilized for potentially resectable(PR)disease.However,little research has focused on patient-centered metrics among patients undergoing NT,including patient experiences,preferences,and recommendations.
What a torment57 her stout58 old attendants and servants sometime thought her when she insisted on staying awake, and making them chatter52 or do something, when they could hardly keep their eyes open! Sometimes, however, the princess would pretend to go to sleep, and then, after all her women had gladly followed her example, she would get up and go out by herself, her veil hanging loosely about her
Research motivation
A better understanding of all aspects of the patient experience during NT may help identify opportunities to design interventions aimed at improving quality of life.It may also facilitate the completion of NT and receipt of surgery,ultimately optimizing long-term outcomes.
A total of 12 patients with localized PDAC were interviewed.All patients indicated that choosing NT was the doctor’s recommendation and most reported not being familiar with the concept of NT(
= 11,92%).Five patient experience themes emerged: physical symptoms,emotional symptoms,coping mechanisms,access to care,and life factors.Improved education before and during NT was the most commonly cited recommendation for improving the experience during NT(
= 7,58%).Patients highlighted the need for more information on the rationale behind choosing NT prior to surgery,the anticipated surgery and its likelihood of surgery occurring after NT,as well as general information prior to starting NT treatment.
Research objectives
This research aims to understand the experience of patients initiating and receiving NT to identify opportunities to improve neoadjuvant cancer care delivery.
Research methods
Semi-structured,open-ended interviews of patients with localized PDAC during NT were conducted to explore their experience initiating and receiving NT.Interviews were conducted over the phone.All interviews were audio recorded,transcribed,and coded by two independent researchers using NVivo 12,iteratively identifying themes until thematic saturation was achieved.
Research results
The Prince without paying any further heed68 to him or to his whistling returned to the pretty gazelle, saying: Well! are you satisfied now? Since you can talk, pray tell me instantly what all this is about, and how you happen to know my name
Research conclusions
This study provides a framework to allow for a better understanding of the PDAC patient experience during NT and highlights opportunities to improve quality and quantity of life outcomes.
Research perspectives
This exploratory research utilizes qualitative interviews to examine the patient experience when initiating and receiving NT.
Marshmallows, I said, as though I d said it every summer. Last night Daddy and I walked down toward the lake and it looks as though they re just about ready to pick. It s a good thing we re here now. They only come out one day a year.
ACKNOWLEDGEMENTS
The authors extend their sincere appreciation to all participants of this study who shared their experience with us.We thank Angela Sarna for her assistance with participant recruitment and administrative support.The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
FOOTNOTES
Cloyd JM designed the research study;Stevens L,Zeh R,Monsour C and Cloyd JM performed the research;Stevens L,Brown ZJ and Cloyd JM analyzed the data and wrote the manuscript;Wells-Di Gregorio S,Santry H,Ejaz AM and Pawlik TM provided expert opinion and edits to the manuscript;and All authors have read and approve the final manuscript.
Girl: It s so much fun being with you. So much happiness is what I m feeling now.Guy: Are you satisfied in our relationship? Girl: Satisfaction isn t enough for me to explain this. Guy: Do you really love me?Girl: You silly(,)! What kind of question is that? You already knew my nswer... aight?Guy: Please baby. I just want to hear it from you. Girl: Of course. I love you so much! I really am. [silence]Girl: Is something wrong?Guy: Nah... my helmet() s bothering me. Can you take it off from me?Girl: Okay...guy: Now…wear it. Put it on…please baby. [girl putting it on]Guy: Now, hug me as tight as you can.Girl: I wonder what s got in to you.Guy: Tell me you love me then hug me.Girl: I love you more than anything else, sealed with this hug.
All study participants provided informed verbal consent prior to study enrollment.
But as I have plighted34 my word to another maiden35, you will see yourself, and so will this young woman, that I cannot go back from my word, and be faithless to her whom I love
There are no conflicts of interest to report.
No additional data are available.
The authors have read the STROBE Statement - checklist of items,and the manuscript was prepared and revised according to the STROBE Statement - checklist of items.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial(CC BYNC 4.0)license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
United States
Lena Stevens 0000-0002-0958-0035;Zachary J Brown 0000-0003-1899-5467;Ryan Zeh 0000-0003-2746-289X;Christina Monsour 0000-0002-7003-3751;Sharla Wells-Di Gregorio 0000-0002-5193-7448;Heena Santry 0000-0003-2352-1557;Aslam M Ejaz 0000-0002-1387-2604;Timothy Michael Pawlik 0000-0002-4828-8096;Jordan M Cloyd 0000-0002-2373-8433.
Ma YJ
A
The King s son declared that he would fulfil his promise, and when his parents mildly remarked that the girl was only a keeper of sheep, and a very ugly one too, the maiden boldly said that she was born a princess, and that, if they would only give her some water and leave her alone in a room for a few minutes, she would show that she could look as well as anyone in fine clothes
Ma YJ
1 Cloyd JM,Shen C,Santry H,Bridges J,Dillhoff M,Ejaz A,Pawlik TM,Tsung A.Disparities in the Use of Neoadjuvant Therapy for Resectable Pancreatic Ductal Adenocarcinoma.
2020;18: 556-563[PMID: 32380462 DOI: 10.6004/jnccn.2019.7380]
2 Aquina CT,Ejaz A,Tsung A,Pawlik TM,Cloyd JM.National Trends in the Use of Neoadjuvant Therapy Before Cancer Surgery in the US From 2004 to 2016.
2021;4: e211031[PMID: 33688961 DOI: 10.1001/jamanetworkopen.2021.1031]
3 Cloyd JM,Heh V,Pawlik TM,Ejaz A,Dillhoff M,Tsung A,Williams T,Abushahin L,Bridges JFP,Santry H.Neoadjuvant Therapy for Resectable and Borderline Resectable Pancreatic Cancer: A Meta-Analysis of Randomized Controlled Trials.
2020;9[PMID: 32326559 DOI: 10.3390/jcm9041129]
4 Piperdi M,McDade TP,Shim JK,Piperdi B,Kadish SP,Sullivan ME,Whalen GF,Tseng JF.A neoadjuvant strategy for pancreatic adenocarcinoma increases the likelihood of receiving all components of care: lessons from a single-institution database.
2010;12: 204-210[PMID: 20590888 DOI: 10.1111/j.1477-2574.2009.00150.x]
5 de Geus SW,Eskander MF,Bliss LA,Kasumova GG,Ng SC,Callery MP,Tseng JF.Neoadjuvant therapy versus upfront surgery for resected pancreatic adenocarcinoma: A nationwide propensity score matched analysis.
2017;161: 592-601[PMID: 28341441 DOI: 10.1016/j.surg.2016.08.040]
6 Lutfi W,Talamonti MS,Kantor O,Wang CH,Liederbach E,Stocker SJ,Bentrem DJ,Roggin KK,Winchester DJ,Marsh R,Prinz RA,Baker MS.Perioperative chemotherapy is associated with a survival advantage in early stage adenocarcinoma of the pancreatic head.
2016;160: 714-724[PMID: 27422328 DOI: 10.1016/j.surg.2016.05.029]
7 Artinyan A,Anaya DA,McKenzie S,Ellenhorn JD,Kim J.Neoadjuvant therapy is associated with improved survival in resectable pancreatic adenocarcinoma.
2011;117: 2044-2049[PMID: 21523715 DOI: 10.1002/cncr.25763]
8 Khorana AA,Mangu PB,Berlin J,Engebretson A,Hong TS,Maitra A,Mohile SG,Mumber M,Schulick R,Shapiro M,Urba S,Zeh HJ,Katz MH.Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline.
2016;34: 2541-2556[PMID: 27247221 DOI: 10.1200/JCO.2016.67.5553]
9 Abrams RA,Lowy AM,O'Reilly EM,Wolff RA,Picozzi VJ,Pisters PW.Combined modality treatment of resectable and borderline resectable pancreas cancer: expert consensus statement.
2009;16: 1751-1756[PMID: 19390900 DOI: 10.1245/s10434-009-0413-9]
10 Hamad A,Crossnohere N,Ejaz A,Tsung A,Pawlik TM,Sarna A,Santry H,Wills C,Cloyd JM.Patient Stated Preferences for Neoadjuvant Therapy in Pancreatic Ductal Adenocarcinoma Pancreas.In press
11 Cloyd JM,Hyman S,Huwig T,Monsour C,Santry H,Wills C,Tsung A,Bridges JFP.Patient experience and quality of life during neoadjuvant therapy for pancreatic cancer: a systematic review and study protocol.
2021;29: 3009-3016[PMID: 33030596 DOI: 10.1007/s00520-020-05813-2]
12 Evans DB.The Complexity of Neoadjuvant Therapy for Operable Pancreatic Cancer: Lessons Learned From SWOG S1505.
2020;272: 487[PMID: 32657915 DOI: 10.1097/SLA.0000000000004131]
13 Bradley EH,Curry LA,Devers KJ.Qualitative data analysis for health services research: developing taxonomy,themes,and theory.
2007;42: 1758-1772[PMID: 17286625 DOI: 10.1111/j.1475-6773.2006.00684.x]
14 Neoptolemos JP,Moore MJ,Cox TF,Valle JW,Palmer DH,McDonald AC,Carter R,Tebbutt NC,Dervenis C,Smith D,Glimelius B,Charnley RM,Lacaine F,Scarfe AG,Middleton MR,Anthoney A,Ghaneh P,Halloran CM,Lerch MM,Oláh A,Rawcliffe CL,Verbeke CS,Campbell F,Büchler MW;European Study Group for Pancreatic Cancer.Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial.
2012;308: 147-156[PMID: 22782416 DOI: 10.1001/jama.2012.7352]
15 Neoptolemos JP,Palmer DH,Ghaneh P,Psarelli EE,Valle JW,Halloran CM,Faluyi O,O'Reilly DA,Cunningham D,Wadsley J,Darby S,Meyer T,Gillmore R,Anthoney A,Lind P,Glimelius B,Falk S,Izbicki JR,Middleton GW,Cummins S,Ross PJ,Wasan H,McDonald A,Crosby T,Ma YT,Patel K,Sherriff D,Soomal R,Borg D,Sothi S,Hammel P,Hackert T,Jackson R,Büchler MW;European Study Group for Pancreatic Cancer.Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer(ESPAC-4): a multicentre,openlabel,randomised,phase 3 trial.
2017;389: 1011-1024[PMID: 28129987 DOI: 10.1016/S0140-6736(16)32409-6]
16 Neoptolemos JP,Stocken DD,Friess H,Bassi C,Dunn JA,Hickey H,Beger H,Fernandez-Cruz L,Dervenis C,Lacaine F,Falconi M,Pederzoli P,Pap A,Spooner D,Kerr DJ,Büchler MW;European Study Group for Pancreatic Cancer.A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer.
2004;350: 1200-1210[PMID: 15028824 DOI: 10.1056/NEJMoa032295]
17 Oettle H,Neuhaus P,Hochhaus A,Hartmann JT,Gellert K,Ridwelski K,Niedergethmann M,Zülke C,Fahlke J,Arning MB,Sinn M,Hinke A,Riess H.Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial.
2013;310: 1473-1481[PMID: 24104372 DOI: 10.1001/jama.2013.279201]
18 Cloyd JM,Tsung A,Hays J,Wills CE,Bridges JF.Neoadjuvant therapy for resectable pancreatic ductal adenocarcinoma: The need for patient-centered research.
2020;26: 375-382[PMID: 32063686 DOI: 10.3748/wjg.v26.i4.375]
19 Merkow RP,Bilimoria KY,Tomlinson JS,Paruch JL,Fleming JB,Talamonti MS,Ko CY,Bentrem DJ.Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer.
2014;260: 372-377[PMID: 24374509 DOI: 10.1097/SLA.0000000000000378]
20 Cloyd JM,Katz MH,Prakash L,Varadhachary GR,Wolff RA,Shroff RT,Javle M,Fogelman D,Overman M,Crane CH,Koay EJ,Das P,Krishnan S,Minsky BD,Lee JH,Bhutani MS,Weston B,Ross W,Bhosale P,Tamm EP,Wang H,Maitra A,Kim MP,Aloia TA,Vauthey JN,Fleming JB,Abbruzzese JL,Pisters PW,Evans DB,Lee JE.Preoperative Therapy and Pancreatoduodenectomy for Pancreatic Ductal Adenocarcinoma: a 25-Year Single-Institution Experience.
2017;21: 164-174[PMID: 27778257 DOI: 10.1007/s11605-016-3265-1]
21 Beaver K,Williamson S,Briggs J.Exploring patient experiences of neo-adjuvant chemotherapy for breast cancer.
2016;20: 77-86[PMID: 26078034 DOI: 10.1016/j.ejon.2015.06.001]
22 Légaré F,Witteman HO.Shared decision making: examining key elements and barriers to adoption into routine clinical practice.
2013;32: 276-284[PMID: 23381520 DOI: 10.1377/hlthaff.2012.1078]
23 Tariman JD,Berry DL,Cochrane B,Doorenbos A,Schepp K.Preferred and actual participation roles during health care decision making in persons with cancer: a systematic review.
2010;21: 1145-1151[PMID: 19940010 DOI: 10.1093/annonc/mdp534]
24 Gattellari M,Butow PN,Tattersall MH.Sharing decisions in cancer care.
2001;52: 1865-1878[PMID: 11352412 DOI: 10.1016/s0277-9536(00)00303-8]
25 Hack TF,Degner LF,Watson P,Sinha L.Do patients benefit from participating in medical decision making?
2006;15: 9-19[PMID: 15669023 DOI: 10.1002/pon.907]
26 Kehl KL,Landrum MB,Arora NK,Ganz PA,van Ryn M,Mack JW,Keating NL.Association of Actual and Preferred Decision Roles With Patient-Reported Quality of Care: Shared Decision Making in Cancer Care.
2015;1: 50-58[PMID: 26182303 DOI: 10.1001/jamaoncol.2014.112]
27 de Ligt KM,Spronk PER,van Bommel ACM,Vrancken Peeters MTFD,Siesling S,Smorenburg CH;Nabon Breast Cancer Audit group.Patients' experiences with decisions on timing of chemotherapy for breast cancer.
2018;37: 99-106[PMID: 29128583 DOI: 10.1016/j.breast.2017.10.016]
28 Herrmann A,Hall A,Zdenkowski N.Women's Experiences with Deciding on Neoadjuvant Systemic Therapy for Operable Breast Cancer: A Qualitative Study.
2018;5: 68-76[PMID: 29379837 DOI: 10.4103/apjon.apjon_60_17]
29 Kunneman M,Engelhardt EG,Ten Hove FL,Marijnen CA,Portielje JE,Smets EM,de Haes HJ,Stiggelbout AM,Pieterse AH.Deciding about(neo-)adjuvant rectal and breast cancer treatment: Missed opportunities for shared decision making.
2016;55: 134-139[PMID: 26237738 DOI: 10.3109/0284186X.2015.1068447]
30 de Mik SML,Stubenrouch FE,Balm R,Ubbink DT.Systematic review of shared decision-making in surgery.
2018;105: 1721-1730[PMID: 30357815 DOI: 10.1002/bjs.11009]
31 D'Agostino TA,Shuk E,Maloney EK,Zeuren R,Tuttle RM,Bylund CL.Treatment decision making in early-stage papillary thyroid cancer.
2018;27: 61-68[PMID: 28124394 DOI: 10.1002/pon.4383]
32 Sanders JJ,Curtis JR,Tulsky JA.Achieving Goal-Concordant Care: A Conceptual Model and Approach to Measuring Serious Illness Communication and Its Impact.
2018;21: S17-S27[PMID: 29091522 DOI: 10.1089/jpm.2017.0459]
33 Jang JY,Han Y,Lee H,Kim SW,Kwon W,Lee KH,Oh DY,Chie EK,Lee JM,Heo JS,Park JO,Lim DH,Kim SH,Park SJ,Lee WJ,Koh YH,Park JS,Yoon DS,Lee IJ,Choi SH.Oncological Benefits of Neoadjuvant Chemoradiation With Gemcitabine Versus Upfront Surgery in Patients With Borderline Resectable Pancreatic Cancer: A Prospective,Randomized,Open-label,Multicenter Phase 2/3 Trial.
2018;268: 215-222[PMID: 29462005 DOI: 10.1097/SLA.0000000000002705]
34 Versteijne E,Suker M,Groothuis K,Akkermans-Vogelaar JM,Besselink MG,Bonsing BA,Buijsen J,Busch OR,Creemers GM,van Dam RM,Eskens FALM,Festen S,de Groot JWB,Groot Koerkamp B,de Hingh IH,Homs MYV,van Hooft JE,Kerver ED,Luelmo SAC,Neelis KJ,Nuyttens J,Paardekooper GMRM,Patijn GA,van der Sangen MJC,de Vos-Geelen J,Wilmink JW,Zwinderman AH,Punt CJ,van Eijck CH,van Tienhoven G;Dutch Pancreatic Cancer Group.Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial.
2020;38: 1763-1773[PMID: 32105518 DOI: 10.1200/JCO.19.02274]
35 Versteijne E,Vogel JA,Besselink MG,Busch ORC,Wilmink JW,Daams JG,van Eijck CHJ,Groot Koerkamp B,Rasch CRN,van Tienhoven G;Dutch Pancreatic Cancer Group.Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer.
2018;105: 946-958[PMID: 29708592 DOI: 10.1002/bjs.10870]
36 Brown ZJ,Cloyd JM.Trends in the utilization of neoadjuvant therapy for pancreatic ductal adenocarcinoma.
2021;123: 1432-1440[PMID: 33831253 DOI: 10.1002/jso.26384]
37 Christians KK,Heimler JW,George B,Ritch PS,Erickson BA,Johnston F,Tolat PP,Foley WD,Evans DB,Tsai S.Survival of patients with resectable pancreatic cancer who received neoadjuvant therapy.
2016;159: 893-900[PMID: 26602840 DOI: 10.1016/j.surg.2015.09.018]
38 Tzeng CW,Fleming JB,Lee JE,Xiao L,Pisters PW,Vauthey JN,Abdalla EK,Wolff RA,Varadhachary GR,Fogelman DR,Crane CH,Balachandran A,Katz MH.Defined clinical classifications are associated with outcome of patients with anatomically resectable pancreatic adenocarcinoma treated with neoadjuvant therapy.
2012;19: 2045-2053[PMID: 22258816 DOI: 10.1245/s10434-011-2211-4]
39 Sohal DPS,Duong M,Ahmad SA,Gandhi NS,Beg MS,Wang-Gillam A,Wade JL 3rd,Chiorean EG,Guthrie KA,Lowy AM,Philip PA,Hochster HS.Efficacy of Perioperative Chemotherapy for Resectable Pancreatic Adenocarcinoma: A Phase 2 Randomized Clinical Trial.
2021;7: 421-427[PMID: 33475684 DOI: 10.1001/jamaoncol.2020.7328]
40 Ye M,Zhang Q,Chen Y,Fu Q,Li X,Bai X,Liang T.Neoadjuvant chemotherapy for primary resectable pancreatic cancer: a systematic review and meta-analysis.
2020;22: 821-832[PMID: 32001139 DOI: 10.1016/j.hpb.2020.01.001]
41 Beesley VL,Janda M,Burmeister EA,Goldstein D,Gooden H,Merrett ND,O'Connell DL,Wyld DK,Chan RJ,Young JM,Neale RE.Association between pancreatic cancer patients' perception of their care coordination and patient-reported and survival outcomes.
2018;16: 534-543[PMID: 28669376 DOI: 10.1017/S1478951517000608]
 World Journal of Gastrointestinal Oncology2022年6期
World Journal of Gastrointestinal Oncology2022年6期
- World Journal of Gastrointestinal Oncology的其它文章
- Circular RNAs in hepatocellular carcinoma:Recent advances
- Practical considerations for colorectal cancer screening in older adults
- Can dietary flavonoids be useful in the personalized treatment of colorectal cancer?
- Fibrolamellar hepatocellular carcinoma:A rare but unpleasant event
- Glutamine deprivation impairs function of infiltrating CD8+ T cells in hepatocellular carcinoma by inducing mitochondrial damage and apoptosis
- Does the addition of Braun anastomosis to Billroth II reconstruction on laparoscopic-assisted distal gastrectomy benefit patients?
