Sulfur-substitution-enhanced crystallization and crystal structure of poly(trimethylene monothiocarbonate)
Xiohn Co, Hongling Wng, Jiling Yng, Ruiyng Wng, Xin Hong,Xinghong Zhng,*, Junting Xu,*, Hi Wng
a MOE Key Laboratory of Macromolecular Synthesis and Functionalization, Department of Polymer Science and Engineering, Zhejiang University, Hangzhou 310027, China
b Center of Chemistry for Frontier Technologies, Zhejiang University, Hangzhou 310027, China
c Department of Chemistry, Zhejiang University, Hangzhou 310027, China
d Department of Polymer Science and Engineering, College of Chemical Engineering, Dalian University of Technology, Dalian 116024, China
ABSTRACT In this paper, the crystallization behavior of a novel poly(monothiocarbonate), poly(trimethylene monothiocarbonate) (PTMMTC), was investigated and compared with its polycarbonate analogue,poly(trimethylene carbonate) (PTMC).It is found that PTMMTC exhibits strong crystallizability, while unstretched PTMC is amorphous.DSC and DMA results reveal that PTMMTC possesses higher glass transition temperature (Tg) and β-transition temperature (Tβ) than PTMC.Simulation based on density functional theory (DFT) shows that, the bond angle of C-S-C is evidently smaller than that of C-O-C, and thus a larger dipole moment.This leads to the stronger intermolecular interaction and more rigid chain conformation in PTMMTC, which is the origin of sulfur-substitution enhanced crystallization.The crystal structure of PTMMTC was preliminarily determined for the first time.PTMMTC has an orthorhombic crystal structure with a planar zig-zag chain conformation.The parameters of unit cell are a = 10.74 , b = 4.79 , and c (fiber axis) = 7.74 .
Keywords:Poly(monothiocarbonate) Polycarbonate Crystallization behavior Crystal structure Sulfur substitution enhanced crystallization
The alternating copolymerization of carbonyl sulfide (COS) with epoxides to synthesize functional poly(thiocarbonate)s, as an effi-cient method of introducing sulfur to backbone, has received considerable attention in the past several years [1–4].The presence of thiocarbonate linkages endow it with some favorable properties[5–9], and thus poly(thiocarbonate)s can be utilized as heavy-metal scavengers, photoconductive fibers, solid polymer electrolytes,etc.[5,6,10,11].Recently, semicrystalline poly(trimethylene monothiocarbonate) (PTMMTC) with a melting temperature (Tm) above 120 °C, derived from the copolymerization of COS with oxetane(OX), was reported [7,12].By contrast, its corresponding polycarbonate analogue, (poly(trimethylene carbonate) (PTMC) is crystalline only upon tensile loading [13].We call this phenomenon“sulfur-substitution-enhanced crystallization”, and it is common in polyether-polythioether and polyester-polythioester systems.For example, poly(ethylene sulfide) [14] and poly(ε-thiocaprolactone)[15] have higherTms compared with their oxygen analogues i.e.poly(ethylene oxide) and polycaprolactone, respectively.In addition, Marchessault [16,17] and Matsumura [18,19] found that both theTmand crystallization temperature (Tc) of various linear aliphatic polythioesters were always higher when compared to those of the corresponding polyoxyesters.Sasanuma and coworkers investigated the conformational characteristics of aliphatic polythioethers and aromatic polythioesters by a rotational isomeric state analysis of ab initio molecular orbital calculations and compared with those of their corresponding polyethers [20–24].They found that the moretransconformers and a larger characteristic ratio were observed for sulfur-substituted polyethers due to the strong S···S repulsion along the polymer chains.It is worth noting that the steric S···S repulsion may only exist when adjacent S atoms are closely spaced.For example, the S···S repulsion is negligible in poly(trimethylene sulfide) with three bonds between adjacent sulfur atoms [21].Therefore, unlike the poly(ethylene sulfide), the C–C bond adjacent to the C–S bond in poly(trimethylene sulfide) exhibits its inherentgaucheconformation without the S···S repulsion [21].On the other hand, Bhaumik and Mark [25] pointed out that the difference of intermolecular interaction energy between poly(ethylene sulfide) and poly(ethylene oxide) was primarily due to van der Waals interactions rather than dipolar effect.However, it is known that the S atom has a larger atomic radius than O atom and the bond length of the C–S bond is larger than that of C–O bond, which should result in a weaker intermolecular interaction.It seems that the mechanism for sulfur-substitutedenhanced crystallization of polymers is still controversial.Herein,we compared the crystallization behaviors of PTMMTC and PTMC using differential scanning calorimetry (DSC) and wide-angle X-ray diffraction (WAXD).The crystal structure of PTMMTC was preliminarily determined as well.
As shown in Fig.1, the synthesis of PTMMTC and PTMC were performed following the alternating copolymerization method described in our earlier work [26].The details for synthesis were shown in the Supporting Information.The molecular weight and polydispersity index (PDI) of the samples were listed in Table S1(Supporting information).Fig.1 shows the typical DSC thermograms of PTMMTC and PTMC powders.It is observed that PTMMTC displays a strong exothermic peak at 88°C when cooled from the melt at a rate of 10°C/min (Fig.1a).The melting peak of PTMMTC is located at 133°C with a melting enthalpy (ΔHm)of 74.4 J/g.In addition, PTMMTC can still crystallize even if it is quenched at a cooling rate (Rc) of 50°C/min (Fig.S3 in Supporting information).All of these results show that PTMMTC can crystallize quickly.On the contrary, only glass transition can be detected by DSC for PTMC upon both cooling and heating (glass transition temperatureTg= -17.7°C), indicating that PTMC cannot crystallize under the experimental conditions (Fig.1b).It was reported that PTMC could crystallize only upon tensile loading and was amorphous in the relaxed state [13].That is to say, PTMMTC possesses stronger crystallizability than corresponding PTMC.In the present work, the only difference of PTMMTC and PTMC chains lies in sulfur-substitution.Therefore, we call this phenomenon as “sulfursubstitution-enhanced crystallization”.The stronger crystallizability of PTMMTC further leads to its better mechanical properties, as compared with PTMC (Figs.S4 and S5 in Supporting information).
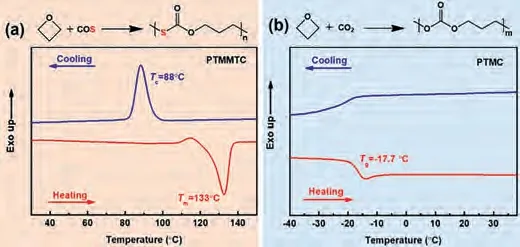
Fig.1.DSC thermograms of (a) PTMMTC and (b) PTMC powders.
In order to clarify the mechanism of sulfur-substitutionenhanced crystallization, the mobility of chain segments and subsegment units in PTMMTC and PTMC were investigated using dynamic mechanical analysis (DMA).The tanδand loss modulus (E′′)of PTMMTC and PTMC as a function of temperature are shown in Fig.2.It is observed that, both tanδ~TandE′′~Tcurves of PTMMTC and PTMC exhibit a strong peak at high temperature and a weak peak at low temperature, which are ascribed toα-transition (glass transition) andβ-transition, respectively.In general,Tgreflects the mobility of chain segments in middle length scale, which involves several repeating units.Theβ-transition reflects the local mobility of PTMMTC and PTMC chains in short length scale, which usually involves 2-4 bonds, possibly originating from the vibration or rotation of thiocarbonate and carbonate groups.The values ofTgand theβ-transition temperature (Tβ) determined by different methods are summarized in Table S2 (Supporting information).TheTgof the PTMMTC obtained by DMA is higher than that of PTMC,which is consistent with the result of DSC.This indicates that the chain segments of PTMC possess higher mobility than those of PTMMTC.As shown in Table S2, theTβs of PTMC, determined from both tanδ~TandE′′~Tcurves, are lower than those of PTMMTC.This shows that the carbonate groups exhibit higher mobility than monothiocarbonate groups.
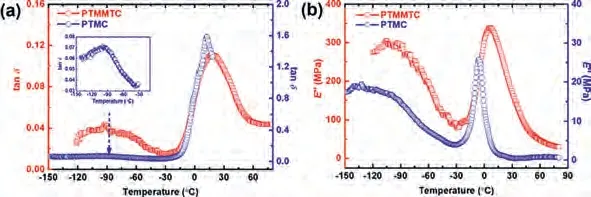
Fig.2.Variations of (a) tanδ and (b) loss modulus of PTMMTC and PTMC with temperatures.
The configurations of two small molecule model compounds(Fig.S7 in Supporting information) comprising the same repeating unit of PTMMTC and PTMC were also simulated using Gaussian 09 software based on the density functional theory (DFT) [27,28].One can see that there is a striking difference in the bond angles of C–S–C and C–O–C, which are 98.08° and 114.28°, respectively.Similar phenomena were reported in the polyether-polythioether systems.The bond angle of C–S–C (99.45° ~99.5°) in polythioethers is also smaller than that of C–O–C (111.5° ~112.8°) in polyethers[20,21].The smaller bond angle of C–S–C may result in a larger antiparallel dipole-dipole interaction along the bisector of the C–S–C angle (Fig.S7).In addition, it is reported that C–S bond in the polythioether systems possess bigger dipole moment than C–O bond[20,21].That is to say, the total intermolecular dipole-dipole interaction energy in PTMMTC is larger than that in PTMC.On the other hand, the smaller bond angle of C–S–C and the larger atomic radius of S atom will lead to more protrusion of S atoms along the direction vertical to polymer main chain.As a result, besides the C=O and C–H intermolecular interaction, S atoms can interact with the H atoms in –CH2–O– of other polymer chains more easily due to a shorter distance in PTMMTC (Fig.3a).By contrast, in PTMC,the intermolecular interaction between O atoms in C–O–C and the H atoms in –CH2–O– may be absent because of the long distance(Fig.3b).Combining the above two aspects, the intermolecular interaction in PTMMTC with the planarzig-zagconformation (this will be confirmed from the crystal structure of PTMMTC discussed below) is stronger than that of PTMC.
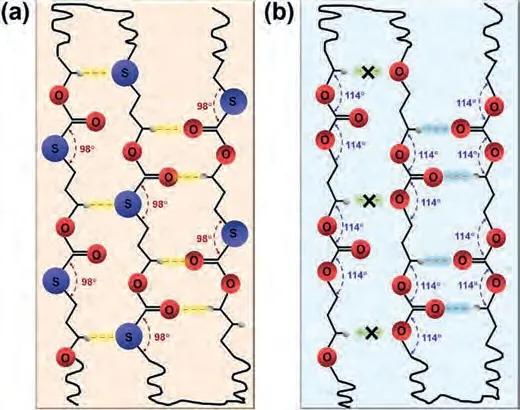
Fig.3.Possible interactions for (a) PTMMTC and (b) PTMC with extended chain conformation.
After clarifying the chain flexibility and intermolecular interaction of PTMMTC and PTMC, their different crystallizability and the origin of sulfur-substitution-enhanced crystallization can be understood from the thermodynamic viewpoint.The equilibrium melting temperature (Tm0) of polymers can be written as:

whereΔHm0is the melting enthalpy of crystals.The value ofΔHm0is mainly determined by the intermolecular interaction of polymers.Above results indicate that PTMMTC possesses the stronger intermolecular interaction than PTMC, which will lead to a largerΔHmof PTMMTC than the corresponding PTMC.In general, the entropy change upon melting (ΔSm) of polymer can be expressed as:

whereSmandScare the entropies of polymer chains in the amorphous melt and in the crystal, respectively.Since polymer chains can hardly change their conformation in crystal,Scis approximately zero.The DSC and DMA results show that more rigid chain conformation of PTMMTC.That is to say theSmof PTMMTC is smaller, thus itsΔSmis also smaller accordingly.As a consequence,PTMMTC possesses higherTmthan that of corresponding PTMC.
The crystal structure of PTMMTC was analyzed using the 2 dimensional wide-angle X-ray diffraction (2D-WAXD) pattern as shown in Fig.4a.Combined with the fiber pattern and 1D-WAXD curve (Fig.4b), the unit cell of the PTMMTC crystal structure model are estimated by the constrained least-squares method.PTMMTC can be indexed by an orthorhombic unit cell with parametersa= 10.74,b= 4.79, andc(fiber axis) =7.74(Fig.S8 in Supporting information).The unit cell of PTMMTC includes two molecular chains with one repeating unit in each chain.In the ideal extended chain of PTMMTC, the length of one repeating units is 7.62which is very close to the value ofcaxis, further indicating the planarzig-zagconformation of PTMMTC (Fig.3).Furthermore,the planarzig-zagconformation in polymers can easily lead to the odd-even effect, which means that the thermal properties of polymers fluctuate with the number of methylene unit in the chain and is widely observed for polyamides [29].This agrees well with our previous result that PTMMTC with 3 methylene units in each repeating unit has a higherTmthan the poly(ethylene monothiocarbonate) with 2 methylene units in each repeating unit [26].By contrast, the stretched PTMC forms an orthorhombic unit cell ofa= 7.02,b= 5.81andc(fiber axis) = 12.30, in which there are also two PTMC chain but the PTMC chains adopt a helical conformation [13].The cell parameters of PTMMTC also calculated based on the powder X-ray diffraction data (Fig.4b) using the Jade software, and the obtained unit cell parameters are given in Table S3 (Supporting information).It is found that the cell parameters obtained by two methods are similar.This also indicates that there is no obvious change in the crystal structure of PTMMTC after stretching.
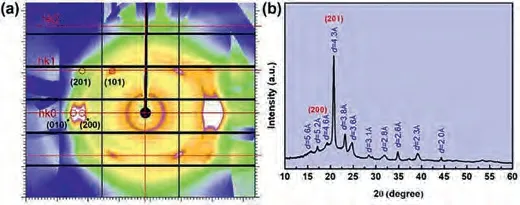
Fig.4.(a) 2D WAXD pattern and (b) 1D-WAXD curve of PTMMTC.
In this study, the crystallization properties of PTMMTC and PTMC were contrastively investigated.It is found that PTMMTC possesses stronger crystallizability than corresponding PTMC.DSC and DMA results reveal that the PTMMTC chains are more rigid than PTMC.Computer simulation shows that the bond angle of C-S-C bond is evidently smaller than that of C-O-C, leading to stronger intermolecular dipole-dipole and -S-/H2C-O interactions in PTMMTC.The sulfur-substitution-enhanced crystallization phenomenon in PTMMTC can be interpreted from above two aspects.The crystal structure of PTMMTC was also preliminarily determined.The unit cell of PTMMTC crystals is orthorhombic and contains two PTMMTC chains with one repeating unit in thec-axis.The PTMMTC chains in the crystals adopt an extended conformation.
Declaration of competing interest
The authors report no declarations of interest.
Acknowledgments
We gratefully acknowledge the financial support of the National Natural Science Foundation of China (Nos.21774108 and 21574116),the Distinguished Young Investigator Fund of Zhejiang Province(No.LR16B040001), the China Postdoctoral Science Foundation (No.2020M681818) and the Center of Chemistry for Frontier Technologies of Zhejiang University.We also thank beamline 16B1 at Shanghai Synchrotron Radiation Facility (SSRF) for providing beamtime and kind help for WAXD experiments.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.07.014.
 Chinese Chemical Letters2022年2期
Chinese Chemical Letters2022年2期
- Chinese Chemical Letters的其它文章
- Comment on “Acid-induced tunable white light emission based on triphenylamine derivatives”
- Strategies for efficient photothermal therapy at mild temperatures:Progresses and challenges
- Liposome-based delivery of biological drugs
- Macrophage-targeted nanomedicine for chronic diseases immunotherapy
- Advances, opportunities, and challenge for full-color emissive carbon dots
- Fluorine-containing agrochemicals in the last decade and approaches for fluorine incorporation
