Pt/TiO2–x nanofibrous aerogel for effective nitrogen reduction: A simple strategy for simultaneous Pt formation and TiO2–x vacancy engineering
Meng Zhng, Jin Di, Shengmei Hung, Dn Fng, Yito Liu,*, Jinyong Yu,Bin Ding,*, Andres Greiner
a Innovation Center for Textile Science and Technology, College of Textiles, Donghua University, Shanghai 201620, China
b Macromolecular Chemistry and Bavarian Polymer Institute, University of Bayreuth, Bayreuth 95440, Germany
ABSTRACT Electrocatalysis plays an increasingly important role in converting atmospheric molecules (e.g., N2, CO2 and H2O) to value-added products (e.g., NH3, C2H4 and H2).However, developing a simple strategy for preparing catalysts with high performance for the effective conversion of clean energy is still full of challenges.Herein, we describe a straightforward, one-step reduction method to achieve the formation of Pt nanoparticles (NPs) and the vacancy engineering of TiO2–x nanofibers (NFs) simultaneously, which can be accomplished in 5 min.Furthermore, a Pt/TiO2–x nanofibrous aerogel (NA) with an ordered cellular architecture is prepared through a directional freezing technology.The Pt/TiO2–x NA with excellent mechanical properties can be made into a self-supporting electrode for electrocatalytic N2 reduction reaction (NRR),showing high NH3 yield rate (4.81 × 10–10 mol/s cm–2) and Faraday efficiency (14.9%) at –0.35 V vs. RHE.
Keywords:Vacancy engineering Pt nanoparticles TiO2–x nanofibers Nanofibrous aerogel Electrocatalysis Nitrogen reduction
Energy, which vitally relates to human life from manufacturing to transportation, is essential for modern economies.With the growth of global population, the increase of energy demand and intensification of global climate change, developing sustainable pathways with fossils to produce fuels and chemicals is crucial to energy security [1].Electrocatalysis, which can utilize renewable power (solar, wind and hydro) as the driving force to convert atmospheric molecules (e.g., N2, CO2and H2O) to products with higher values (e.g., NH3, C2H4and H2), is a good choice [2–4].During the conversion process, catalysts for improving the reaction rate, efficiency and selectivity play a key role.Hence, developing advanced catalysts with high performance is imperative.
In recent years, supported metal catalysts have been widely concerned in electrocatalysis benefiting from the strong metal–support interaction (SMSI), which is very important to tune the stability and selectivity of catalysts [5].Currently, two approaches are mainly adopted to improve the SMSI: reducing the size of the loaded metal particles and constructing vacancies (e.g., by heteroatom doping or amorphization) on the support [6,7].Decreasing the particle size to nanoscale, especially to single atoms (SAs), can ensure the maximum exposure of active sites and improve the catalyst performance, since the processes of reactants adsorption and products desorption take place on the active sites [8].However,the unachievable high-loading and difficulty in large-scale production of SAs greatly limit their practical applications [9].Loading ultrasmall-size nanoparticles (NPs) on the support still dominates current research.
Vacancy engineering of the support is another approach to enhance the SMSI by changing the chemical structure of the support and improving the charge transfer [7,10].However, the present vacancy engineering strategies are either time-consuming with multiple steps or energy-consuming with harsh synthesis conditions[11–13].Although the introduction of doping ions is a simple method to construct vacancies in the support, one more process is needed to load metal NPs [14].Besides, carbon fiber paper is usually used as the matrix to load the catalyst by polymer binder,which makes most active sites unable to contact the reactants in electrocatalysis [15].Under these circumstances, developing a new method for metal NPs loading and vacancy engineering simultaneously is imperative.

Fig.1.(a) Illustration of the preparation procedure of Pt/TiO2–x NA.(b) Digital image showing the ultralight feature of Pt/TiO2–x NA (2 mg/cm3).FESEM images showing (c)the ordered lamellar structure, (d) the interlaminar cellular structure and (e) the lamellar wall of Pt/TiO2–x NA.(f) Low- and (g) high-magnification TEM images of Pt/TiO2–x NF and the inset in (g) presents the size distribution of Pt NPs.(h) HRTEM image and (i) HAADF image and the corresponding EDX maps of Pt/TiO2–x NF.
Herein, the formation of Pt NPs and the vacancy engineering of TiO2–xNFs are implemented simultaneously through a simple NaBH4reduction.By pouring NaBH4solution (80 °C) into the dispersion with H2PtCl6and TiO2NFs without other harsh conditions,the Pt4+is reduced and loaded on the TiO2NFs immediately, and the TiO2NFs are reduced by NaBH4in 5 min to form TiO2–xNFs with abundant oxygen vacancies (OVs).Furthermore, the Pt/TiO2–xNFs are prepared into a Pt/TiO2–xNA with good mechanical properties by a freeze-drying process.As an application presentation, the Pt/TiO2–xNA is used as a self-supporting catalyst for the N2reduction reaction (NRR) and the optimum NH3yield rate and Faraday efficiency (FE) are at –0.35 Vvs.RHE, being 4.81 × 10–10mol s–1cm–2and 14.9%, respectively.
The illustration for the preparation of Pt/TiO2–xNA is shown in Fig.1a.Firstly, 0.5 g of flexible TiO2nanofibrous membrane, whose average fiber diameter was 184 nm (Fig.S1 in Supporting information), was cut into small pieces and dispersed in 20 mL of water to prepare a TiO2NFs dispersion.Then, a small amount of H2PtCl6solution was dropped into the dispersion.After being sonicated for 1 h and stirred for another 2 h, the dispersion was transferred to a screw bottle.Subsequently, 180 mL of hot water (80 °C) with NaBH4was poured into the screw bottle under vigorous stirring.The dispersion was changed from yellow to gray immediately and the TiO2NFs were reduced in 5 min in the presence of NaBH4[16].After being cooled naturally to ambient temperature, the dispersion was centrifuged and washed several times alternately with 0.1 mol/L HCl and water to obtain 47.5 mL of Pt/TiO2–xNFs dispersion.After that, 2.5 mL of SiO2sol working as the binder was dropped into the dispersion and stirred for 2 h [13].The color of the dispersion remained unchanged during this process, showing the stability of the Pt/TiO2–xNFs (Fig.S2 in Supporting information).Finally, the dispersion was poured into a homemade mold, directionally frozen by a cold plate, and freeze-dried to obtain a Pt/TiO2–xNA.For comparison, TiO2–xNA and TiO2NA were also prepared (Fig.S3 in Supporting information).Benefiting from its ultralight feature, the Pt/TiO2–xNA can stand on the tip of a flower (Fig.1b).An ordered cellular architecture of Pt/TiO2–xNA can be seen in Figs.1c and d, the field emission scanning electron microscopy (FESEM) images, which is caused by the fiber selfassembly with oriented growth of ice crystals during the directional freezing progress [13,17].The bonding structure caused by the SiO2sol wrapping around the TiO2NFs in Fig.1e can maintain the stability of the Pt/TiO2–xNA [18].Under the transmission electron microscopy (TEM) observation in Figs.1f–h, it can be seen the successful loading of Pt NPs (3.9 nm) on the surface of TiO2NFs.The energy dispersive X-ray (EDX) mapping spectra of a Pt/TiO2–xnanofiber in Fig.1i further prove the uniform distribution of Pt NPs.The loading amount of Pt NPs is 4.9 wt%, as tested by inductively coupled plasma optical emission spectrometer (ICP–OES).Moreover, the Pt/TiO2–xNA has a large Brunauer-Emmett-Teller(BET) surface area of 70.99 m2/g (Fig.S4 in Supporting information), which is a vital factor to the electrocatalytic reaction.
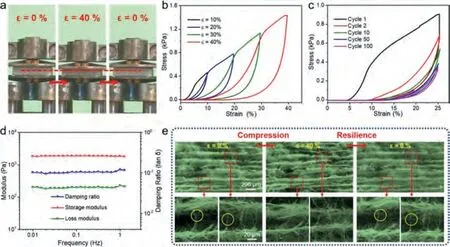
Fig.2.(a) Digital images showing the compression and resilience process at a large compressive strain (ε = 40%).(b) Compressive stress–strain plots of Pt/TiO2–x NA under different maximum strains.(c) Selected compressive stress–strain plots of Pt/TiO2–x NA over 100 cycles compression (ε = 25%).(d) Dynamic mechanical property of Pt/TiO2–x NA at an oscillatory ε = 1%.(e) In-situ FESEM images showing the microstructure evolution of Pt/TiO2–x NA during the compression and resilience process (compressive ε = 40%).
Good mechanical properties are the prerequisite for selfsupporting catalysts.Benefiting from the excellent mechanical properties of TiO2NFs (Fig.S5 in Supporting information) and the resilient bonding among the TiO2NFs caused by the elastic Si-O-Si bonds, the Pt/TiO2–xNA (10 mg/cm3, bulk density)can bear a compression strain up to 40% (Fig.2a) [13,19].From the quantitative characterization of the mechanical properties, the Pt/TiO2–xNA has a plastic deformation about 16.9% at a compression strain up to 40% during the first stress–strain cycle under different maximum compression (Fig.2b).Furthermore, the stability of the mechanical properties was measured through a compression–resilience cycle test at 25% compression strain in Fig.2c and more than 12% plastic deformation occurs after 100 compressions.The viscoelastic properties of the Pt/TiO2–xNA were also demonstrated in Fig.2d, and the stability of modulus and damping ratio from the frequency-dependent tests (0.1–1 Hz) indicates the dynamic mechanical response of the Pt/TiO2–xNA is good [19].Unmissably, the mechanical properties and viscoelastic properties of the Pt/TiO2–xNA are not so good as those of the TiO2NA in our previous work because of the poor mechanical properties of TiO2nanofibrous membrane (Fig.S5) and the shorter average fiber length (Fig.S6 in Supporting information)in the Pt/TiO2–xNA, which is not conducive to enhance the interaction among fibers through entanglement [13,20,21].To provide insight into the elasticity mechanism,in-situFESEM observations at a maximum compression strain of 40% were performed in Fig.2e.During the compression process, the compression work was transformed into elastic potential energy by the bending of nanofiber bundles among the ordered cellular architecture (Fig.S7 in Supporting information).The elastic potential energy was released with the stress decreasing during the resilience process [15].However, when the bending degree exceeded the bearing limit, the fiber bundles would be broken, as marked by the yellow circles in Fig.2e and caused the plastic deformation of the Pt/TiO2–xNA.
The X-ray diffraction (XRD) patterns of the TiO2NA, TiO2–xNA and Pt/TiO2–xNA are shown in Fig.3a, revealing that the crystalline structures of TiO2remain unchanged after NaBH4reduction.The diffraction peaks of Pt (JCPDS #04–0802) can be observed, in consistent with the above TEM results [22].The TiO2NA displays the typical Raman active modes of anatase TiO2with the characteristic peaks at 143.9, 197.2 and 639.8 cm–1(Eg), 397.3 cm–1(B1g), and 515.6 cm–1(A1g) in Fig.3b [23].Compared to the TiO2NA, the strongest band coming from external vibration of the Ti–O bond at 143.9 cm–1shifts to higher wavenumbers in the TiO2–xNA and Pt/TiO2–xNA by 5.01 cm–1and 2.92 cm–1, respectively (Fig.3b, inset), demonstrating the existence of oxygen vacancies caused by the decrease of oxygen species [24].Because of the charge balance, the formation of each OV will, in turn, produce a pair of Ti3+,which can be confirmed by the electron paramagnetic resonance(EPR) and X-ray photoelectron spectroscopy (XPS) measurements[25].The distinct signal at g = 2.003 and g = 1.96 corresponding to OVs and Ti3+, respectively, can be found in Fig.3c [26].Notably,the instability of surface Ti3+makes it easy to be oxidized in aerobic environment (air or water) [27].So the obvious Ti3+EPR signals in Fig.3c indicate the presence of bulk Ti3+, accounting for its high stability during the NA preparation process, which is inevitably illuminated in air or water.In the high-resolution Ti 2p XPS spectrum of TiO2NA (Fig.3d), two peaks assigned to Ti4+at 457.5 and 464.3 eV can be observed, corresponding to Ti 2p3/2and Ti 2p1/2, respectively.After reduction, these peaks shift to lower binding energies in the TiO2–xNA and Pt/TiO2–xNA, demonstrating that electrons are withdrawn from deficient oxygen atoms [28].The O 1s XPS spectra (Fig.3e) for the three samples contain two peaks which are associated with lattice oxygen (529.9 eV) and OVs (531.8 eV) [12].The increased peak area of OVs in the TiO2–xNA and Pt/TiO2–xNA indicates that more surface defects were produced after NaBH4reduction.The Pt 4f XPS spectrum in Fig.3f provides further evidence for the presence of metallic Pt in Pt/TiO2xNA from the peaks of Pt0at 71.2 and 74.5 eV [29].Considering that H2PtCl6(Pt4+) was chosen as the Pt source, some metallic Pt species should be reduced by the surface OVs of TiO2–xwith charge transfer from OVs to Pt NPs, causing the peak intensity or shift degree of the Pt/TiO2–xNA lower than that of the TiO2–xNA in Figs.3b–e [30].
An H-type cell (three-electrode system), separated by a Nafion 212 membrane, was used for the NRR performance.According to the linear sweep voltammetry (LSV) curves (Fig.4a), the current density in N2-saturated 0.1 mol/L Na2SO4electrolyte is much higher than that in Ar-saturated 0.1 mol/L Na2SO4electrolyte between –0.8 V to –0.2 Vvs.RHE, implying the effective N2reduction performance of the Pt/TiO2–xNA [31].From Fig.4b, the chronoamperometric curves, no obvious fluctuation in the current can be observed at different potentials, indicating good stability during the electrocatalytic process [32,33].In order to quantify the NH3yield of the Pt/TiO2–xNA at different potentials, indophenol blue method was employed.According to the standard curves and UVvis absorption spectra of electrolyte, which were dyed by indophenol blue, after 2 h reaction in Fig.S8 (Supporting information),the NH3yield rate and FE could be further calculated.As plotted in Fig.4c, the optimum NH3yield rate occurred at –0.35 Vvs.RHE for the Pt/TiO2–xNA, which were 4.81 × 10–10mol s–1cm–2and 14.9%, respectively.These values rank our Pt/TiO2–xNA as an advanced catalyst towards electrocatalytic NRR, as compared with other noble metal/TiO2or TiO2-based catalysts (Table S1 in Supporting information).As a comparison, the NRR performance for TiO2NA, TiO2–xNA and Pt/TiO2–xNA at –0.35 Vvs.RHE were also measured in Fig.4d.The Pt/TiO2–xNA shows the best performance,proving that the higher vacancy concentration and the interaction between Pt and TiO2–xis beneficial to improve the catalytic activity in electrocatalytic NRR.It is also proved by the N2temperatureprogrammed desorption (N2–TPD) spectra in Fig.S9 (Supporting information) that the Pt/TiO2–xNA has the highest desorption temperature, meaning that the presence of Pt NPs and OVs effectively enhance the N2chemisorption, which is favorable for the improvement of NRR performance [34].Considering the stability of catalysts is a critical parameter in practical applications, cycling tests and a longtime electrolysis for the Pt/TiO2–xNA at –0.35 Vvs.RHE were assessed.As observed in Fig.4e, the NH3yield rate and FE do not significantly change during 5 successive tests, showing the excellent recyclability for N2reduction.Besides, no obvious fluctuation of current density and FE after 12 h electrolysis in Fig.4f suggests that the Pt/TiO2–xNA has good electrochemical durability.The possible byproduct hydrazine was not detected after 12 h electrolysis by the Watt and Chrisp method (Fig.S10 in Supporting information).
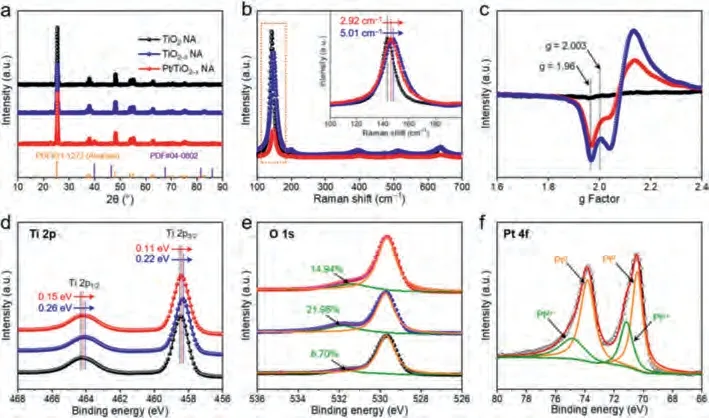
Fig.3.(a) XRD patterns of TiO2 NA, TiO2–x NA and Pt/TiO2–x NA.(b) Raman spectra of TiO2 NA, TiO2–x NA and Pt/TiO2–x NA and inset is the magnification of Eg peak.(c) EPR spectra of TiO2 NA, TiO2–x NA and Pt/TiO2–x NA.(d) Ti 2p and (e) O 1s XPS spectra of TiO2 NA, TiO2–x NA and Pt/TiO2–x NA.(f) Pt 4f XPS spectrum for Pt/TiO2–x NA.
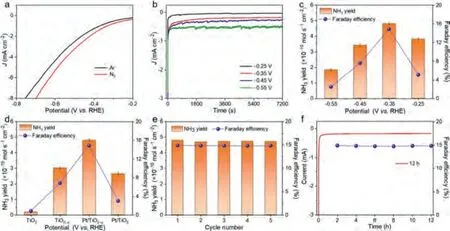
Fig.4.(a) LSV curves of Pt/TiO2–x NA in N2- and Ar-saturated 0.1 mol/L Na2SO4 electrolytes.(b) Chronoamperometric curves and (c) NH3 yield rates and FE for Pt/TiO2–x NA at different potentials (V vs. RHE).(d) The comparison of NH3 yield rates and FE at optimum potential for TiO2 NA, TiO2–x NA and Pt/TiO2–x NA at –0.35 V vs. RHE.(e) Cycling tests and (f) a longtime electrolysis for Pt/TiO2–x NA at –0.35 V vs. RHE.
In conclusion, we utilize a convenient strategy to achieve the Pt NPs formation and the vacancy engineering of TiO2–xNFs simultaneously.Under the function of NaBH4, the whole reduction process can be completed in 5 min.Benefiting from the high stability of OVs in the Pt/TiO2–xNFs in air and water, the Pt/TiO2–xNA can be prepared after a freeze-drying process.The nanofiber bundles among the ordered cellular architecture, induced by the oriented growth of ice crystals,endow the Pt/TiO2–xNA with excellent mechanical properties to work as a self-supporting electrode for electrocatalysis,achieving high NH3yield rate (4.81 × 10–10mol s–1cm–2)and FE (14.9%) at –0.35 Vvs.RHE with excellent stability for electrocatalytic NRR.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos.52173055, 21961132024 and 51925302), the Natural Science Foundation of Shanghai(No.19ZR1401100), the Innovation Program of Shanghai Municipal Education Commission (No.2017-01-07-00-03-E00024), the Fundamental Research Funds for the Central Universities (No.CUSF-DH-D-2019028), and the DHU Distinguished Young Professor Program (No.LZA2020001).D.Fang and A.Greiner acknowledge financial support from DFG (No.431073172).
Supplementary materials
Supplementary data associated with this article can be found,in the online version, at 10.1016/j.cclet.2021.08.069.
 Chinese Chemical Letters2022年2期
Chinese Chemical Letters2022年2期
- Chinese Chemical Letters的其它文章
- Comment on “Acid-induced tunable white light emission based on triphenylamine derivatives”
- Strategies for efficient photothermal therapy at mild temperatures:Progresses and challenges
- Liposome-based delivery of biological drugs
- Macrophage-targeted nanomedicine for chronic diseases immunotherapy
- Advances, opportunities, and challenge for full-color emissive carbon dots
- Fluorine-containing agrochemicals in the last decade and approaches for fluorine incorporation
