Facile preparation of nano-g-C3N4/UiO-66-NH2 composite as sorbent for high-efficient extraction and preconcentration of food colorants prior to HPLC analysis
Xiown Zhng, Yixin Yng, Peige Qin, Lizhen Hn, Wenli Zhu, Shofeng Dun,Minghu Lu,*, Zongwei Ci
a Henan International Joint Laboratory of Medicinal Plants Utilization, School of Chemistry and Chemical Engineering, Henan University, Kaifeng 475004,China
b Institute for Innovative Drug Design and Evaluation, School of Pharmacy, Henan University, Kaifeng 475004, China
c State Key Laboratory of Environmental and Biological Analysis, Department of Chemistry, Hong Kong Baptist University, Hong Kong, China
ABSTRACT In this work, the nano-g-C3N4/UiO-66-NH2 composite was prepared by one-step solvothermal method.The as-prepared composite was characterized by scanning electron microscopy, Brunner-Emmet-Teller measurement, energy dispersive spectrometer, X-ray diffraction, and Fourier transform infrared spectroscopy.By using nano-g-C3N4/UiO-66-NH2 composite as sorbent, a dispersive solid-phase extraction coupled with high-performance liquid chromatography was developed to sensitive analysis of food colorants including tartrazine, amaranth, carmine, sunset yellow, allura red and bright blue.The experiment parameters including the amount of sorbent, adsorption time, the pH of adsorption solution, desorption time, desorption solvent, the pH of desorption solution as well as the proportion between desorption solvent and buffer solvent were investigated.Under the optimized conditions, the limits of detection(S/N = 3) and limits of quantitation (S/N = 10) were determined in the ranges of 0.08–0.8 and 0.2–2.0 ng/mL, respectively.With the developed sample pretreatment method, carmine and brilliant blue were determined from blueberry juice by HPLC-DAD.The contents were calculated as 1.53 μg/mL and 0.17 μg/mL, respectively.
Keywords:Nano-g-C3N4/UiO-66-NH2 composite Metal-organic frameworks Dispersive solid-phase extraction Sample pretreatment Food colorants Food additives High-performance liquid chromatography
With improving the standard of living, food safety received considerable attention.Colorants as a type of food additives are commonly used to improve food appearance to make them more attractive and appetizing [1].According to their origin, food colorants can be divided into natural colorants and synthetic colorants.Natural colorants with property of relatively safe are mainly extracted from plant tissues, animals and microorganisms [2].Due to complex extraction procedures, poor stability and high cost, the application of natural colorants is limited in food industry.So, the natural colorants are usually replaced by synthetic colorants because of their stable properties, bright colors and low prices [3].However, some synthetic colorants are considered to have chronic toxicity, lethality, carcinogenesis and gene mutation to customer,particularly to children [4].Therefore, it is very necessary to develop simple, reliable and high-efficient method for determination of synthetic colorants in foodstuffs.
Various analytical methods including high performance liquid chromatography (HPLC) [5], liquid chromatography-mass spectrometry (LC-MS) [6], capillary electrophoresis [7], electrochemical sensor [8], as well as Raman spectra and surface-enhanced Raman scattering [9,10] were reported to determine synthetic colorants in different food matrices.Moreover, mass spectrometry (MS) with an interchangeable thermal desorption electrospray ionization source was used to rapid screening of basic colorants in processed vegetables [11].Among above-mentioned techniques for determining colorants, HPLC equipped with UV or DAD earned great attention because of easy operation, low-cost in maintenance and operation,as well as reliable results.However, low-sensitivity of this detector limited the widely application of HPLC for ultra-trace analytes in complex samples.To improve the detection sensitivity of universal methods, developing high-efficient sample pretreatment techniques was paid more and more attention.
With the fast developing of nanoscience and nanotechnology,various sample pretreatment methods based on nanomaterials were developed for clean-up and preconcentration of trace targets in complex samples [12,13].Nanoparticles as sorbents gained widespread interest in sample pretreatment since they can significantly affect the sensitivity and selectivity of the methods.Due to large surface area, high porosity, wide range of pore shapes,abundant organic linkers, and tunable frameworks, metal-organic frameworks (MOFs), a kind of organic-inorganic hybrid materials, are gaining increasing attention in various fields, such as energy storage [14], pollutants adsorption [15], chemical sensor [16],biomedicine [17], and catalysis [18].Recently, MOFs and MOFbased composites were found as an ideal sample pretreatment material in biological, environmental and food analysis [19–22].However, the application of MOFs in sample pretreatment was limited because of their poor chemical stability in liquid matrices (e.g., water or solvents).
Among MOFs, UiO-66 is a zirconium-based building brick with high stability and specific surface area, which was synthesized in 2010 [23].The crystal form of UiO-66 can keep basically unchanged in water and organic solvents (e.g., DMF, acetone and benzene).Graphitic carbon nitride (g-C3N4), a new non-metallic material, has drawn considerable attention with its unusual numerous intrinsic properties, such as good dispersion, large specific surface area, excellent thermal and chemical stability, as well as good biocompatibility and non-toxicity [24–26].Our group has demonstrated that g-C3N4or g-C3N4-based composites as the useful sorbent for clean-up and preconcentration of ultra-trace compounds in sample pretreatment [27–30].
In this work, the composite of two-dimensional nano-g-C3N4and three-dimensional MOF (UiO-66-NH2) were prepared by onestep solvothermal method.The composite was used as sorbent of dispersive solid-phase extraction (dSPE) for clean-up and preconcentration of colorants in different foodstuffs.The tartrazine, amaranth, carmine, sunset yellow, allura red and brilliant blue (chemical structures see Fig.S1 in Supporting information) were selected as target analytes to evaluate the performance of the nano-g-C3N4/UiO-66-NH2(CN/UiO-66-NH2).The as-prepared CN/UiO-66-NH2composite showed the best extraction performance compared with that of other composites, such as bulk-g-C3N4/UiO-66, nanog-C3N4/UiO-66 (CN/UiO-66), and nano-g-C3N4/UiO-66 (CN/UiO-66-COOH).
First, the nano-g-C3N4was prepared by thermal polymerization and calcination of melamine at high temperature according our previous work [28].Then, the composites including bulk-g-C3N4/UiO-66, CN/UiO-66-NH2, CN/UiO-66, and CN/ UiO-66-COOH were synthesized by similar solvothermal method with a modification.The detailed preparation information and protocol were provided in the experiments section of SM.
The morphology of pure UiO-66 and CN/UiO-66-NH2composite was characterized by SEM.It can be seen from Fig.1a that pure UiO-66 showed with large particle size and uniform octahedron.However, the CN/UiO-66-NH2composite had a similar crystalline but with smaller particle size (Fig.1b).What is more, the Brunner-Emmet-Teller (BET) measurement was performed to evaluate the difference of specific surface area and pore structure.The specific surface area of pure UiO-66 was determined as 752.12 m2/g(Fig.1c).As presented in Fig.1d, the CN/UiO-66-NH2provided larger specific surface area (835.06 m2/g) than that of pure UiO-66.The total pore volumes of CN/UiO-66-NH2were determined as 0.69 m3/g with average pore size of 3.41 nm, which revealed that the CN/UiO-66-NH2composite was the mesoporous material.In additional, the energy dispersive spectrometer analysis was carried out to confirm elements type and their corresponding content(Fig.S2 in Supporting information).
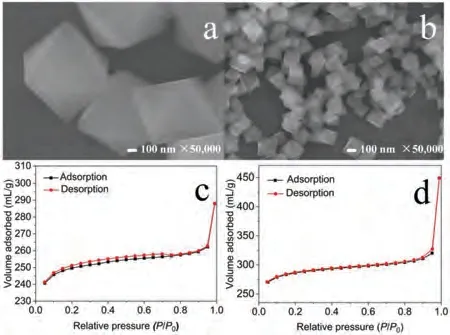
Fig.1.SEM images of UiO-66 (a) and CN/UiO-66-NH2 (b).The BET of UiO-66 (c)and CN/UiO-66-NH2 (d).
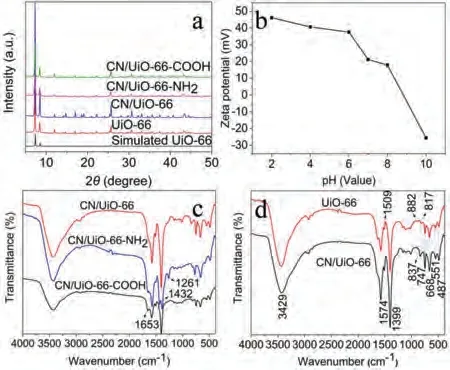
Fig.2.(a) Typical XRD patterns of UiO-66, CN/UiO-66, CN/UiO-66-NH2 and CN/UiO-66-COOH.(b) Zeta charge of CN/UiO-66-NH2 at different pH value.(c) FT-IR spectra of UiO-66 and CN/UiO-66 powders.(d) FT-IR spectra of CN/UiO-66, CN/UiO-66-NH2 and CN/UiO-66-COOH powders.
The crystallographic structure and phase purity of the asprepared materials was investigated by X-ray diffraction (XRD).It can be seen from the Fig.2a that the characteristic peaks of UiO-66 were consistent with that reported in the literature [23] and simulated standard of UiO-66.The main characteristic peaks of CN/UiO-66, CN/UiO-66-NH2and CN/UiO-66-COOH demonstrated that their crystal structures had not been destroyed during the preparation process (Fig.2a).The characteristic peak of nano-g-C3N4was not clearly demonstrated may be associated with low content of nanog-C3N4in composites.
The zeta potential of CN/UiO-66-NH2composite under different pH conditions was studied.The results were shown in the Fig.2b.The charges of the material surface changed from positive to negative with increasing pH value from 2 to 10, and the isoelectric point is 9.
To verify the chemical functional groups in the CN/UiO-66,CN/UiO-66-NH2and CN/UiO-66-COOH composites, the Fourier transform infrared (FT-IR) spectra was employed and results are shown in Figs.2c and d.As for UiO-66, the low wavelength bands mainly including 487, 551, 668, 747, 817 and 882 cm-1may be associated with stretching vibration of O–H, C–H, and Zr–O [24].The wavelength of the aromatic compound of C–O organic linker is 1450–1580 cm-1.The 1509 cm-1is assigned as the vibration of aromatic ring of organic ligand.The wavelength at 3200–3700 cm-1is attributed to the characteristic peak of O–H stretching vibration, because of the absorption of water.The characteristic peak in the spectrum of CN/UiO-66 at 837 cm-1which associates to the ring plane bending vibration of triazine unit.The new absorptionpeaks of CN/UiO-66-NH2at 1432 cm-1and 1261 cm-1can be explained as following.The characteristic peak of C–O stretching vibration appears at 1432 cm-1[31], indicating the existence of C–O bond in 2-aminoterephthalic acid (abbreviated to NH2-TPA) [32].The characteristic peak of C–N stretching vibration is considered as 1261 cm-1, belonging to CN/UiO-66-NH2[33].In additional, the characteristic peaks of 1400 cm-1and 1600 cm-1are the stretching vibration peaks of carboxylate (COO-) (Fig.2d).Compared with CN/UiO-66, the new absorption peak of CN/UiO-66-COOH is appeared at 1653 cm-1[23].
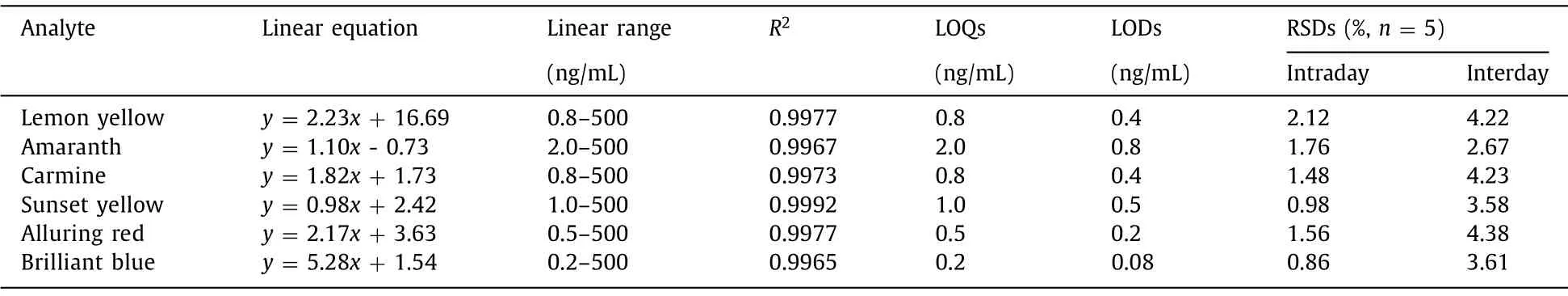
Table 1 The linear equation, linear range, correlation coefficients (R2), LOQs, LODs and precision of the developed dSPE-HPLC method for the analysis of food colorants.
To evaluate the adsorption ability of as-prepared composites including bulk-g-C3N4/UiO-66, CN/UiO-66, CN/UiO-66-COOH and CN/UiO-66-NH2, six food colorants were selected as target analytes.Among all prepared materials, the CN/UiO-66-NH2provided highest adsorption capacity (Fig.3).This can be attributed to that compared with bulk-g-C3N4, the nano-g-C3N4possessed large specific area.In additional, the amino group (cationic group) of CN/UiO-66-NH2can form strong electrostatic interaction with anionic food colorants.
To achieve the best performance of CN/UiO-66-NH2as dSPE sorbent for extraction and preconcentration of food colorants prior to HPLC-DAD analysis, the main experiment parameters including the amount of sorbent, adsorption time, pH of adsorption solution, desorption time, desorption solvents, pH of desorption solution as well as the proportion between desorption solvents and buffer solution were investigated and subsequently optimized by using 10 mL of standard solution containing the six food colorants at concentration of 100 ng/mL.The experimental procedures and explanations were provided in Supporting information.In conclusion, 12 mg as the amount of sorbent, 10 min as adsorption time,4 as the pH value of adsorption solution, 6 min as desorption time,acetone as desorption solvent, 11 as the pH of desorption solution, 60:40 (v/v) as the proportion of acetone and Britton-Robinson buffer.
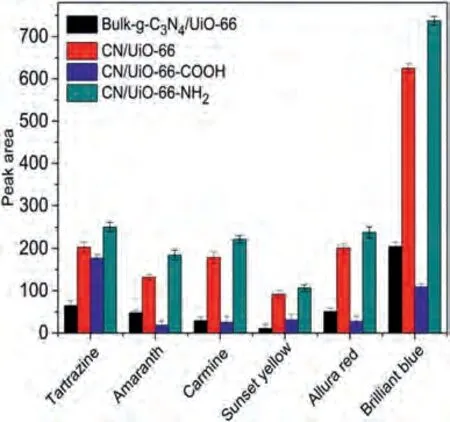
Fig.3.Comparison of the performance of bulk-g-C3N4/UiO-66, CN/UiO-66, CN/UiO-66-COOH, and CN/UiO-66-NH2 as sorbents of dSPE for extraction of food colorants.
Under the optimized conditions, a dSPE method using CN/UiO-66-NH2as sorbent was developed to clean-up and preconcentration of food colorants prior to HPLC-DAD analysis.The limit of detection (LOD) was determined as 3:1 of signal-to-noise ratio and limit of quantitation (LOQ) was determined as 10:1 of signal-tonoise ratio.As shown in the Table 1, LODs and LOQs were calculated in the range of 0.08–0.8 and 0.2–2.0 ng/mL.The precision of the method was investigated with relative standard deviations(n= 5) of intraday and interday in the range of 0%–2.12% and 2.67%–4.38%, respectively.
Three foodstuffs (yellow croaker, orange fruit sugar and blueberry fruit juice) were selected as real samples to study the reliability of the method.No target analyte was detected in yellow croaker and fruit sugar.The carmine and brilliant blue were detected in blueberry juice (Fig.4).The concentrations of carmine and brilliant blue were calculated as 1.53 μg/mL and 0.17 μg/mL,respectively.
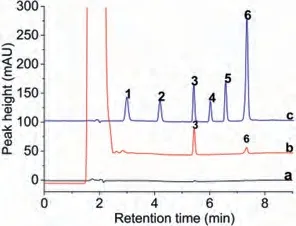
Fig.4.The chromatograms for the analysis of blueberry juice by HPLC-DAD, (a) direct injection of 1 μL blueberry juice, (b) pretreated by dSPE with CN/UiO-66-NH2 as sorbent, (c) standard solution with a concentration of 10 μg/mL.Peaks identification: (1) lemon yellow, (2) amaranth, (3) carmine, (4) sunset yellow, (5) alluring red, (6) brilliant blue.
To evaluate the reliability of the method, three different concentration (5, 50, 200 ng/mL) of standard solution were added to blueberry juice.The samples were extracted and analyzed with the developed method.Each concentration was determined three times in parallel.The results were listed in the Table 2.It can be seen from Table 2 that the recoveries were achieved in the range of 82.6%–105.8%.The reusability of sorbent was investigated with a series of adsorption and desorption.Experimental results demonstrated that the adsorption capacity can maintain more than 90%with 10 times continuous analysis.
The CN/UiO-66-NH2as sorbent of dSPE for extraction and preconcentration of food colorants was compared with other reported methods for the analysis of food colorants.As illustrated in Table S1 (Supporting information), the sorbent including PEI@PDA@Fe3O4[34], GO@Fe3O4[35], Forisil [36], rGO/Fe3O4[37],and MSN-NH2[38] were used to enrichment of colorants.
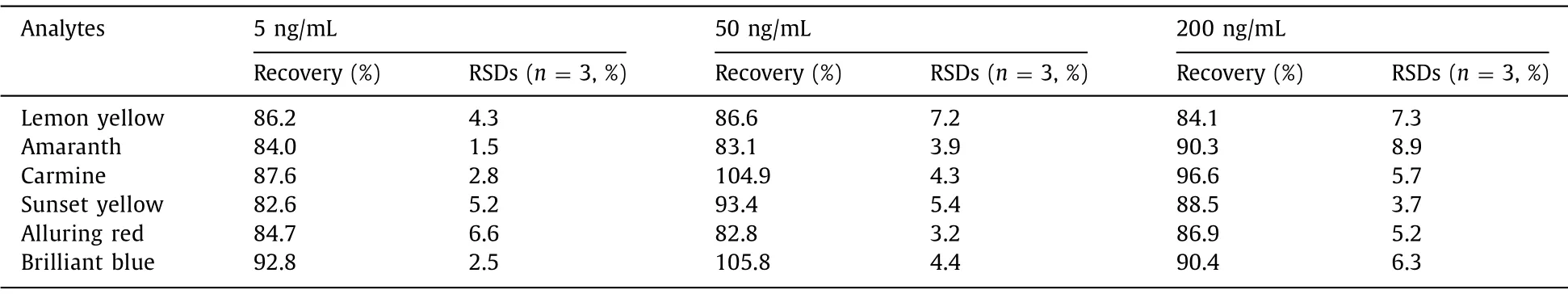
Table 2 The recoveries of method for the analysis of food colorants.
In conclusion, the nano-g-C3N4/UiO-66-NH2composites were prepared by one step solvothermal method.The nano-g-C3N4/UiO-66-NH2was demonstrated as a useful sorbent of dSPE for cleanup and preconcentration of colorants in different foodstuffs.Comparing with bulk-g-C3N4/UiO-66, the nano-g-C3N4/UiO-66 possessed higher adsorption capacity because of large specific area.Among the composites from nano-g-C3N4, the amino-group modified UiO-66 can provide the highest performance in the extraction of food colorants.The possible reason can be attributed the amino group can form strong electrostatic interaction with anionic food colorants.By using nano-g-C3N4/UiO-66-NH2as sorbent,a dSPE method was developed for clean-up and preconcentration of colorants from different foodstuffs prior to HPLC-DAD analysis.The method showed a simple, reliable, low-cost, and environmentfriendly properties for the analysis of ultra-trace food colorants in complex samples.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was sponsored by the National Nature Science Foundation of China (No.22076038), and Natural Science Foundation of Henan (No.202300410044).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.07.003.
 Chinese Chemical Letters2022年2期
Chinese Chemical Letters2022年2期
- Chinese Chemical Letters的其它文章
- Comment on “Acid-induced tunable white light emission based on triphenylamine derivatives”
- Strategies for efficient photothermal therapy at mild temperatures:Progresses and challenges
- Liposome-based delivery of biological drugs
- Macrophage-targeted nanomedicine for chronic diseases immunotherapy
- Advances, opportunities, and challenge for full-color emissive carbon dots
- Fluorine-containing agrochemicals in the last decade and approaches for fluorine incorporation
