Designing DNA cage-based immuno-fluorescence strategy for rapid diagnosis of clinical cervical cancer tissues
Junjun Li, Hongjie Luo, Xueqiong Zhu, Jinfu Zho,*, Tinfeng Chen,*
a Department of Oncology, The First Affiliated Hospital, and Department of Chemistry, Jinan University, Guangzhou 510632, China
b Department of Obstetrics and Gynecology, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou 325000,China
ABSTRACT Exploiting a tissue diagnosis method to abstain the involuted operating and consume valuable reagents while realizing high-speed and inexpensive pathological grading technology to supply a better scheme for cancer therapy is a significant method of cancers detection.A promising immuno-fluorescence strategy was rationally designed and synthesized by loading ruthenium complex into cervical cancer-targeted DNA-cage, which was well used to realize high-speed and inexpensive diagnosis of clinical cervical cancer tumor tissues avoiding the traditional multi-stage process, thus demonstrating high application potential in clinical pathological grading and surgical judgment.Moreover, it has been finding that Apts-DNA@Ru can enrichment in the tumor region, interestingly, no enrichment in normal cervical cancer tissue.It has the potential to realize the integration of in vivo diagnose and further synchronous treatment in the near future.Thence, this study demonstrates a strategy for integration of cancer-targeted DNA-cage and fluorescent RuPOP as alternative IHC reagents for next-generation more rapid convenient cancer detection.
Keywords:Rapid diagnostic Pathological grading Cervical cancer Immuno-fluorescence Cancer-targeted DNA-cage
The number of chronic diseases has dramatic increased with the accelerating processes of industrialization and urbanization.Chronic disease has become a major human health problem that seriously affects economic and social development [1].In spite of the efficacy of disease precaution and therapy is improved with advances in medical level, cancer remain responsible for human mortality [2,3].The most effective cancer treatment is early diagnosis and treatment, and the clinical cure rate of early-stage cancer can reach over 90% [4–7].Although liquid biopsy diagnostic approaches are revolutionizing early tumor diagnosis by allowing clinicians to monitor the blood, these approaches are still in the trial phase [8–13].Currently, tissue biopsy is the primary, and most widely used as before diagnostic technology for cancer histopathological detection, and is regarded as the decisive clinical diagnosis.Immunohistochemical (IHC) method, which can be applied in the vast majority of clinical cases, is applied to screen and detection cancers as well as instruct therapy strategies [14], but this approach has limitations.For instance, it usually takes a long time to get IHC results, and this approach is expensive and unstable because it requires multi-stage hatching including primary and secondary antibody application.Therefore, there is a need for the development of tumor diagnosis technology that provides improved guidance for clinicians, avoids sophisticated operation steps, and achieves rapid pathological grading diagnosis.Exploiting a tissue diagnosis method to abstain the involuted operating and consume valuable reagents while realizing high-speed and inexpensive pathological grading technology to supply a better scheme for cancer therapy is a significant method of cancers detection [15–22].Herein, a promising immuno-fluorescence Apts-DNA@Ru system was rationally designed and synthesized by loading a ruthenium complex into a cervical cancer-targeted DNA-cage.Ruthenium complexes can be integrated into DNA tetrahedrons with active targeting groups [23].Ruthenium(II) (Ru(II)) polypyridyl complexes possess many qualities that make them superior to organic dyes as fluorescent probesin vivo.The excellent photophysical properties of Ru(II)polypyridyl complexes provide advantages making them suitable as tumor diagnostic reagents [24–27].In this study, to improve the diagnostic ability of metal Ru(II) complexes, a cancer-targeted DNAcage was fabricated as biocompatible nanocarrier following Andrew Turberfield’s method [28].MUC-1, a cancer-targeted ligand,and nucleolin (AS1411) were attached to the 5′ end of the DNA strands by one-step linkage.This prevents chemical modificationmediated DNA denaturation and removes the danger of off target effects in blood circulation.The obtained Apts-DNA@Ru could be applied as diagnostic reagents to realize high-speed and inexpensive cancer detection for clinical specimens after simple dewaxing of tissue slices without requiring multi-stage hatching (Scheme 1).This approach avoids the traditional multi-stage diagnosis process and demonstrates high application potential in clinical pathological grading and surgical judgment.27 cases of clinical cancer samples from patients with cervical cancer, and seven non-cancer samples were detected.Clinical tissue samples were reviewed by the Ethics Committee and obtained safely and legally from the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University with signed informed consents obtained from either the patients or from the next of kin.The diagnostic results of clinical specimen certificated that Apts-DNA@Ru can specifically distinguish cancer tissue from non-cancer specimens.These results show that the targeted Apts-DNA@Ru has the potential to be a diagnostic tool for high-speed and inexpensive tumor tissue diagnosis in clinical specimens.Moreover, testing in clinical samples reveals that Apts-DNA@Ru tumor region enrichment differs in different malignancies in clinical tumors.Apts-DNA@Ru has the potential to realize the integration ofin vivodiagnose and further synchronous treatment in the near future.Therefore, this study presents a strategy for cancer-targeted DNA-cage and fluorescent RuPOP integration as an alternative to immunohistochemical reagents for nextgeneration, rapid, and convenient tumor diagnostics.
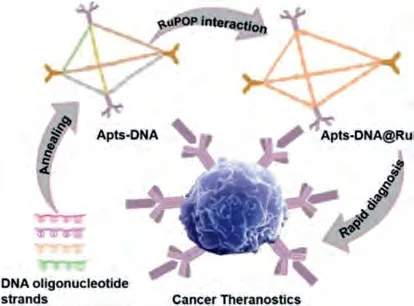
Scheme 1.Proposed schematic illustration for synthesis of Apts-DNA@Ru for rapid diagnosis of clinical tumor.
During our nanomedicine design, sample databases were exploited to open up an ideal target.Mucin (MUC-1), is abundantly expressed and aberrantly glycosylated in a large number of carcinomas of breast, ovary, colon, rectum, pancreas, and has been used as a targeting marker for tumor diagnosis and therapy [29,30].As shown in Fig.1A, the relationship betweenMUC-1expression and survival rate in patients with cervical and endocervical cancer (CESC) was investigated by the 291 clinical studies in Cancer Genome Atlas database.The data show that low/mediumMUC-1mRNA expression is related to poor survival (P= 0.68) and that demonstrate highMUC-1mRNA expression indicates a good prognosis for patients with CESC.Furthermore, obviousMUC-1overexpression is observed in patients with CESC (n= 298) when compared to that in healthy people (n= 3) (Fig.1B).Therefore, the data in these two databases indicates thatMUC-1mRNA is increased in cervical cancer and that highMUC-1expression is a tumor biomarker.Therefore, we assume that MUC-1 can be useful for the quick diagnosis of cancer and for distinguishing cancer cells from normal tissues.
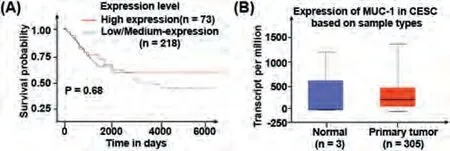
Fig.1.(A) Relationship between MUC-1 expression and survival data from TCGA dataset.(B) Expression levels of MUC-1 mRNA in normal tissue and cervical cancer form ONCOMINE database.
In this study, a DNA tetrahedron was fabricated as an ideal RuPOP (ruthenium complexes) nanocarrier.To strengthen the tumor-targeting ability of DNA cages, the MUC-1 and nucleolin(AS1411) aptamers were connected to the 5′ terminus of each DNA strand.A cancer-targeted DNA nanosystem (Apts-DNA@Ru) was fabricated as a diagnostic reagent by loading fluorescent RuPOP(Fig.2A).The positively charged RuPOP complex (+2.4 mV) was loaded on the DNA-cage (–12.57 mV) to form Apts-DNA@Ru, and the zeta potential was increased to +1.5 mV (Fig.2B).The results indicate that the RuPOP complex is loaded onto the carrier DNAcage.To identify the interaction between RuPOP and the DNAcage, UV-vis and fluorescence spectroscopy of RuPOP, DNA-cage,and Apts-DNA@Ru was performed.The peaks of Apts-DNA@Ru at 456 nm show a red shift compared to those of RuPOP, demonstrating the interaction between RuPOP and the Bio-cage (Figs.2C and D).As shown in Fig.2C, the decreased absorbance peak of RuPOP shows a hypochromic effect with the addition of Apts-DNA, which may bring about by RuPOP interactions with DNA through noncovalent bounds, or RuPOP unfold the helix and subsequently discovering more embedded bases in DNA [31].What is more, the fluorescence emission spectrum of Apts-DNA@Ru at 600 nm decreased, suggesting that RuPOP interacts with Apts-DNA.The intense absorption bands of RuPOP and Apts-DNA@Ru (350-550 nm)could be assigned to mixed charge-transfer modes such as triplet metal to ligand charge transfer (1MLCT and3MLCT).Simultaneously, Apts-DNA@Ru exhibited characteristic emission maxima at 607 nm, similar to the starting RuPOP, and Apts-DNA@Ru revealed no change in charge-transfer modes during the reaction.We studied the load efficiency of the DNA-cage using inductively coupled plasma mass spectrometry and found that the concentration of the RuPOP complex is 12.4 μmol/L in the Apts-DNA@Ru nanometer system.20 μL of the probe Apts-DNA@Ru to be used for the fluorescent imaging experiment.Taken together, these results demonstrate that RuPOP was successfully loaded on Apts-DNA@Ru.
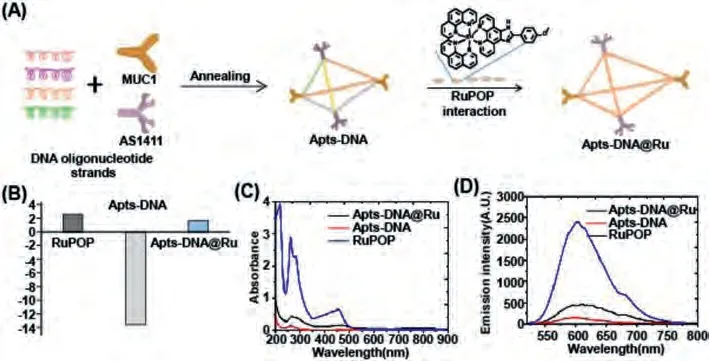
Fig.2.Synthesis and structural characterization of Apts-DNA@Ru.(A) The illustration of the preparation of the DNA-cage and Apts-DNA@Ru with a tetrahedral structure.(B) Zeta potential of RuPOP, DNA-cage, and Apts-DNA@Ru.Values were expressed as means ± SD of triplicate.(C) The UV-vis spectra of RuPOP (80 μmol/L), DNA-cage, and Apts-DNA@Ru in TAE/MgCl2 buffer.(D) Emission spectra of RuPOP (80 μmol/L), DNA-cage, and Apts-DNA@Ru in TAE/MgCl2 buffer (Excitation wavelength: 365 nm).
Moreover, comparison of the RuPOP and Apts-DNA@Ru fluorescence spectra revealed that characteristic IHC staining, as an objective method to measure and assessment of many pharmacological responses, is widely used in the diagnosis of cancer.We examined whether the Apts-DNA@Ru can be used in a way similar to MUC-1 expression, to allow for quick cancer diagnosis and grading and to distinguish cancer cells from normal tissues.We first made the specificity of Apts-DNA@Ru to normal tissues and responsiveness for cervical cancer by immunofluorescence.Apts-DNA@Ru fluorescence staining results that Apts-DNA@Ru specifically binds to tumor tissue.Briefly, the slicing, dewaxing and antigen repair steps maintain constant unless instead of multi-stage hatching with Apts-DNA@Ru.The tissue sections were then incubated with Apts-DNA@Ru at room temperature for 2 h and washed with PBS for 3 times.Finally, observing and collecting data under microscope.Strong Apts-DNA@Ru fluorescence signals, consistent with the position as well as intensity of MUC-1 expression,were observed (Fig.3A).The experimental results show that there is apositive correlation between fluorescence intensity and the degree of tumor malignancy.Interestingly, Apts-DNA@Ru shows very weak fluorescence in normal cervical tissue (Fig.3B).Consistent with this, while normal tissues showed low or little MUC-1 expression (Fig.3A).These results indicate that Apts-DNA@Ru has the potential to diagnose different grades of cervical cancer.To more accurate evaluate whether the Apts-DNA@Ru could be utilized as a detection reagent for tumors samples, we detected 18 clinical cancer specimens as well as seven normal tissue specimens using Apts-DNA@Ru.As shown in Fig.4 and Fig.S1 (Supporting information), Apts-DNA@Ru showed different fluorescence staining intensities in 18 CESC tissue samples, while no or low fluorescence staining was detected in the seven normal tissue samples.These data confirm the specific tumor-binding reactivity of Apts-DNA@Ru(Figs.4E–H) and that Apts-DNA@Ru can distinction between cervical cancer and normal cells through specific antigen-antibody binding mediated by MUC-1 peptides.Compared with conventional IHC method for cancer diagnosis, our novel Apts-DNA@Ru provides improved guidance for clinicians.This approach avoids the requirement for multiplexed process while acquiring rapid pathological grading detection within 1–2 h of incubation.The RuPOP immunofluorescence control group involved the same group of cervical cancer sections being incubated with RuPOP alone.In this group we found that RuPOP cannot distinguish between normal and cervical cancer tissues (Figs.4A–D).Taken together, our results show that Apts-DNA@Ru has a universal capacity to recognize CESC specifically expressing MUC-1.This provides a novel strategy for CESC diagnosis and has potential application value in cancer diagnosis.
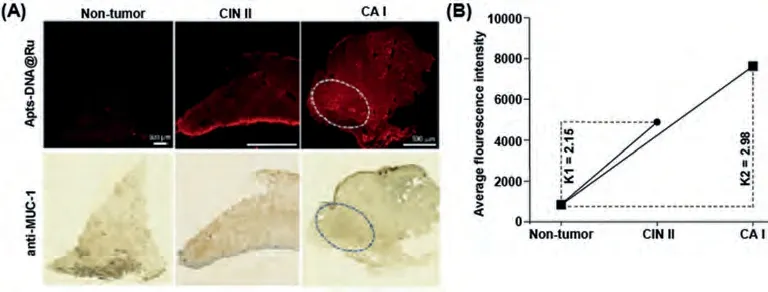
Fig.3.(A) Histological examination of the diagnostic with Apts-DNA@Ru and anti-MUC-1 antibody in non-tumor and different grades of cervical cancer.The white circle marks the place where the contrast is obvious.(B) Fluorescence intensity of Apts-DNA@Ru in non-tumor and different grades of cervical cancer.CIN Ⅱ: second stage of precancerous lesions; CA Ⅰ: first stage of cervical cancer; K1: The ratio of average fluorescence intensity between CIN Ⅱand normal tissue.K2: The ratio of average fluorescence intensity between CA Ⅰand normal tissue.

Fig.4.(A-D) Histological examination of the diagnostic with RuPOP and anti-MUC-1 antibody in non-tumor and different grades of cervical cancer.(E-H) Histological examination of the diagnostic with Apts-DNA@Ru and anti-MUC-1 antibody in non-tumor and different grades of cervical cancer.Inset column represents the relative intensity of fluorescence.Adjacent: Precancerous lesions; CA Ⅰ: first stage of cervical cancer; CA Ⅱ: second stage of cervical cancer.The sections of non-tumor and cervical cancers are serial sections of the same patient tissues.Scale bars: 2500 μm.
Exploitation of diagnostic reagents with good biocompatibility and fluorescence characteristics is important for the clinical diagnosis and treatment of cancer.In this study, DNA-cages targeting tumors were used as carriers of fluorescent RuPOP dyes.Apts-DNA@Ru was formed as an alternative to IHC reagents and presents a high-speed and inexpensive tissue diagnostic approach for clinical samples.This design can conquer the disadvantages of traditional IHC agents as well as has the following merits: ⅰ)Apts-DNA@Ru has unique biocompatibility, physiological functions,superior biodegradability, and high efficacy of drug loading capability, to achieve rapid preclinical detection and verification; ⅱ)the targeted nanosystem, with excellent sensitivity and high specificity, can distinguish tumor organization and normal organization through specific antigen-antibody binding, designed in view of clinical data assays, and has guiding significance for future development of clinically targeted diagnostic and therapeutic reagents;ⅲ) Apts-DNA@Ru has the potential for rapid and convenient preparation to assist in the differential diagnosis of clinical tumor samples, and has the potential for realize the integration ofin vivodiagnose and further synchronous treatment in the near future;and ⅳ) the novel Apts-DNA@Ru is promising imaging reagent that provides better guidance for clinicians and avoids the need for intricated procedures while also providing rapid pathological detection within 1–2 h of incubation.In summary, the targeted Apts-DNA@Ru offers promising application value in tumor detection and our results provide a theoretical basis for pathological detection of cervical cancer tissues using this tool.
Declaration of competing interest
The authors report no declarations of interest.
Acknowledgments
This work was supported by the National Key R&D Programs of China (No.2017YFC1103603), National Natural Science Foundation of China (No.21877049), Major Program for Tackling Key Problems of Industrial Technology in Guangzhou (No.201902020013), Dedicated Fund for Promoting High-Quality Marine Economic Development in Guangdong Province (Nos.GDOE-2019-A31, 2020-035), Guangzhou Key Laboratory of Molecular and Functional Imaging for Clinical Translation (No.201905010003), Innovation Team Project in Guangdong Colleges and Universities (No.2019KCXTD008), the Cultivation of Guangdong College Students’Scientific and Technological Innovation – ‘‘Climbing Program’’Special Funds (No.pdjh2019b0065).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.08.088.
 Chinese Chemical Letters2022年2期
Chinese Chemical Letters2022年2期
- Chinese Chemical Letters的其它文章
- Comment on “Acid-induced tunable white light emission based on triphenylamine derivatives”
- Strategies for efficient photothermal therapy at mild temperatures:Progresses and challenges
- Liposome-based delivery of biological drugs
- Macrophage-targeted nanomedicine for chronic diseases immunotherapy
- Advances, opportunities, and challenge for full-color emissive carbon dots
- Fluorine-containing agrochemicals in the last decade and approaches for fluorine incorporation
