Effects of local matrix environment on the spectroscopic properties of ensemble to single-particle level carbon dots
Zhihong Wei, Boyng Wng, Mingi Xie, Doheng Hong, Xin Yng, Sushu Wn,Weiqing Yng, Siyu Lu,*, Yuxi Tin,*
a Key Laboratory of Mesoscopic Chemistry of MOE, School of Chemistry and Chemical Engineering, Jiangsu Key Laboratory of Vehicle Emissions Control,Nanjing University, Nanjing 210023, China
b Green Catalysis Center, and College of Chemistry, Zhengzhou University, Zhengzhou 450001, China
c Key Laboratory for Advanced Technology in Environmental Protection of Jiangsu Province, Yancheng Institute of Technology, Yancheng 224051, China
ABSTRACT Carbon dots (CDs), because of their unique properties, are being rapidly developed as important luminescent materials for imaging, sensing, and use in photonic devices.However, most of the reported fundamental properties of the CDs are results of investigations conducted in the solution state, which may be completely different from those conducted in the solid state.In this work, we study the luminescence properties, photostability, and the dynamics of CDs in different matrix environments, from ensemble to the single-particle level.We observed that the properties associated with the emission centers and photostability of CDs were extremely sensitive to the local chemical environment.A better understanding of the dependence of the spectroscopic properties of CDs on the complex local chemical environment is an important step toward finding new ways of controlling the optical properties of CDs and optimizing their use in various applications.
Keywords:Carbon dots Matrix effects Single-particle Photostability Blinking
In recent years, carbon dots (CDs) have emerged as promising materials in biological [1-3] and optoelectronic applications [4-6],owing to their biocompatibility, low toxicity, and environmentally friendly property [7-11].Since the discovery of CDs in 2006 [12],there has been great progress in the design and synthesis of fluorescent CDs with highly tunable bandgaps and high quantum yield(QY) over 90% [13,14]; the performance of the fluorescent CDs, synthesized using heteroatom doping, surface engineering or passivation, and product separation and purification, is comparable to that of the best performing Cd2+/Pb2+-based QDs [15-21].Although there has been an increase in the number of research studies on CDs in the recent past, most of them are still at the ensemble level,averaging out the individual properties of single emission centers[22-24].Alternatively, single-particle spectroscopy (SPS) eliminates the average effect of the ensemble level [22,25-27], and, thus, enables us to investigate the intrinsic characteristics and mechanism(including but not limited to spectroscopic and kinetic properties)of CDs at the single-particle level, which is crucial to discerning the photoluminescence mechanism and other advanced applications of CDs.
A few studies on CDs at the single-particle level have been conducted.Three types of intermittent photoluminescence (PL) characteristics were observed based on the kinetic correlation studies:(1) individual CDs and graphene quantum dots (GQDs) without any blinking behavior (Sunet al.[12] and Xuet al.[25]); (2) conversely,single CDs with obvious blinking and multiple fluorescence intensity levels (Daset al.[28]), an observation that was similar to the results in other reports [29-31]; (3) a gradual decrease in the intensity with respect to time and finally bleached in a single CD was observed (Mandal’s group [32]).Meanwhile, Khanet al.[31] analyzed the mechanism of spontaneous blinking; they inferred that the Dexter-type electron transfer between the surface groups of nanoparticles plays a major role in the transition of CDs to “off” or“gray” states.For spectroscopy correlation studies, SPS has shown that most of the luminescence properties of CDs largely resemble those of organic molecules, which behave as electric dipoles, and that their emission originates from the defect centers due to the strong interaction between electrons and phonons [33,34].Nguyenet al.[35] directly imaged optically active surface defects and their bandgaps, and observed that the interaction between the graphitic core and localized defects determined the electronic gaps.Damet al.[36,37] elucidated the origin of the excitation-dependent PL of CDs, which showed multiple active and independent excitable emission sites within a single CD.However, for SPS, CDs under study are usually spin-coated on the surface of a glass cover slide or immobilized in a polymer matrix that ignores the effect on luminescent properties between CDs and the matrix material.In addition, some previous studies have suggested that the matrix material can affect the molecular properties by changing their conformation and/or inducing local stress [38-44].It should be noted that while these early studies have focused mostly on the conjugated polymers and molecular aggregates, there is a lack of homogeneous systematic studies on CDs.Therefore, understanding the interaction between CDs and matrix materials and the influence of the latter is a prerequisite to studying the properties of singleparticle CDs.
In this paper, we systemically investigated the effects of local chemical environments on the fluorescence properties, photostability, and the dynamics of CDs, from ensemble to single-particle level.Three CDs, prepared using three different methods, and five different matrices were selected for study.Nevertheless, irrespective of the method of preparation, all the CDs showed higher luminous efficiency and better photostability when PVA was used as the matrix.Meanwhile, the properties associated with the emission centers of CDs were found to be significantly sensitive to the local chemical environment, which resulted in the variations in the emission spectra and excited state lifetimes.In addition, we also observed that the CDs were prone to photobleaching with obvious photoblinking, and have poor photostability compared with that of the ensemble level.This paper provides a better understanding of the dependence of the spectroscopic properties of CDs on the complex local chemical environment for further research and applications.
In general, while CDs exhibit favorable photostability in solution under long-term excitation conditions, most of them suffer from severe aggregation-induced quenching (AIQ) as solid-state powders or films, accompanied by faster photobleaching, which limits their application in the solid state [45-50].Besides their effect on the optical response of CDs, matrix materials are crucial in determining their photostability.To further improve the luminous efficiency and photostability of CDs films, we explored the impact of different matrices on them.Four most commonly used matrices were selected, which included PVA, PMMA, PS, and PVP(Scheme S1 in Supporting information).We investigated the effect of the matrices on CD samples prepared using two different methods: (1) the method in which the glass coverslips were covered by a polymer film before the CDs were spin-coated on them,wherein the CDs were located on top of the polymer films and(2) the method in which the CDs were mixed with the polymer in solution and then spin-coated onto the surface of the clean glass slides, wherein the CDs were embedded in the polymer film (denoted as CDs@matrix).The three CDs were synthesized using citric acid and thiourea (S-CDs),o-phenylenediamine and H2SO4(RCDs), ando-phenylenediamine and dopamine (oPD-CDs) as carbon sources.The details can be found in Supporting information.The CD solution spin-coated directly on the bare glass slides were used as the control samples.The results are shown in Fig.1 and Fig.S1 (Supporting information).We found that while S-CDs prepared using the first method could be well dispersed on PVA, PVP, and PS to form a relatively uniform fluorescent film, serious aggregation was observed on the surface of PMMA (Figs.1a–e).This may be due to the poor hydrophilicity of PMMA, which is not conducive to the S-CDs richness of a large number of carboxyl groups.The luminescence intensity (luminous efficiency) and dispersibility of the S-CDs prepared using the second method (Fig.S1) were found to be greatly decreased; only PVP-diluted S-CDs were found to have a uniformly distributed CD film and maintained a brighter luminescence, and their photostability also was found to decrease.The luminescence of S-CDs in the control sample was almost completely quenched, which may be due to the interfacial interaction and electron transfer between CDs and glass substrates [46].In addition, the electronic coupling between the CDs and glass substrates was observed to generate new excitonic states, resulting in luminescence quenching.The luminescence intensities of the S-CDs on the different matrix surfaces were compared (Fig.1f); it was seen that the S-CDs on the surface of PVA and PVP exhibited the highest luminescence intensity, which was approximately two orders of magnitude higher than the luminescence intensity observed for other matrices.
We then studied the bleaching dynamics of S-CDs on different matrices under nitrogen atmosphere (Fig.1g).An excitation power of 48 W/cm2was used for the experiments.It was obvious that the S-CDs on PVA had the best photostability.Since the role of atmospheric oxygen in photobleaching was minimal, the bleaching of S-CDs could be attributed to the photodestruction of the chromophore itself on the matrix polymer.It is also worth noting that the bleaching of S-CDs was slower on PVA than on other matrices, when both the excitation power density and the light exposure of 300 s were constant.The above results indicate that the matrices and preparation methods have a large effect on the luminous efficiency and photostability of CDs.The effect may be related to many factors such as host-guest interaction, and even different conformation of CDs on different matrixes in comparison to PVA.
Properties such as dispersibility, luminescence intensity, and photostability of the other CDs on different matrices were compared under the same conditions; the results showed similar trends.All of them were found to have excellent dispersibility, high intensity, and the best light stability when PVA film was used as the matrix (Figs.S2 and S3 in Supporting information).In addition,the results of CDs mixed with the matrix were shown in Figs.S4 and S5 (Supporting information).We found that the luminescence intensity of the other two CDs@matrix is much lower than on PVA.R-CDs prepared using the second method could be well dispersed only in PVA, and serious aggregation and quenching were observed in PMMA, PVP and PS, respectively; for oPD-CDs, it could be dispersed in PVA, PMMA and PVP, and serious quenching were observed in PMMA and PS.The luminescence intensities of the RCDs and oPD-CDs in the different matrix were compared (Figs.S4e and S5e).It is obvious that the luminescence intensity of the three kinds CDs@matrix is much lower than that on PVA.The above results indicated that CDs maintain excellent dispersibility and luminescence intensity when using the first method.We highlight that the luminous efficiency and photostability of CDs in solid state are the two main liminations for single-particle experiments, and the luminous efficiency/luminescence intensity should be the top priority for futher experimental observation.The comparison of photostablity may be meaningless when the significant difference in luminescence intensity and serious aggregation occurred.The aggregation was affected by intermiscibility or dispersibility of polymer solution with CDs solution.A typical example is that PMMA is soluble in benzene, methylbenzene, anisole and other organic solvents.However, R-CDs and oPD-CDs can be dispersed in ethyl alcohol, but not in methylbenzene and other organic solvents.In addition, the interaction between matrix and CDs should be taken into consideration.For the three CDs were functionalized with abundant functional groups -OH, -COOH, and -NH2, which are prone to conbine with similar functional groups forming a uniform fluorescent film, but to form aggregation or quenching with other local matrix environments.The zeta potentials of the three CDs suggested that the above results were not directly related to their electronegativity values (Fig.S6 in Supporting information).All the above results suggested that PVA is a better matrix than the other three.Since the high luminous efficiency is expected to be effectively quenched by the host-guest interaction, the PVA matrix must play a key role.As reported that hydrogen bonds is important factors for fluorophores, which minimizes the non-irradiation transfer and results high fluorescence quantum yields [51-55].PVA contains abundant hydroxy groups and could form hydrogen bonds effectively with C-N/C=N/C=O functional groups on three CDs, limiting the intramolecular motions and preventing the nonradiative relaxation [56,57].In combination with the hydrogen bond being easily destroyed by water molecules [58], we investigated the changes in the PL performance of the CDs on PVA film before and after being affected by tiny amounts of water (20 μL) and water vapor to confirm the inference.As shown in Fig.S7 (Supporting information), obvious quenching phenomenon was observed for the three CDs, and the fading rate of PL intensity was also increased.The above results indicated that hydroxy groups of PVA could effectively form hydrogen bonds with the functional groups on CDs,preventing the nonradiative relaxation.Meanwhile, PVA was observed to effectively block the interfacial interaction between the CDs and the glass substrate.In addition, different polymer cohesive energy density might have influence on the luminescent properties of carbon dots.In addition, different cohesive energy density (CED)in the polymer may have a different binding energy between the CDs and the polymer, which will affect its luminescence performance.Among them, the CDE of PVA (>750 J/cm3) is greater than PMMA and PS (<400 J/cm3).As we all know, the CED increase with the increasing of the strong polar groups, which is prone to form hydrogen bonds with the surface groups of CDs.
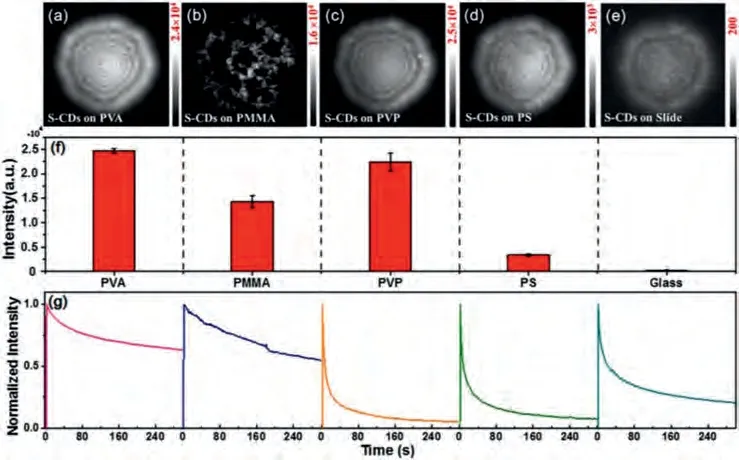
Fig.1.Fluorescence images of S-CDs on different polymer films: (a) PVA, (b) PMMA, (c) PVP, (d) PS, and (e) bare coverslip.(f) The luminescence intensity of S-CDs on different polymer films.(g) Photobleaching dynamics of CDs placed on different matrices (the five histograms and different color lines correspond to luminescence images)under an excitation power of 48 W/cm2.
To further understand the effects of the different matrices on CDs, we obtained the luminescence spectra of CD solutions and CDs on different matrices using a home-built luminescence microscopy system.The emission spectra are shown in Fig.2.The S-CDs in DMF exhibited a broad emission between 600 nm and 800 nm, with the maximum peak at around 663 nm; the RCDs and oPD-CDs showed well-resolved vibronic structures in dilute solutions, with two peaks and the maximum peak located at around 600 and 685 nm, respectively (the red dashed lines).However, the emission spectra of the three CDs vary obviously in dilute solutions and on different matrices.The emission spectra of S-CDs on different matrices were similar with slight differences in the bandwidths and obvious blue shift from 663 nm to 593 nm (the incomplete spectra were detected after passing through a 550-nm longpass filter).The emission spectra of RCDs on PVA maintained well-resolved vibronic structures with a slight blue shift (less than 20 nm).However, broadening and fine structure weakening were observed in the emission spectra of RCDs on the other three matrices, especially PVP.Similar phenomena were observed for oPD-CDs; the emission spectra were generally broad with vibronic structureless from the dilute solution to thin-film spectra, as exemplified in Fig.2c.Upon comparison,the emission spectra of S-CDs and R-CDs obtained on PVA showed line shapes similar to those obtained in solution, which illustrated that the emission spectra of CDs in solution and in film state are congeneric and that both originate from local excited states.The blue shift in the spectra of the three CDs was attributed to aggregation-induced fluorescence blue shift.When the torsional motion of the CD sub-fluorophores was restricted in the aggregates, the emission spectrum shifted correspondingly.A similar phenomenon was discovered by the Tang group [59].In addition,the changes in the spectra of the CDs on matrices without solvent molecules are usually related to intermolecular aggregation,resulting from the mix of neighboring molecules with degenerate energy [45,46,57,60,61].In our case, the observed thin-film emission of oPD-CDs may have originated mainly from singlet excimers,which are formed by the aggregation and interaction between an excited chromophore and an unexcited chromophore.The PL spectra of the three CDs were observed to remain the same during the PL decline (Fig.S8 in Supporting information).No significant spectral change was observed.The above results indicated that different matrices have different but obvious effects on the intrinsic luminescence of the CDs.Among the matrices, PVA was seen to significantly maintain the intrinsic luminescence of the CDs, an observation that was conducive to continuing single-molecule related research.
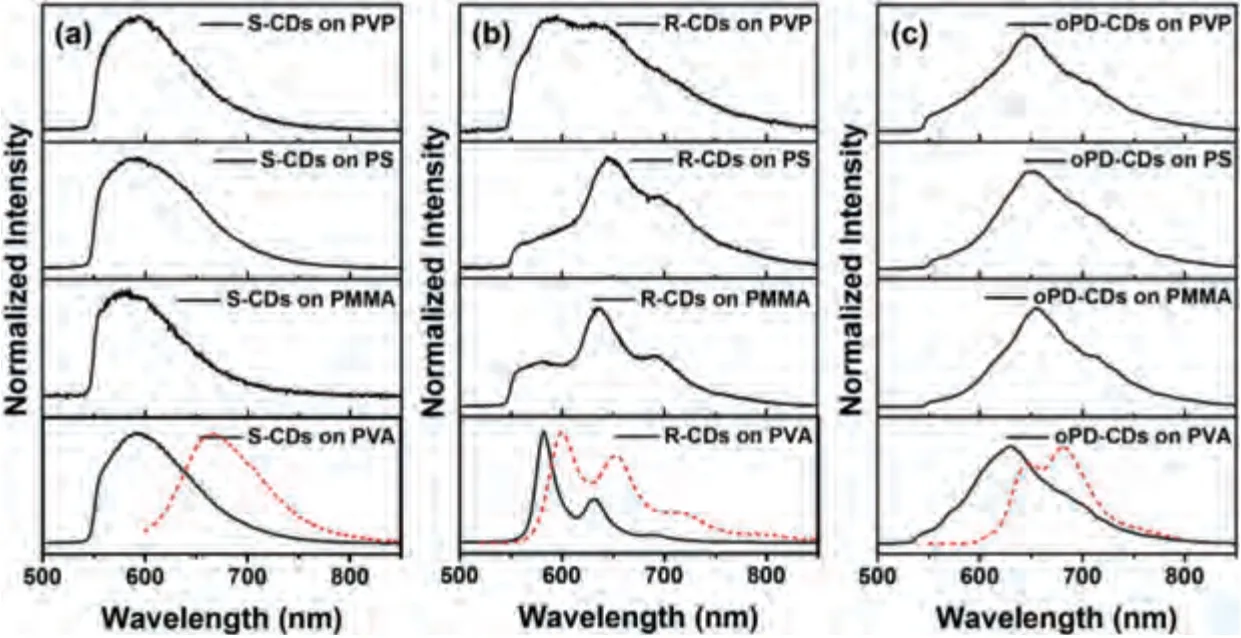
Fig.2.Luminescence spectra of (a) S-CDs, (b) R-CDs and (c) oPD-CDs on different matrices.The red dashed lines represent the luminescence spectra of S-CDs, R-CDs and oPD-CDs in appropriate solvents.
To gain more insight into the exciton recombination dynamics,the difference in the time-resolved PL decay dynamics in solution and film states were observed, and the results are shown in Fig.3,Fig.S9 and Table S1 (Supporting information), respectively.The PL lifetimes of all the three CDs in solid films were shorter than those in solution, indicating the formation of other recombination channels in the aggregated state [49].When the CDs were dispersed in/on different matrix with solvent evaporated, the CDs will directly contact with polymer molecules.There are different interactions can happen between the CDs and polymers including hydrophobic interaction, mechanical pressure, electron transfer, and chemical bonding [46].Some of the interactions can directly or indirectly affect the luminescent properties of CDs including the lifetime.For example, the electron transfer interaction can efficiently quench the luminescence of CDs showing short lifetime, while the hydrogen bonding can protect the CDs from quenching showing longer lifetime [61].The mechanical pressure can change the conformation of the CDs and thus showing different luminescence lifetime.In the above results, all three CDs exhibited the longer PL lifetime on PVA than others, an observation that was consistent with their PL intensities, which could be attributed to protection effectviahydrogen bonding between the CDs and polymers.In contrast, all three CDs deposited on the glass substrate exhibited the shortest PL lifetime, which may be attributed to the formation of non-radiative channels as a result of the interaction of the CDs and the glass substrate.Moreover, the R-CDs in dilute solutions exhibited single exponential decay dynamics of the PL lifetime, while the thin films of R-CDs showed nonexponential decay dynamics,which was best described by the biexponential corresponding to PL lifetimes ~3.99 and ~2.43 ns, respectively.This may be a result of complex kinetics that include distinct species of excimers and aggregated complexes.
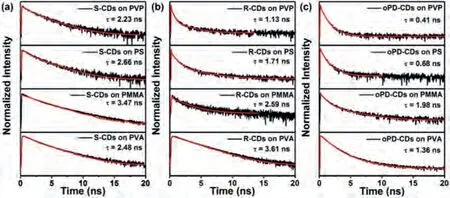
Fig.3.PL lifetimes of (a) S-CDs, (b) R-CDs, and (c) oPD-CDs on different matrices.The red lines represent the corresponding fitting curves; supercontinuous 532-nm laser was chosen for these experiments.
To identify whether PVA is an ideal matrix for SPS, we conducted single-particle imaging on different matrices and compared the results.Our experimental results show that homogeneously dispersed individual CDs could be detected only when PVA was used as the matrix.The ensemble samples were diluted gradually to a concentration from 5 × 10-6mg/mL to 5×10-7mg/mL.As depicted in Fig.S10 (Supporting information), the number of CDs was decrease with the concentration decreasing.Meanwhile, we also investigated the stability and blinking behavior of individual CDs.As seen in Figs.4a–c, the widefield luminescence images of the three CDs exhibited many bright spots, whereas all three CDs showed heterogeneous bright spots.The difference in the brightness can be attributed to the well-documented blinking behavior of the three CDs.We then obtained the dynamics of individual CD particles and observed that many of them were very unstable and survived less than 2 min of excitation with obvious photobleaching.Randomly selected single-particle intensity versus time trajectories were collected, as shown in Fig.S11 (Supporting information).Single-step transitions between the off-state and the highintensity on-state suggest that the high-intensity on-state corresponds to an emissive state of a single CD but not to a superposition of signals from several particles or aggregation [62].
For a better comparison of the photostability of the three single-particle CDs, we obtained the statistical histograms of the total dwell times in the fluorescent “on” state (“total on time”note asτon).As shown in Figs.4d–f, it is obvious thatτonsignificantly displays a power-law distribution.More than half of the CDs exhibited an on-time duration of less than 10 s, implying that most CDs have poor photostability at the single-molecule level.We also recorded the luminescence intensity versus time traces of individual CD particles during widefield illumination.We observed that many individual CD particles show a similar behavior with transient luminescence intensities, which is often characterized as luminescence blinking and in contrast to an earlier report that showed non-blinking of CDs [12,25].Six typical luminescence intensity traces of individual CDs are shown in Figs.4g and h.The six typical blinking behaviors include: (a) blinking with a constant emission rate followed by single-step bleaching; (b)blinking with a transition from “off” to “on” state; (c) blinking with luminescence intensity fluctuations between multiple intensity levels; (d) blinking with extremely short (minimum resolution time) and long (several tens of seconds) “off” state time durations; (e) blinking with a constant emission rate followed by high-frequency blinking, and (f) high-frequency blinking.When the emitter is on, it can be photo-excited to the excited state and return back to ground state by emitting photons.They also have a chance to transfer to some other states such as charged state.When the emitter is charged, it cannot be photo-excited and thus do not emit photons anymore.In this work, we showed different blinking behaviors of each CDs which is just for presentation of typical blinking behaviors of single CDs indicating complex photophysical processes in CDs.Since the blinking is a random process, it can be different from one particle to another.Thus, the most valuable result for this work is the statistics of on state duration time which directly shows relates to the luminescence effi-ciency and stability.Moreover, in this work, three different singleparticle CD measurements revealed that single-particle CDs universally exhibit blinking behavior and become photobleached within a few seconds.It is evident that the poor photostability is a serious limitation in many applications, such as single-molecule tracking and single-photon light sources, and so forth.Therefore, improving the CDs, including an increase in the photostability and comprehension of the photophysical process of blinking, is a prerequisite to move single-particle CDs into mainstream photoluminescence and biological research.It should be noted that the mechanism of spontaneous blinking of CDs still remains controversial,and we will be interesting to investigate in more details in the future.

Fig.4.Relevant data of single-particle CDs.Luminescence images of (a) S-CDs, (b) R-CDs and (c) oPD-CDs.Statistics of the total “on” state dwell time of (d) S-CDs, (e) R-CDs and (f) oPD-CDs; (g-i) Typical single-particle intensity versus time trajectories extracted from widefield imaging (100 ms exposure) with 532-nm excitation (48 W/cm2) of three CDs.
In summary, our study shows that the local chemical environment has a certain impact on CDs properties such as luminous efficiency, photostability, and related electronic energy, which leads to a change in the lifetime of the excited states and the spectral position of emission.We investigated the luminous efficiency and photostability of CDs prepared using three different methods and five different matrices.All of these experiments show that CDs on PVA have higher luminous efficiency and better photostability, which may be ascribed to the hydroxyl groups on the surface of PVA forming hydrogen bonds with the functional groups on CDs, limiting the intramolecular motions and preventing nonradiative relaxation.Meanwhile, PVA effectively blocks the interfacial interaction between CDs and the glass substrate.The obvious blue shift of CD emissions on PVA compared with CDs dissolved in solution may be attributed to the hydrogen bonds of formation and intermolecular aggregation.Meanwhile, the formation of other recombination channels or excimers in the aggregated state created the differences in PL decay dynamics.Moreover, we highlight that all the three CDs show a similar behavior with obvious transient luminescence intensities, and that most CDs have poor photostability at the single-particle level (on-time duration less than 10 s), which limits their application in advanced fields such as single-biomolecular tracking and single-photon light sources, and so forth.In the future, we will address two issues of CDs including poor photostability (both blinking and photobleaching) and the mechanism of blinking in much greater detail.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors gratefully acknowledge financial support from National Natural Science Foundation of China (No.22073046) and Fundamental Research Funds for the Central Universities.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.08.014.
 Chinese Chemical Letters2022年2期
Chinese Chemical Letters2022年2期
- Chinese Chemical Letters的其它文章
- Comment on “Acid-induced tunable white light emission based on triphenylamine derivatives”
- Strategies for efficient photothermal therapy at mild temperatures:Progresses and challenges
- Liposome-based delivery of biological drugs
- Macrophage-targeted nanomedicine for chronic diseases immunotherapy
- Advances, opportunities, and challenge for full-color emissive carbon dots
- Fluorine-containing agrochemicals in the last decade and approaches for fluorine incorporation
