Allosteric conformational changes of G proteins upon its interaction with membrane and GPCR
Longmei Li, Jin Zhng, Wenjing Sun, Weimin Gong, Chnglin Tin, Pn Shi,*,Chowei Shi,*
a Hefei National Laboratory of Physical Science at Microscale and School of Life Sciences, University of Science and Technology of China, Hefei 230027, China
b Department of Chemical Physics at School of Chemistry and Materials Sciences, University of Science and Technology of China, Hefei 230027, China
ABSTRACT Current resolved structures of GPCRs and G protein complexes provided important insights into G protein activation.However, the binding or dissociation of GPCRs with G protein is instantaneous and highly dynamic in the intracellular environment.The conformational dynamic of G protein still needs to be addressed.In this study, we applied 19F solution NMR spectroscopy to monitor the conformational changes of G protein upon interact with detergent mimicking membrane and receptor.Our results show that there are two states equilibria in the Gα in apo states.The interaction of Gα with detergents will accelerate this conformational transformation and induce a state that tends to bind to GPCRs.Finally, the Gα proteins presented a fully activation state when they coupled to GPCRs.
Keywords:19F solution NMR G protein-coupled receptors Conformational dynamics G protein
G protein-coupled receptors (GPCRs) perceive many extracellular signals and transduce them to heterotrimeric G proteins, which in turn initiate a multitude of signaling cascades that alter cellular function.Theβ2adrenergic receptor (β2AR) is a prototypical class A GPCRs [1], which preferentially couples to the stimulatory G protein (Gαs) compared to inhibitory G protein (Gαi)[2–4].The first crystal structure of the quaternary complex composed of agonist-activated monomericβ2AR and heterotrimeric G protein [5] opened a new avenue in the study of the interaction between GPCRs and G proteins.These high-resolution complex structures would provide structure basis for the downstream signaling-selective GPCR-targeting drug development.However, the static crystal structure and cryo-EM structure could not provide sufficient information about the dynamic process of G protein coupling to GPCRs.Furthermore, the Gαi coupling is much less effi-cient than Gαs coupling in most cytological and biochemical analysis making the complex ofβ2AR-Giis less stable thanβ2AR-Gs[6].Therefore, the current research on the mechanism of the interactions betweenβ2AR and Gαi is limited [7].
G proteins transduce signals from GPCRs to a variety of effector proteins, which might be localized to membrane microdomains with different lipid composition [8].Membrane association and binding have been proved to play a crucial role in G protein function [9–11].Direct membrane contact of the photoreceptor G protein heterotrimer has been observed by low resolution (~30)electron crystallography [12].Although there have been numerous studies of G protein-membrane interactions using mutagenesis,biochemical and functional approaches [13,14], the molecular basis underlying the interaction of the distinct G proteins subunits with the plasma membrane are still not fully understood [15].This may in part be due to the fact that the structural heterogeneity of lipid-GPCRs ternary complex hindered the high-resolution structure determination by Cryo-EM or X-ray crystallography [16–18].
Nuclear magnetic resonance (NMR) spectroscopy in solution is one of the key approaches to explore dynamic features, which can be accessed at physiological temperature and with minimal modification of proteins [19–21].13C-methylated lysine,13CH3-labelled methionine and cysteine-based modification approach such as 2,2,2-trifluoroethanethiol (TET) or 3-bromo-1,1,1-trifluoroacetone(BTFA) labelling have been extensively performed for conformational dynamics study of GPCRs [22–24].Previous spectroscopic strategies have shown thatβ2AR adopts multiple conformational states activated by agonist in the presence or absence of G protein [25].However, the dynamic conformational change of G protein coupling to GPCRs is remained elusive.
Herein, we utilize19F NMR spectroscopy to monitor the conformational change of the Gαproteins coupling to theβ2AR.The19F atoms are introduced by site-specific incorporation method with high specificity, efficiency and fidelity [26].Compared with other methods for the fluorine introduction [27–30], site-specific incorporation of a19F labelled amino acids into proteins is a powerful tool for monitoring protein conformational changes, protein processing and protein stability studiesin vivoandin vitro[19,31].Our data show that the apo-form of Gαprotein adopts two conformations.Further structural changes were observed when Gαprotein interacted with detergent mimicking cell membrane, which confirmed that interaction with the cell membrane is pivotal for G protein activation.The conformation of both Gαs and Gαi proteins were further stabilized by coupling to activatedβ2AR.Our studies provided insights into the dynamic process of GPCR-G protein signaling pathway.
To obtain19F labelled target proteins, we used two plasmids in the system: One plasmid expressing theMethanococcus Jannaschiityrosyl amber suppressor tRNA (MjtRNA-TyrCUA)/tyrosyl-tRNA synthetase (MjTyrRS) pair, and the other plasmid containing a TAG mutant of the G protein gene (Fig.S1 in Supporting information)[32].E.colicells carrying both plasmids are grown in the presence of L-4-trifluoromethyl-phenylalanine (tfmF) (Fig.1a) to produce the19F-labelled Gαprotein.Due to the rapid rotation of the CF3group,the sensitivity of NMR signals can be improved by tfmF labelling.
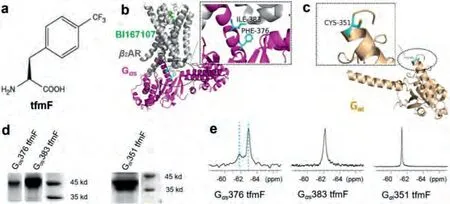
Fig.1.Site-specifically incorporates tfmF into Gα proteins in response to a UAG stop codon in E.coli.(a) The chemical structure of L-4-trifluoromethylphenylanine (tfmF).(b)Ribbon representation of Gα s subunit in the β2AR-Gα s complex (PDB accession number 3SN6).The 19F probe tfmF was incorporated at residue F376 and I383 located at the interface of β2AR and Gα s labelled in cyan.(c) Ribbon representation of Gα i subunit (PDB accession number 6DDE).The 19F probe tfmF was incorporated at residue C351 labelled in cyan.(d) SDS-PAGE analysis of tfmF incorporation into Gα s (47 kDa, left) and Gα i (41 kDa, right).(e) 19F NMR spectra of conformational equilibria for Gα s376 tfmF, Gα s383 tfmF and Gα i351 tfmF.The observation of two uncoupled conformational equilibria shows that there are two distinct states for apo-form Gα protein.The two states S1 and S2 are indicated by two cyan dash lines.
We individually introduced unnatural amino acid tfmF to the receptor coupling interface of the Gαs and Gαi that can be used as19F NMR reporters to monitor structural changes in response toβ2AR.One-dimensional NMR spectra were collected with the proton coil tuned to the19F frequency for 1-3 h to further confirm that the tfmF was successfully incorporated into target proteins.Finally, the19F-labelled proteins with high labelling efficiency and friendly thermal stability were selected for the following NMR experiment, such as Gαs376 tfmF, Gαs383 tfmF and Gαi351 tfmF(Figs.1b-d).All these residues located in the C- terminal ofα5 helix, which is a key determinant of GPCR-G protein coupling selectivity [33].Previous fluorescence lifetime based FRET studies have revealed that both Gαs and Gαi presented two distinct conformations in the apo-state [34].Consistent with previous studies, our results show that there are two conformations (hereinafter called states S1 and S2) for each tfmF incorporation site (Fig.1e).In the apo-form, we observed two obvious peaks for Gαs376 tfmF, suggesting the existence of conformational heterogeneity in this location with slow exchange between two conformational states on slow time scale (k <<1.5 ms-1) at room temperature.Similar to the structural heterogeneity in19F NMR spectra of Gαs376 tfmF,Gαs383 tfmF show a broad line shape, derived from the overlap of two signals.The resulting19F NMR spectra identify two states of Gαs protein that likely correspond to the free-binding states S1 and S2.Compared to the 1D19F NMR spectra of Gαs site-specifically incorporation proteins, there is only one19F peak in the 1D NMR spectra of Gαi351 tfmF.The difference in the19F NMR spectra of Gαs and Gαi proteins is probably due to the Gαi351 located at the C-tail ofα5 helix in Gαi containing a total of 354 amino acids,which is highly flexible and the two free-binding states S1/S2 are in fast exchange timescale resulting only one sharp peak on the NMR spectrum.
Recently, nanodisc has been widely used for structural and functional studies of membrane proteins [35].Membrane scaffold protein MSP1D1 and the lipid mixture of 1-palmitoyl-2-oleoylsn-glycero-3-phosphocholine (POPC) and 1-palmitoyl-2-oleoyl-snglycero-3-phospho-(1′-rac-glycerol) (POPG) were used to assemble the nanodisc with the suitable diameter for titration analysis (Fig.S2 in Supporting information).May due to the invisibility of giant size Gα-nanodisc complex, we could mainly observed the gradually peak intensity decreases of Gαprotein titrated with nanodiscs from the19F NMR spectroscopy (Fig.S3 in Supporting information).Fortunately, we observed a new broad peak on the Gαs376 tfmF spectrum titrated with nanodisc, indicating the new Gα-nanodisc complex state (Fig.S3a in Supporting information).In order to determine the dynamic process of Gαprotein interacting with plasma membrane, we use micelle composed of small molecule detergents mimicking membrane to monitoring the conformational changes of different Gαproteins compared of their apo state.The mixed micelles ofn-dodecyl-β-D-maltoside (DDM) and cholesteryl hemisuccinate (CHS), with DDM/CHS (2:1), was gradually titrated into Gαprotein.The structural changes that occurred upon the addition of detergent to the Gαprotein were performed using19F NMR.Interestingly, the19F NMR spectrum of Gαprotein changes accompany with the detergent concentration increases.Starting with the two weakly peaks on the spectrum of Gαs376 tfmF, addition of the detergent resulted in the appearance of a broad line shape signal peak, indicated that the rate of chemical exchange between S1 and S2 has increased induced by detergent monomers (Fig.2a).Subsequent addition of an excess of the detergent made the peak shape of the resonance sharp and the signal stronger.Therefore, it can be reasonably deduced based on the peak shape and intensity, there covered another new state of Gαs376 tfmF apart from S1-S2, which we referred as state S3.Since the new broad peak of Gα-micelle complex state shows the same chemical shift with Gα-nanodisc complex, we consider that the mixed micelles could mimic the native plasma membrane in our case (Fig.S3b in Supporting information).Similarly, as detergent is gradually added to the Gαs383 tfmF, the broad line shape getting sharper at low detergent concentration, indicating faster chemical exchange of state S1 and S2.The binding of detergent micelles promotes the generation of equilibrium between S1 and S2, driving the Gαs from S1-S2 towards S3 (Fig.2b).In addition, observation of the19F NMR signals originating from Gαi provided similar results to those obtained from observation of Gαs.In contrast to Gαs376 tfmF and Gαs383 tfmF,addition of the detergent micelles resulted in a pronounced shift towards S3 for Gαi351 tfmF, making position of the NMR peaks is clearly separated from the S1-S2 (Fig.2c).Collectively, detergent will accelerate the rate of equilibrium between S1-S2 and induce a new state S3, which is associated with the membrane interaction.This state probably represents the transition state before the G protein and receptor interaction.

Fig.2.19F NMR spectra of the Gα s or Gα i in the apo-form or with increasing amount of detergent: Detergent titration of (a) Gα s376 tfmF, (b) Gα s383 tfmF, and(c) Gα i351 tfmF.The concentration of detergent increases from top to bottom (0,0.04%, 0.08%, 0.12%, 0.2%).The transition states S3 was indicated by cyan dash lines.
To characterize the dynamic properties of different G proteins coupling to GPCR, we acquired19F NMR spectra of Gαproteins bound toβ2AR in the presence of the BI167107, an ultra-highaffinity full agonist that increase basal Gαs coupling activity [36].Due to the activation of theβ2AR results in TM6 movement that lead to a receptor-binding site for intracellular effector or regulatory proteins, a given concentration of agonist is added throughout the experiment [37].We observed spectral changes in Gαprotein upon detergent binding, and further changes were observed followingβ2AR coupling (Figs.3 and 4).No significant chemical shift changes of Gαs376 tfmF was observed uponβ2AR binding.However, the interaction withβ2AR slow down the global tumbling of Gαs376 tfmF leading to a slightly broad line shape of the NMR peaks (Fig.4a).This shows that Gαs376 tfmF has produced a receptor-binding conformation, named state S4.Moreover, the Gαprotein andβ2AR were indeed coupled into a complex which was verified through size exclusion chromatography (SEC) (Fig.S4 in Supporting information).
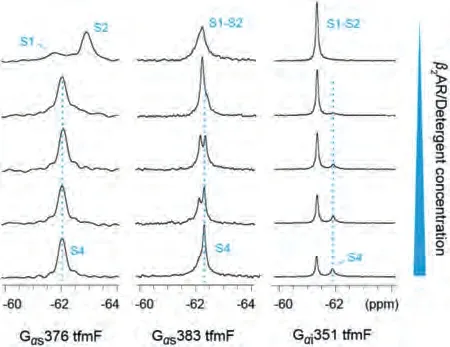
Fig.3.19F NMR spectra of the Gα s and Gα i in the apo-form or with increasing amount of β2AR/detergent.Five 1D 19F spectra for each mutant were recorded with Gα:β2AR ratios of 1:0, 1:0.25, 1:0.5, 1:0.75 and 1:1.

Fig.4.19F NMR spectra of the Gα s and Gα i in the apo-form or in the presence of detergent, β2AR/detergent respectively: (a) Gα s376 tfmF, (b) Gα s383 tfmF and (c)Gα i351 tfmF.Compared with adding detergent alone (the second row), the widths and intensity of the NMR peaks for Gα proteins were slightly changed in the presence of the β2AR (the third row).
In the presence of theβ2AR, the signal peaks representing S1-S2 of Gαs383 tfmF disappear almost completely.The newly upfield peak almost completely replaces the downfield peak, illustrated the population of states shifted towards the binding state S4 at the expense of S1-S2 and S3.Similar to Gαs383 tfmF, upon coupling toβ2AR we see the Gαi351 shifted the S1-S2 equilibrium towards the S4 state by an increment in intensity comparing with the Gαi-detergent binding state (Fig.4c).However, same amount ofβ2AR and detergent can only induce a small fraction ofβ2AR-Gαi complex (~36% based on the integrated peak area).This may be due to the dynamic nature of the Gαi351 tfmF that a small fraction of Gαi351 existing in a state is capable of coupling with theβ2AR, which confirmed the weak coupling specificity ofβ2AR-Gαi complex.In summary, except for the widths and intensity of the NMR peaks, chemical shift of Gαprotein remain practically unchanged after coupling to theβ2AR compared to detergent binding state and confirmed that there may be a shallow energy barrier between S3 and S4, allowing rapid chemical exchange of the two state populations.In this study, the relatively weak interaction between Gαi351 andβ2AR was detected using19F NMR spectroscopy(Fig.4c).Compared with Gαs376 and Gαs383, less population of Gαi351 could form complex S4 states withβ2AR.These differences in one-dimensional NMR spectra consistent with the result of a previous study thatβ2AR can interact with both Gαi and Gαs and it may have a preference over coupling Gαs.
As noted above, the tfmF was site-specific incorporated into Gαproteins with high fidelity and used to monitor G proteinmembrane interactions and G protein-receptor binding conformational changes.In 1D NMR, each peak defines a given conformation or state.Our data suggests that Gαproteins adopt allosteric conformational changes upon interacting with membrane and coupling with GPCRs (Fig.S5 in Supporting information).One-dimensional19F NMR studies provide evidence for conformational heterogeneity as we observe more than one peak for F376, I383 in apo Gαs and C351 in apo Gαi.Previous study showed that the Gαprotein was anchored on the cell membrane through palmitoylated posttranslational modification.Based on our data, the C-terminalα5 helix also involve in the Gα-membrane interaction.The19F NMR spectra of Gαs and Gαi in the presence of detergent may represented the membrane binding state of Gαs and Gαi, which is ready to coupling to GPCR.The membrane interactions with Gαsimulated by nanodisc or micelle both induce a transition from the equilibrium state of the two free conformations to the membranebinding state, which may be similar to the transition state before the G protein coupling to the receptor.Combine the results of Gα-β2AR coupling, we suspect that the interaction with membrane may be indispensable for the activation of different Gαproteins.These data provide evidence for the importance of G protein and membrane interaction for GPCR coupling.Additionally, we captured the protein conformational changes and differences in the interactions betweenβ2AR -Gαi and theβ2AR-Gαs complex by monitoring of the19F NMR chemical shifts.The19F NMR may thus prove to be a convenient and effective tool for the studies of dynamic process of GPCR-G protein signaling pathway .
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the National Key Research and Development Project of China (Nos.2019YFA0904100 and 2017YFA0505400), the National Natural Science Foundation of China (Nos.22077117 and 31971152) and the USTC Research Funds of the Double First-Class Initiative.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.07.042.
 Chinese Chemical Letters2022年2期
Chinese Chemical Letters2022年2期
- Chinese Chemical Letters的其它文章
- Comment on “Acid-induced tunable white light emission based on triphenylamine derivatives”
- Strategies for efficient photothermal therapy at mild temperatures:Progresses and challenges
- Liposome-based delivery of biological drugs
- Macrophage-targeted nanomedicine for chronic diseases immunotherapy
- Advances, opportunities, and challenge for full-color emissive carbon dots
- Fluorine-containing agrochemicals in the last decade and approaches for fluorine incorporation
