Washing-free chemiluminescence immunoassay for rapid detection of cardiac troponin I in whole blood samples
Hun Zho, Enben Su,*, Li Hung, Yunfeng Zi, Yun Liu, Zhu Chen, Song Li,Lin Jin,,*, Yn Deng,*, Nongyue He,,*
a State Key Laboratory of Bioelectronics, National Demonstration Center for Experimental Biomedical Engineering Education, School of Biological Science and Medical Engineering, Southeast University, Nanjing 210096, China
b Getein Biotechnology Co., Ltd., Nanjing 210000, China
c Hunan Key Laboratory of Biomedical Nanomaterials and Devices, Hunan University of Technology, Zhuzhou 412007, China
ABSTRACT Chemiluminescence immunoassay (CLIA) has always been a great challenge in detecting cardiac troponin I (cTnI) in whole blood samples without centrifugation because of the interference of red blood cells and low sensitivity.In this study, the antigens and erythrocytes in the blood were captured by the antibodies immobilized on the magnetic particles, recognized by another biotinconjugated cTnI antibody and detected by streptavidin/acridine aster-conjugated polychloromethylstyrene microspheres (PCMS).After magnetic separation, the supernatant was transferred and measured.No significant difference was noted between the cTnI concentrations of the serum samples,plasma samples and whole blood.The prepared PCMS provided more functional areas to conjugate streptavidin and acridinium ester, so the immunoassay has highly sensitive, the limits of blank at 0.012 ng/mL, and functional sensitivity at 0.019 ng/mL with a CV of 20%, and 0.058 ng/mL with a CV of 10%.Total precision of any sample type ranged from 2.62%~5.67%.The assay was linear over the studied range of 0.01–50.00 ng/mL, and no hook effect was found when cTnI concentrations reached 1900 ng/mL.No significant interference was noted with the potential endogenous interfering substances.Compared with the commercial kit (Abbott assay kit), the correlation coefficient was 0.9859.A washing-free CLIA was established for the rapid detection of cTnI in human whole blood, using erythrocyte capture antibodies-conjugated magnetic nanoparticles for eliminating the influence of erythrocytes and PCMS for signal amplification, which showed great potential in clinical application.
Keywords:Washing-free Whole blood Chemiluminescence immunoassay Polychloromethylstyrene microspheres
In recent years, the main cause of death and attack is cardiovascular disease worldwide [1].Biomarkers are the most commonly used indicators in screening patients with acute myocardial infarction (AMI).When stress or myocardial injury leads to cardiomyocyte necrosis, cardiac troponins expressed exclusively in cardiomyocytes are released into the circulation [2].Therefore, the accurate determination of cTnI is the preferred biomarker for the diagnosis of AMI or emergency patients with suspected symptoms and signs of AMI presenting to the emergency department [3,4].To detect a low concentration of cTnI in patients with early myocardial necrosis, the experts recommended that the assays with high analytical sensitivity are considered as high-sensitivity cTnI (hscTnI) [5].According to the recommendations, low concentration precision and limits of the assay are the two most critical performances of hs-cTnI [3-7].Various analysis techniques for cTnI detection have been developed to improve precision and sensitivity, including enzyme-linked immunosorbent assays (ELISA) [8], CLIA [9],and lateral flow immunoassays (LFIA) [10,11].With the rapid development of signal amplification strategy and magnetic nanoparticles technology, CLIA has become the most ideal method for the detection of hs-cTnI [9,12-14].
For the sample types used for cTnI assay, the whole blood samples without any pre-treatment are more preferred than serum or plasma because of a short reporting time [4,15-17].Shortened sampling and outcome times will lead to rapid medical decisions when treating patients with symptoms of AMI and have a significant positive impact on their lives.Conventional approaches for cTnI CLIA detection, including those of the world’s major manufacturers, are usually complex, expensive and time-consuming and do not support the whole blood type.Therefore, the development of a new method for simple, rapid, sensitive and whole blood samples supported detection of cTnI is highly desirable.In comparison with these clinically used methods, the established immunoassay has a greater clinical application prospect due to its sensitive and convenient washing-free advantages.Meanwhile, wash-free CLIA immunoassays based on signal amplification for highly sensitive detection of cTnI in whole blood have not been reported.In this study, a cTnI assay with whole blood samples was established, and the assay duration between sampling and the result reporting was 15.6 min, which met the needs of the central laboratory to initiate effective, evidence-based medical management and revascularization [18,19].
To shorten the detection time and maintain high sensitivity in this study, a sandwich washing-free CLIA applying cTnI/erythrocyte antibodies-conjugated magnetic nanoparticles in combination with streptavidin/acridinium ester-conjugated PCMS (SA/AE-PCMS) for further signal amplification was performed, and it is schematically shown in Fig.1.To eliminate the need for washing steps, the change of the chemiluminescence intensity of the supernatant of the reaction solution was measured to indicate the concentration of the analyte in the sample.However, the sensitivity of the change rate of the supernatant was insufficient compared with that of the magnetic bead precipitation.Employing PCMS to provide a larger specific surface area, the labeling efficiency was greatly improved,so that the change rate of the supernatant was enough to indicate the concentration of the analyte.Secondly, the erythrocytes in the whole blood samples have a great influence on the detection intensity of chemiluminescence.The application of erythrocytes antibody-coated magnetic nanoparticles makes the erythrocytes in the supernatant almost invisible to the naked eye.Finally,the low sensitivity of the supernatant and the interference of red blood cells was improved, and the washing-free CLIA method could be easily realized.The analytical performance of cTnI was evaluated according to Clinical Laboratory Standards Institute (CLSI)documents on the Getein MAGICL6000.
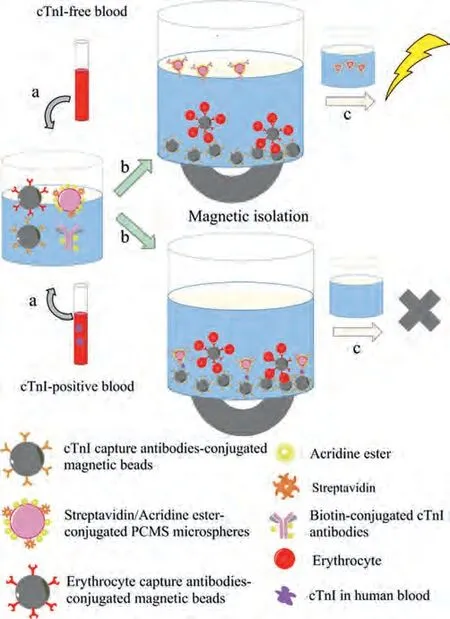
Fig.1.Schematic illustration of a washing-free CLIA for rapid detection of cTnI in whole blood samples.(a) The sample was added to the reaction cup containing cTnI/erythrocyte antibodies-conjugated magnetic nanoparticles, biotin-conjugated cTnI antibodies, and streptavidin/acridine aster-conjugated PCMS reagents.(b) After incubation for 10 min at 37°C, the analyte was sandwiched between the capture antibodies-magnetic nanoparticles and PCMS, and erythrocytes were captured by the erythrocyte antibodies-magnetic nanoparticles, then magnetic nanoparticles were magnetically isolated together with the analyte-bound PCMS and erythrocytes.However, the PCMS remained suspended in the supernatant when there was no analyte in the sample to form the sandwich complex.(c) The luminous intensity of the supernatant was measured after the supernatant was transferred to a new reaction cup.PCMS in the positive samples were captured by the analyte and magnetic beads, resulting in a low luminescence intensity of the supernatant, while the luminescence intensity of the negative samples was high.
In order to amplify the signal, PCMS were prepared per the published procedure slightly modified [20,21].First, a 500 mL three-neck round bottom flask with a condenser was used as the reaction vessel.200 mL of deionized water was added to the threeneck flask, and a layer of nitrogen was kept over the reaction solution during polymerization after nitrogen was bubbled into the water for 20 min to remove oxygen.50 mg of sodium styrene sulfonate was added to the flask and mechanically stirred at 35°C for 10 min.Then 8 mL of styrene, 0.5 mL of divinylbenzene (DVB,50% in ethyl vinylbenzene), and 1 mL of chloromethyl styrene were placed in the reaction flask, and the mixture was continuously stirred for at 75°C for 20 min.Next, 300 mg of potassium persulfate as the initiator was added to the reaction solution stirred for 50 min.Finally, 1 mL of chloromethyl styrene monomer was added slowly and stirred for 18 h.The prepared microspheres were washed with ethanol for 4 times by centrifuged at 3000g for 20 min, and then stored in water for a short time or freeze dried for a long time.The prepared PCMS were used as a carrier to conjugate streptavidin and acridine ester for further signal amplification,the method of preparation process was described in Supporting information.
We have performed some investigation and biomedical applications of magnetic nanoparticles recently [22-25], especially on bioassays [26-28].The carboxyl magnetic nanoparticles were applied in this study, and the procedures of preparation of cTnI/erythrocyte capture antibodies-conjugated magnetic nanoparticles and preparation of biotin-labeled antibodies can be found in Supporting information in detail.
After obtaining the above products, the immunoassays were all performed by the MAGICL6000 chemiluminescence analyzer, and the assembly of the immunoassay kit were shown in Supporting information.
Firstly, the consistency of different types of samples was studied.Serum (with thrombin-based clot activator and without separator), plasma (K2 EDTA as the anticoagulant), and whole blood(K2 EDTA as the anticoagulant) samples were collected from each patient to evaluate the potential impact of anticoagulants and the blood cells on the measured cTnI concentration.Forty patients (3 different sample types were collected from each patient) with various cTnI concentrations were collected from AMI patients admitted to Nanjing Second Hospital.All of the enrolled patients provided informed consent before undergoing the procedure and this study was approved by Nanjing Second Hospital Research Ethics Committee.Accurate cTnI concentrations in whole blood could be corrected by software calculation of hematocrit values (Hct), following the formula: the whole blood cTnI = measured cTnI/[(1-Hct/100)].
Secondly, to evaluate the analysis performance of the proposed method, the limits of blank (LoB) and functional sensitivity, precision, linearity, high-dose hook effect, effect of potentially interfering substances and comparison study were studied, and the methods of evaluation can be found in supporting information.
The microspheres were characterized by scanning electron microscopy (SEM, JSM-5600LV, Japan) and zeta potential analyzer(Zeta Plus, USA).The SEM image of the prepared PCMS is shown in Fig.2A, and the enlarged image is shown in Fig.2B, both images show a discernible structure with an average size of ~100 nm.The Z-average particle sizes of the PCMS were 116.0 nm, and the polydispersity was 0.005.Therefore, the particle size distribution confirmed the SEM characterization.To compare the labeled ratio of acridine ester to streptavidin between direct labeling and indirect labeling amplified with PCMS, the RLUs of acridinium ester labeled streptavidin were measured at the same protein concentration, and the RLU curves were shown in Fig.2C.The amount of proteins(streptavidin) coated to the microspheres was measured by the BCA protein assay kit.The concentration of acridinium ester in the PCMS was calculated by the acridinium ester standard solution-RLU standard curve.Considering the molecular weight of streptavidin (MW: 66KD) and acridine ester (MW: 740.85), the acridinium ester labeling efficiency (the molar ratio of acridinium ester to streptavidin) of the PCMS amplified method was about 12.35, and that of the direct labeling method as the control was 2.55.The proposed labeling method improves the efficiency of acridinium esterlabeling by about 5 times, which is essential to improve the analytical sensitivity.Erythrocyte antibodies coated magnetic nanoparticles were applied to capture erythrocytes in the whole blood samples to eliminate the influence of erythrocytes on immunoassay.As shown in Fig.2D, the relatively large amounts of whole blood and erythrocyte antibodies coated magnetic nanoparticles are used to distinguish the change of turbidity in solution with the naked eye.
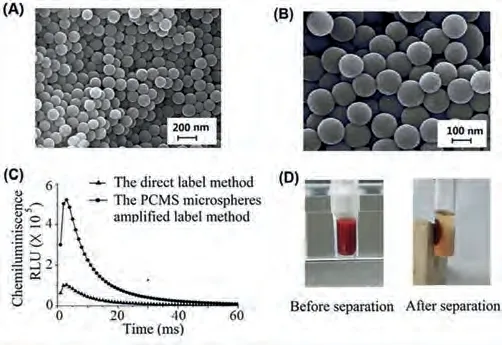
Fig.2.Characterization of PCMS and erythrocyte antibodies-conjugated magnetic nanoparticles.(A and B) SEM photomicrographs of PCMS.(C) The chemiluminescence intensity (RLU) of acridinium ester by direct label method and PCMS amplified label method was measured by MAGICL6000 immunoassay analyzer.(D)Demonstration of the washing-free immunoassay based on erythrocyte antibodiesconjugated magnetic nanoparticles.Before magnetic isolation, the reaction solution was affected by the whole blood sample and was very turbid.After magnetic isolation, erythrocytes were separated by magnets and the supernatant became very clear.
A matrix comparison study was performed using serum,plasma, and whole blood samples.Serum reference tubes, EDTA plasma, and EDTA whole blood tubes were used.40 blood donors were tested using each sampling tube was spiked with cTnI range from 0.026–48.51 ng/mL.No significant difference was noted between the cTnI concentrations of the serum samples and plasma samples or EDTA whole blood samples as shown in Fig.S1 (Supporting information).
The cTnI assay needs to be highly sensitive to become a preferred biomarker for the diagnosis of heart attacks.Following Clinical Laboratory Standards Institute (CLSI) EP17-A2-Evaluation of Detection Capability for Clinical Laboratory Measurement Procedures; Approved Guideline—Second Edition, LoB and functional sensitivity of the proposed method were studied.A representative standard curve (RLU values against cTnI concentrations of 0.01–50 ng/mL) was successfully obtained as shown in Fig.3A.It showed negative slopes with increasing cTnI concentrations, and the square of the correlation coefficient was 0.9997 indicating good linearity.LoB was determined to be 0.012 ng/mL (M-2SD,n= 20), and the functional sensitivity was determined to be 0.058 ng/mL with a CV of 10%, and 0.019 ng/mL with a CV of 20% as shown in Fig.3B, which showed high accuracy in low concentration detection of cTnI.
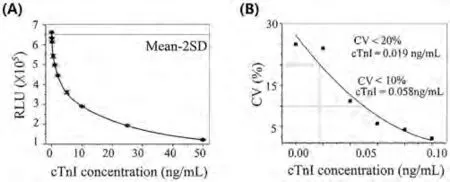
Fig.3.The sensitivity measurements of the proposed method.(A) Standard curve of chemiluminescence intensity (RLU) vs. the concentration of cTnI, with the fitted curve showing the LoB of the immunoassay.(B) The functional sensitivity of cTnI assay at 10% CV and 20% CV.
The determination of an analyte concentration at very low concentrations in a blood sample requires both precision and accuracy.Six samples (including two serums, two plasmas, and two whole blood) were tested in duplicate on 20 different days (2 runs per day) with the established method using MAGICL6000 chemiluminescence analyzer.The intra and inter-day precisions were calculated based on recommendations from the CLSI EP 5-A3 document as shown in Table S1 (Supporting information).Total precision of serum samples for various cTnI concentrations ranged from 2.62%to 4.74%, plasma samples ranged from 2.67% to 4.94%, and whole blood ranged from 3.10% to 5.76%.
Sensitivity is closely related to precision since sensitivity is the concentration of the analyte below which imprecise is unacceptable.The imprecision of the measurement method is caused by the combined influence of several sources of variation, mainly determined by manipulation errors, separation, washing, detection, and antibody characteristics.In conventional magnetic immunoassay,the washing process of immune complexes on magnetic nanoparticles is the main factor leading to imprecision.In the improved washing-free CLIA, the supernatant was directly tested after magnetic separation, which avoids the loss and error in the process of magnetic bead washing.The results showed that the improved CLIA without washing process in the presence of PCMS and erythrocyte antibody-coated magnetic nanoparticles can be applied to quantify cTnI in a variety of human sample types with excellent precision.
165 samples collected from the Second Hospital of Nanjing were analyzed to further assess the feasibility of the established method for clinical application.A comparison study was performed between our method and the clinical method (Abbott kit assay) in Fig.4.The linear regression equation and the square of the correlation coefficient were given according to the comparison data:y= 1.0067x– 0.0569,R2= 0.9859.The Bland-Altman analysis showed that the mean absolute bias was –0.0124 ng/L (95% CI, –0.19 to 0.17), and the mean percentage bias was –8.2% (95% CI,–13.1% to –3.1%), suggesting a good consistency with the control reagent.Therefore, our washing-free CLIA could be used as a clinical examination assay to detect cTnI in real samples, human blood,with excellent precision and high sensitivity, showing a good application prospect for early diagnosis of patients with AMIs.
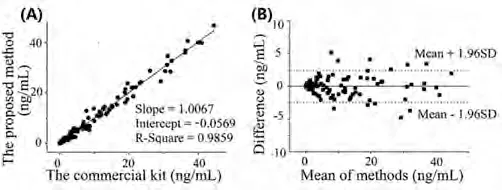
Fig.4.Comparison study between the washing-free CLIA and the clinical method(Abbott assay): (A) Correlation analysis.(B) Bland-Altman analysis.
In conclusions, a washing-free assay for the detection of cTnI in human whole blood based on cTnI/erythrocyte antibodiesconjugated magnetic nanoparticles and streptavidin/acridine asterconjugated PCMS was presented.To reduce the analysis time a washing-free assay was employed to test whole blood samples,including sample addition, incubation, magnetic separation, and measurement of supernatant.In terms of whole blood analysis, it was found that erythrocyte antibodies coated magnetic nanoparticles could be used without effect on test results and PCMS amplified label method could significantly improve the sensitivity.This strategy presents an excellent platform for CLIA that allows direct analysis of whole blood samples without washing steps, improving the detection efficiency by reducing operation steps for the nonprofessionals.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (Nos.81902153, 61871180, 62071119 and 61971187) and Jiangsu Provincial Key Research and Development Program (Nos.BA2020016 and BE 2018695).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.07.017.
 Chinese Chemical Letters2022年2期
Chinese Chemical Letters2022年2期
- Chinese Chemical Letters的其它文章
- Comment on “Acid-induced tunable white light emission based on triphenylamine derivatives”
- Strategies for efficient photothermal therapy at mild temperatures:Progresses and challenges
- Liposome-based delivery of biological drugs
- Macrophage-targeted nanomedicine for chronic diseases immunotherapy
- Advances, opportunities, and challenge for full-color emissive carbon dots
- Fluorine-containing agrochemicals in the last decade and approaches for fluorine incorporation
