Nanosized zinc oxides-based materials for electrochemical energy storage and conversion: Batteries and supercapacitors
Tingting Wei, Nn Zhng,c, Yurui Ji,c, Junhong Zhng, Ynrong Zhu,c,Tingfeng Yi,c,d,**
a School of Materials Science and Engineering, Northeastern University, Shenyang 110819, China
b Shandong Provincial Key Laboratory of Chemical Energy Storage and Novel Cell Technology, College of Chemistry and Chemical Engineering, Liaocheng University, Liaocheng 252059, China
c School of Resources and Materials, Northeastern University at Qinhuangdao, Qinhuangdao 066004, China
d Key Laboratory of Dielectric and Electrolyte Functional Material Hebei Province, Qinhuangdao 066004, China
ABSTRACT Transition metal oxides (TMO) bring a novel direction for the development of energy store materials due to their excellent stability.They not only have high capacity and good cycle performance, but also are cheap and easily available.Zinc oxide (ZnO) as an important part of TMO have gradually attracted attention in the research of electrochemistry.ZnO, as a metal semiconductor with the advantages of wide band gap, possesses high ion migration rate, good chemical stability, simple preparation and low cost, and is widely used in various fields.However, poor conductivity, low permittivity and quick capacity decays quickly impede the commercial application of these electrodes.In recent years, in order to improve the structural stability, ion diffusion and conductivity of zinc oxides-based anodes, various strategies have been raised, such as structural design, surface modification and composition control.In this paper, the recent advances of zinc oxides-based materials for batteries and hybrid supercapacitors (SCs) were introduced.We comprehensively reviewed the prepared process, reaction mechanism and electrochemical performance and discussed the shortcoming of zinc oxides-based nanomaterials.In particular, several insights toward the future research development, practical applications and commercialization of energy storage devices are also proposed for improving the performance of zinc oxides-based materials.
Keywords:Zinc oxides-based materials Battery Supercapacitors Nanomaterials Hierarchical structure
1.Introduction
Problems including the exhaustion of traditional fossil energy and environmental pollution become cumulatively serious in recent years [1,2].Sustainable and renewable green energy sources,including traditional energy storage systems such as supercapacitors (SCs) [3,4] and rechargeable batteries [5–8], are one of the key solutions to mitigate environmental problems caused by the escalating energy crisis and the consumption of fossil fuels [9–12].Electrochemical energy storage has been a key technology in energy storage adhibitions such as electric vehicles, portable electronic devices and power grid energy storage [13–15].
The lithium-ion batteries (LIB) are fascinating energy storage equipment account for their relatively high energy density and excellent cycling capability [16,17].To further meet requirements of enhancing energy density, novel electrode materials are required with higher specific and volume capacities [18–20].At present, the cost of LIBs prevents it from being used in electric cars and renewable energy storage, result from the small and uneven amount of lithium metal in the earth’s crust.Since the discovery of the sodium ion batteries (SIBs), the research team has become increasingly powerful [7,21,22].Metal sodium is a relatively abundant element in nature, additionally, their electrochemical working mechanism is similar to LIBs [5,23–25].Therefore, sodium-ion batteries are more hopeful for commercialization than LIBs [26].However,the radius of Na+is about 70% larger than Li+ions, which gives rise to the mechanism of sodium ion insertion and storage scientifically challenging [27–30].
Scheme 1.Various modification strategies of ZnO-based materials.All insets come from the literatures.
Supercapacitors, as an important energy storage device, exhibit fast energy delivery [31,32], high power density [33,34] and a longlife cycling behavior [35,36], which have attracted extensive attention and can be widely utilized in portable commercial electronic equipment, electric vehicles and backup power systems [37–39].However, they face significant challenges in industrial applications account for their lower energy density than LIBs, SIBs and fuel cells[40,41].To sum up, it is urgent to design efficient electrode materials for supercapacitor with high energy density, high power density and prominent stability, and rationally utilize renewable energy to meet the increasing energy demand [42–45].
As one of the most important electrode materials for supercapacitors, carbon materials including [46,47], mesoporous carbon[48,49], carbon nanotube [50,51] and graphene [52,53] have attracted extensive attention account for their strengths such as large surface area, prominent electrical conductivity, remarkable cycling stability and cheap [54].But each of these carbon materials has many disadvantages.For instance, though AC is electrochemically stable and cheap, its main shortcoming is the low conductivity and the low utilization of the pore structure [4,55].Although its excellent electrochemical properties, graphene is complex and expensive to fabricate [56].Additionally, relatively low specific capacitance cannot meet the growing demand for SCs with high electrochemical properties, which greatly limits their further large-scale applications [57,58].
At same time, the LIBs and SIBs also faced similar predicament.Although carbon materials have many advantages such as high specific capacity, low voltage platform, good cycle performance and low price as the anode electrode of LIBs/SIBs, they are not suitable for application in the field of large-scale energy storage.The work potential of carbon materials is close to the precipitation potential of lithium and sodium.During high-current charging and discharging, metal dendrites are easy to precipitate on the electrode surface, and the dendrites pierce the separator and cause short circuits in the battery leading to serious safety problems.Moreover,the carbon material easily reacts with the electrolyte during charging and discharging, thereby reducing the compatibility with the electrolyte.Therefore, there is an urgent need to find new highperformance anode materials that can replace carbon materials.
Researching an appropriate electrode material becomes a critical problem for enhancing the electrochemical performance of batteries and SCs.As we known, ZnO has been widely applied in different fields, such as optoelectronic devices, gas sensors and secondary batteries owing to high theoretical capacity, cheap and friendly environment [59].But ZnO materials still suffered from the inherent poor conductivity and severe pulverization during charging/discharging [60–62].Some effective strategies were proposed to improve the performances, such as the design and fabrication of nanorods and nanowires [63]; the modificationviacoating of carbon materials [64,65], metal oxides [66,67] and polymer on the electrodes surface [68].To date, an ongoing increase in publications regarding zinc oxide-based pseudocapacitive materials and battery materials has occurred, but the application of zinc oxidebased materials in the field of supercapacitors and batteries was only touched in passing in few review papers [16,17].Apparently,above review articles do not cover on the new development of zinc oxide-based materials.To our knowledge, a critical review that provides comprehensive coverage and in-depth discussion on the supercapacitors and batteries performance of zinc oxide-based materials has not yet been reported.Considering the in-depth research in this field and the prospect of zinc oxide-based electrode materials for practical supercapacitors and batteries, a key review is very much needed.This article reviewed recent progresses and trends in nanostructured ZnO-based materials, Zn-based bimetallic oxides for batteries and supercapacitors.Some highlighted electrochemical performances were summarized, discussed the facing problem and proposed the novel opinion for future direction and commercialization for zinc oxides-based materials (Scheme 1).We expect that this article will offer a specific perspective for the contruction and optimization of zinc oxide-based electrode materials for supercapacitors and Li(Na)-ion batteries.
2.Fundamental charge storage mechanisms of ZnO-based materials
Asayesh-Ardakaniet al.[69] proposed the lithiation process of ZnO by in situ TEM test.The lithium process of the material consisted of two steps.Firstly, the surface of ZnO began to lithium,then it continued to be lithiumed radially.At the same time, the crystal structure of ZnO changed from single crystal to polycrystalline.The lithification of zinc oxide occurs in two stages [70].Firstly, the intercalation of Li+accompanied by an irreversible conversion reaction of ZnO into Zn (Eq.1).Secondly, a reversible alloying process of Zn with Li occurred (Eq.2).

From the cyclic voltammetry (CV) curves of ZnO/rGO (Fig.1a),a clear reduction peak at ~0.36 V at first cycle indicated the transition of ZnO to Zn and the formation of LiZn alloy and solid electrolyte interphase (SEI) film [71], and the reduction peaks moved to 0.88 V at second cycle [72].The oxidation peaks at 0.21, 0.51,0.66 and 1.34 V are conspicuous, corresponding to the multi-step dealloying process of LiZn alloy [73], and the oxidation of Zn to ZnO occurred at 2.56 V [74].The curves nearly repeated in the succeeding cycles, demonstrating good reversibility and cycling stability of ZnO/rGO.The galvanostatic charge-discharge curves (GCD) of ZnO/rGO exhibited a charge/discharge plateau at 1.30 and 0.45 V in first cycle, respectively, which was in correspondence with the result of CVs (Fig.1b).The ZnO/rGO can provide a discharge capacity of 1636 mAh/g and a coulombic efficiency of 61% in first cycle account for the irreversible formation of SEI [75].
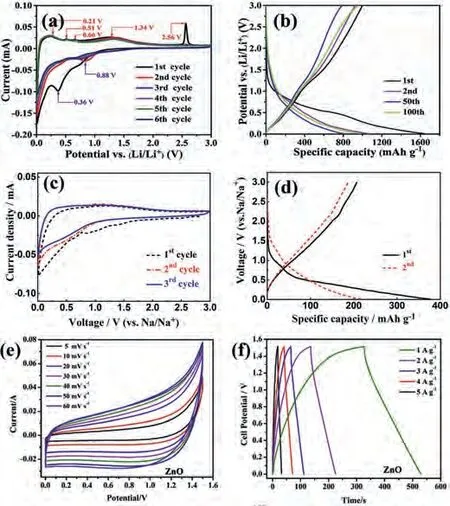
Fig.1.(a) CV curves and (b) GCD curves of ZnO/rGO as an anode material for LIBs.Reproduced with permission [72].Copyright 2020, Elsevier.(c) CV curves and (d)GCD curves of ZnO/rGO as an anode material for SIBs.Reproduced with permission[77].Copyright 2016, Elsevier.(e) CV curves and (f) GCD curves of ZnO nanosphere as electrode material for SCs.Reproduced with permission [78].Copyright 2020,Elsevier.
Similar with LIBs, the transmission of Na+in ZnO-based materials have a same process.Fig.1c showed the CV curves of ZnO/rGO anode for SIBs at the range of 0.01–3 V, the two reduction peaks at ~1.2 V and around 0.75 V were attributed to the reduction of ZnO to Zn and the formation of NaZn alloy and SEI film at first cycle [76].Account for irreversible phase transformation, the reduction peak moved to 0.72 V.The broaden peak between 0.75 V and 1.75 V was described the oxidation of Zn to ZnO and dealloying of NaZn.The ZnO/rGO can offer an initial discharge capacity of about 380 mAh/g at 100 mA/g, according to CV curves, the second discharging capacity have a clear decrease (Fig.1d) [77].
The ZnO-based materials also exhibited an acceptable supercapacitor performance [78,79].Fig.1e shows the CV curves of ZnO nanosphere in KOH solution, which deviates from the rectangular shape revealing pseudocapacitive contribution [80,81].In addition,no oxidation and reduction peaks can be found in CV curves of pristine ZnO, revealing a chemical adsorption/desorption of K+or redox reaction of surface functional groups occurring on the electrode surface [78,79,82,83].The faradaic reaction of pristine ZnO in KOH solution can be summarized as follows:
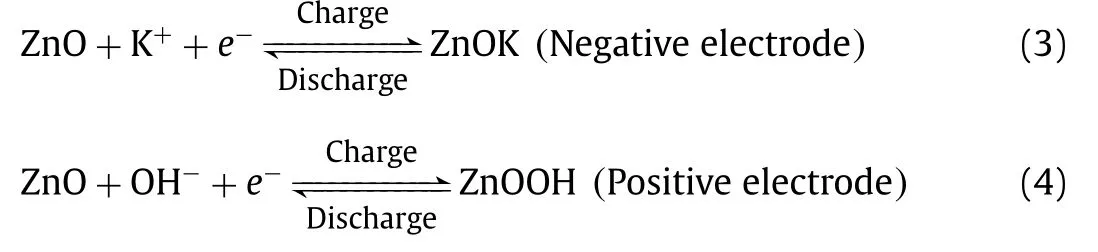
As shown in Fig.1f, all curves exhibit nonlinear shapes, indicating a representative pseudocapacitive characteristic, and the discharge time obviously reduces with the increase of current densities because of the slower diffusion kinetics of ions and insufficient active material participated in reaction at larger current densities[84].
3.Nanostructured ZnO-based electrode materials for energy storage
3.1.Nanostructured ZnO for batteries
When single metal oxides are discovered, they have engaged attention account for their high specific capacity.Over time, people found that oxides also have some shortcomings, such as, poor conductivity, poor cyclic stability [85–87].Of course, zinc oxide (ZnO)is no exception.The performance characteristics of zinc oxide are as follows: nontoxicity, low cost, electrical conductivity (1 S/cm),and theoretical capacity (~978 mAh/g) [88].Nanostructured ZnO can improve the electrochemical performance due to its highly active surface/interface and structure stability.Several ZnO nanostructures, such as nanoparticles [89], nanosheets [90], nanorods[91], nanotubes [92], hollow spheres [93], mesoporous structures[94], hierarchical architectures [95] and thin films, have been synthesized through some synthetic approaches for LIBs, SIBs and SCs.
ZnO nanoparticles were prepared by the hydrothermal–solvothermal followed by heat treat process [96].Figs.2a and bdepicted the ZnO morphology including many nanoparticles connected to each other.The GCD curves of the ZnO were tested at a current density of 1 A/g.The specific capacity weakened from 400 mAh/g to 200 mAh/g after 300 cycles.Huanget al.[97] synthesized porous ZnO nanosheets directly grown on copper substrates through the chemical deposition method thenviathe annealing process.As depicted in Figs.2c and d, the thickness of porous ZnO nanosheets are less than 50 nm and length and width only were several microns.The porous ZnO nanosheets deliver better electrochemical performance than those of commercial ZnO powders.The electrode materials exhibit the first discharge/charge capacities of 1120 and 750 mAh/g at 50 mA/g.Additionally, they also maintain high specific capacity of 400 mAh/g up to 100 cycles at 500 mA/g and display great rate performances (the discharge capacities of 600, 520, 400, 300, 195 mAh/g at 50, 250, 500, 1000,2000 mA/g, respectively).The prominent electrochemical performance of ZnO is partly derived from the structure of porous nanosheets.Wanget al.[98] synthesized the ZnO nanorods through a simple template-free approach.The ZnO array electrode showed the initial discharge/charge capacities of 1461 mAh/g and 980 mAh/g at 0.1 mA/cm2.On the side, they also keep the charge capacity of 310 mAh/g after 40 cycles.The morphology is one the crucial factor affecting the electrochemical properties of materials.It not only was related to specific surface area of the materials but also affected the contact region with electrolyte.
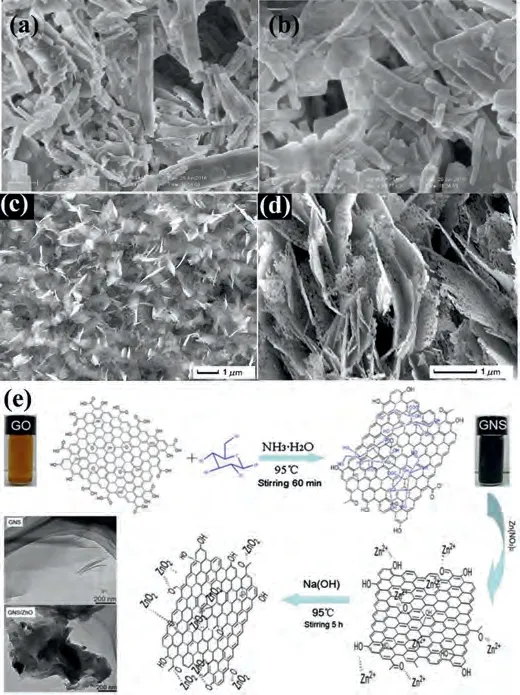
Fig.2.(a, b) SEM images of ZnO nanoparticles.Reproduced with permission [96].Copyright 2017, Elsevier.(c, d) SEM images of porous ZnO nanosheets grown on copper substrates.Reproduced with permission [97].Copyright 2011, Elsevier.(e)The synthesis process and reaction mechanism for GNS/ZnO composites.Reproduced with permission [99].Copyright 2011, Elsevier.
As a single metal oxide, ZnO also has several shortcomings,such as poor conductivity, low capacity and limited cycling performance.In addition to improving the properties by changing the structure mentioned above, the electrochemical performance of ZnO materials can also be effectively enhanced by compounding other materials, such as different carbon materials, metal oxides,metal sulfides and conducting polymers.
3.2.ZnO-carbon materials for energy storage
3.2.1.Nanostructured ZnO-carbon materials for supercapacitor
In recent years, carbon materials have been diffusely applied in energy storage, especially the discovery of graphene has promoted the progress of energy storage equipment.Carbon materials have low capacity and poor safety, but they have excellent conductivity.Therefore, they are widely used to improve the electrochemical performances of other materials.Wanget al.[99] prepared graphene nanosheets/ZnO (GNS/ZnO) compositesviaa green and facile method (Fig.2e).They prepared the GNS and ZnO materials in different proportions for comparison, including 1:3,1:2, 1:1, respectively.The electrode material exhibited specific capacitances of 622 F/g (GNS/ZnO = 1:1) at 5 mA/cm2and theRctof GNS/ZnO were tested through electrochemical impedance spectroscopy measurements.And the specific capacitance maintained 94.9% of the initial capacitance after 2000 cycles.The 3D graphene/ZnO(3DG/ZnO) nanorods composite networks were successfully synthesized through hydrothermal method and chemical vapor deposition (CVD).The 3DG/ZnO electrode displayed a high specific capacitance of 554.23 F/g at 5 mV/s.In addition, it is a prominent cycling stability of 94.40% retention after 2300 cycles[100].
3.2.2.Nanostructured ZnO-carbon materials for batteries
Zhanget al.[101] prepared ZnO nanorods/graphene using a facile hydrothermal reaction and spray drying for LIBs.The ZnO nanorods/graphene materials revealed the initial discharge and charge capacities of ~1600 and ~1000 mAh/g at 0.2 A/g, the discharge capacity decreased 886 mAh/g after 100 cycles.And the rate capacity was exhibited discharge capacities of 938, 835, 718,582 and 508 mAh/g at 0.2, 0,4, 1, 1.6, 2 A/g, respectively.ZnO nanoparticles were anchored on vertically aligned grapheme (ZnOVAGN) through chemical vapor deposition and hydrothermal synthesis approach by Liet al.[65].The ZnO-VAGN materials showed prominent electrochemical performance for LIBs.For instance, the electrode materials gain high discharge/charge capacities of 1560 and 955 mAh/g and the coulombic efficiency of 61.2%.The high reversible capacity could be remained 809 mAh/g after 100 cycles at 80 mA/g.Even at a larger current density of 350 mAh/g, the capacity could remain 451 mAh/g after 50 cycles.And the ZnO-VAGNs also exhibit an excellent rate performance of 201 mAh/g even at 6400 mA/g.
Gaoet al.[102] prepared the ZnO/porous carbon composite materials for LIBs.The electrode materials were fabricated through an in-situ synthesis method utilizing zinc citrate as the precursors.At current density of 100 mA/g, the initial discharge/charge capacities are 1051 and 757 mAh/g, respectively.Even after 60 and 100 cycles, ZnO/porous carbon microspheres still remain the reversible capacities of 561.5 and 557 mAh/g at 100 mA/g.And at different current densities of 0.05, 0.1,0.2, 0.5 and 1 A/g, respectively, the electrode materials exhibit the discharge capacities of 747.8, 544.6, 423.8, 352.6 and 304.0 mAh/g.When restoreding 50 mA/g, the reversible capacity can be restored to ~510 mAh/g.Wuet al.[93] prepared a ZnO hollow spheres with hedgehog-like and triple-shelled though an easy hydrothermal process as Fig.3a shown.And the number of internal multishells can be controlled by the temperature of calcination process.The hedgehog-like ZnO hollow spheres showed an excellent electrochemical performance as Fig.3b, which can exhibit a capacity of about 100 mAh/g at 1 A/g.Tenget al.[103] synthesized ZnO coated amorphous carbon with about 5 nm thickness (C-ZnO)viaa simple hydrothermal process followed by CVD as Fig.3c shown.The C-ZnO anode materials for LIBs can retain a high charge capacity of about 800 mAh/g after 200 cycles at 1 A/g, but Fig.3d revealed the anode was unsatisfactory for SIBs.Due to the slower rate of ions diffusion than lithium and fearful pulverization during cycling, the C-ZnO exhibited only 70 mAh/g after 200 cycles at 300 mA/g, which need more effects to enhance the electrochemical performance for SIBs.Parket al.[104] exploited a new core-shell ZnO@C composites as LIBs anode.As Fig.3e shown, the zeolitic imidazolate frameworks evenly grown on the surface of ZnO, and then the samples were annealed under N2atmosphere to obtain an excellent conductive material with ZnO inserted into nitrogen-doped carbon nanolayer.The ZnO@C showed an ultrahigh initial charge capacity, great cycling stability and rate capacity.When the current density returned to original value from 2 A/g, charge capacity only decreased nearly 4% at rate performance (Fig.3f), due to carbon layer on the surface of sample improved the ions conductivity and interfacial stability.Yanget al.[105] made ZnO nanoparticles evenly distributed on the graphene to synthesized ZnO@graphene composites as LIBs anode materials (Fig.3g).The LIBs full battery was assembled with a pre-lithiation ZnO@graphene anode and commercial LiCoO2(Fig.3h) or LiNi0.8Co0.1Mn0.1O2(Fig.3i) cathode, the former can offer an ultrahigh energy density of ~1480 Wh/kg at 100 mA/g with working potential about 3.8 V.The latter can work at 3.3 V and offered a higher energy density of~1790 Wh/kg and revealed an excellent rate performance.The metal oxides anode was regard as an alternative candidate for commercialized energy storage system owing to pre-lithiation process.
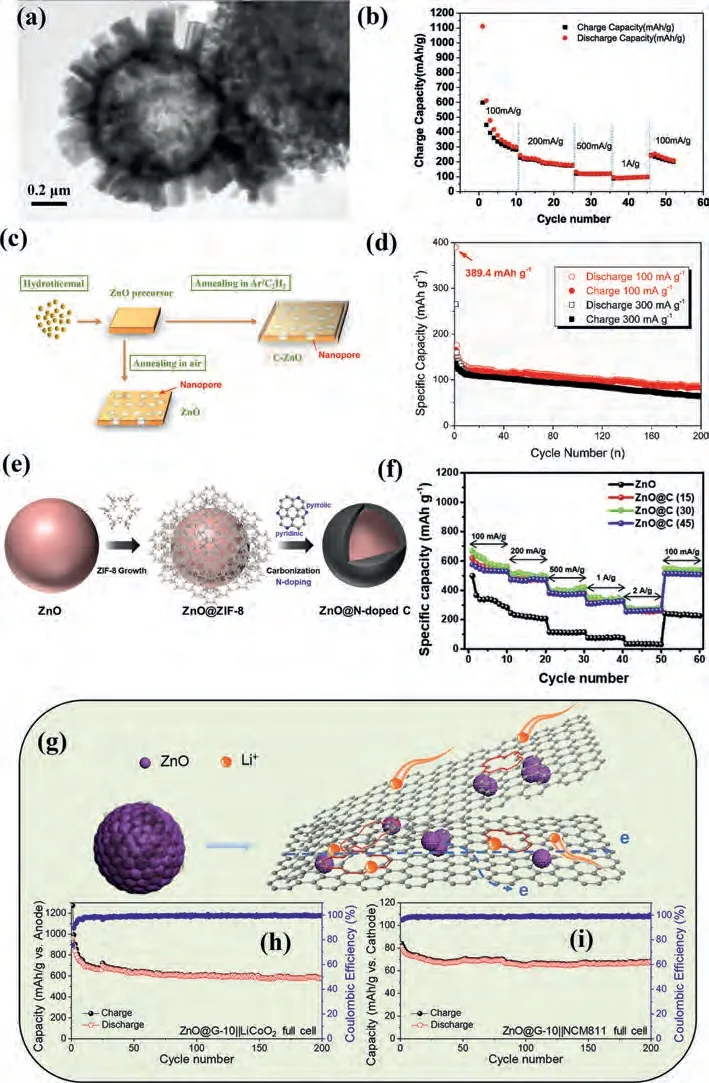
Fig.3.(a) SEM image and (b) rate performance of ZnO/porous carbon composite materials.Reproduced with permission [93].Copyright 2019, Elsevier.(c) The electrochemical principle of C-ZnO and (d) cycling stability for SIBs.Reproduced with permission [103].Copyright 2018, Elsevier.(e) The synthesis and (f) rate capability of core-shell ZnO@C composites.Reproduced with permission [104].Copyright 2019, Elsevier.(g) Preparation process of ZnO@graphene composites, the cycling performance of (h) LiCoO2 and (i)LiNi0.8Co0.1Mn0.1O2 full batteries.Reproduced with permission [105].Copyright 2010, Elsevier.
Although high-performance ZnO anode materials had some progress, it still faced a low reversible capacity at a high current density and poor cycling stability.Designing the reasonable nanostructures and coating carbon layer have been proved was potent ways to enhance batteries performances [106–109], which can shorten the pathways of ion diffusion and increase contact area of interface.Liuet al.[110] utilized Sprite and Fanta as solvent to prepare hierarchical structure called ZnO@SC and ZnO@FC, respectively.As shown in Fig.4a, two samples had a hydrothermal process followed by calcination in Ar atmosphere at 600 °C for 2 h, linked carbon framework can availably increase the conductivity of composites and nanostructure also effectively alleviated pulverization of ZnO-based materials during charging/discharging.The ZnO@SC and ZnO@FC exhibited a high discharge capacity of 1015 and ~1160 mAh/g at 200 mA/g after 200 cycles for LIBs,thank to unique carbon framework and hierarchical construction.The wasted drinks offered a novel probability for improving ZnObased materials.Wanget al.[111] associated electrospinning with water bath to synthesize a free-standing ZnO@carbon nanorod(ZnO@CNF) with hierarchical arrays structure for LIBs (Fig.4b).The CV curves of anode materials were shown in Fig.4c, two distinct reduction peaks can be observed, one at 0.6 V indicated the ZnO was reduced into Zn and formed amorphous Li2O and SEI layer, another at 0.35 V showed the alloying of Li-Al.Accordingly, the three oxidation peaks can be discovered at 0.2, 0.4 and 0.55 V, due to delithiation process of Li-Zn alloy.The segistration of CV curves proved the excellent reversibility and stability of ZnO@CNF for LIBs during charging/discharging.At same time,the Fig.4d also expressed stable cycling of ZnO@CNF, which can provide ~540 mAh/g at 200 mA/g after 150 cycles account for specific nanorod arrays structures and conductive network.Songet al.[112] prepared coating carbon ZnO microspheres utilizing various acid as organic ligands and carbon sources (Figs.4e and f).Among these acids, ZnO microspheres using maleopimaric acid showed best electrochemical performance, which can deliver ultrahigh discharge capacity of ~1050 mAh/g at 0.1 C.The summary of electrochemical performance of ZnO-carbon materials was exhibited in Table 1.

Table 1 The electrochemical performance of ZnO-carbon materials for SCs and LIBs.
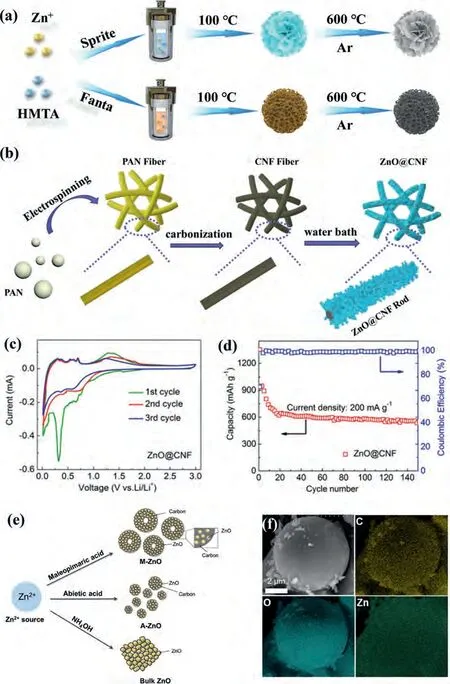
Fig.4.(a) Preparation process of ZnO@C.Reproduced with permission [110].Copyright 2021, Elsevier.(b) Synthesis method, (c) CV curves and (d) cycling stability of ZnO@CNF for LIBs.Reproduced with permission [111].Copyright 2020, Elsevier.(e) Preparation and (f) SEM image of ZnO-based anode.Reproduced with permission [112].Copyright 2020, Elsevier.
ZnO was widely used in energy storage system account for high theoretical capacity, cheap, and environmentally.Whereas, ZnO had the disappointing electrochemical performance including slow reaction kinetics and quick capacity decay account for its severe volume expansion, and low conductivities of electrical and ionic during cycling.To solve these impediments, several methods have been executed to prepare nanostructure ZnO, including nanotube,nanosphere and flower-shape with porous features.Those design can raise the diffusion kinetics of ions and remit structural changes during charging/discharging.Another effective method was coating carbons materials on the ZnO surface, which was a positive try to boost the electrical conductivity, cycling stability, and rate performance of ZnO electrodes.However, the matching hybridization of ZnO materials with the predetermined carbon matrix on a teeny scale was thought to be arduous.Under this circumstance, other modification methods of ZnO electrode also were putted forward.
3.3.Zinc oxide-based nanohybrids for energy storage system
3.3.1.Zinc oxide-based nanohybrids for supercapacitor
NiO has been diffusely researched as alternative materials for energy storage system with cheap, high energy density and power density.Panget al.[113] and Weiet al.[114] investigated that the electrochemistry properties of the ZnO combine with NiO for supercapacitors.They used different synthesis methods to synthesize ZnO–NiO with different morphologies as electrode materials.In 2012, porous ZnO–NiO composite micro polyhedrons were prepared through the heat process of Zn0.9Ni0.1(C2O4)2.nH2O precursors in air.They compared the electrochemical properties of electrode materials in different concentrations of electrolyte.In addition, they also compared the effect of calcination temperature of electrode materials on the electrochemical properties.The P1 (T= 400 °C) exhibits outstanding electrochemistry properties, in which the specific capacitance 649.0 F/g at 5800 mA/g and only reduce 0.9% of this value after 400 cycles in 3.0 mol/L KOH solution.Even at a large current density of 5.8 A/g, the specific capacitance of P1 still remained around 400 F/g.While the specific power enhanced from 1581 to 15,709 W/kg,the specific energy of P1 shorten from 19.9 Wh/kg to 12.1 Wh/kg in similar electrolyte.Additionally, they also synthesized 3D ZnO–NiO mesoporous architecturesviaheat treating the zinc hydroxide carbonate–nickel hydroxide carbonate composite precursor in 2014.In the same way, the specific capacitance of P1 (T= 400 °C)are 2497.8 F/g at 2.6 A/g.Even at 12.6 A/g, the P1 still possess an ultrahigh specific capacitance of 1959.1 F/g far more than P2(T= 450 °C) and P3 (T= 550 °C).The P1 still possessed the 97%retention of first charge capacitance after 2000 cycles at 2.6 A/g.To sum up, the 3D mesoporous electrode material (P1) has the best electrochemical performance in 3 mol/L KOH electrolyte.
Liet al.[115] prepared hierarchical double-shelled NiO/ZnO hollow spheres by a template-free one-step solvothermal approach.The NiO/ZnO hollow spheres showed the electrochemistry properties including that the specific capacitances are about 300 F/g at a large current density of 13.3 A/g.And the specific capacitance raised from nearly 410 F/g to ~480 F/g after 800 cycles, but the next 1200 cycles barely change.Therefore, the NiO/ZnO electrode had an outstanding cycling stability.So as to enhance the electrochemical properties of ZnO, Zhenget al.[116] not only added NiO, but also embedded gold.Fig.5a schematically showed the formation of ZnO@Au@NiO.Primarily, the ZnO nanoarrays directly grew on carbon cloth through a facile hydrothermal approach.Then small quantity of gold nanoparticles was embedded on ZnO nanowires.Finally, with the NiO nanosheets coated on the surface of Au-modified ZnO nanoarrays, ZnO@Au@NiO core-shell hierarchitectures were fabricated (Fig.5b).The electrochemical properties of ZnO@Au@NiO nanocomposites were tested in a threeelectrode system.The CV test results showed that ZnO@Au@NiO nanocomposites have a larger area than ZnO/NiO nanorods in the CVs.GCD curves of ZnO@Au@NiO nanocomposites and ZnO/NiO nanorods in the voltage window of 0–0.5 V at 2 mA/cm2.This suggests that ZnO@Au@NiO nanocomposites possess an excellent electrochemistry property including the areal capacitance of 4.10 F/cm2at 5 mA/cm2and the outstanding cycle performance (loss 19.7%of the initial capacitance after 4000 cycles).Principle of charge transfer between ZnO, Au and NiO during charge and discharge,respectively (Fig.5c).Therefore, embeding Au nanoparticles successfully improve the electrochemistry properties of supercapacitor electrodes.This optimized hybrid electrodes mainly benefited from the increase of electro-electrolyte interface, short electron diffusion path, prominent electrical conductivity.
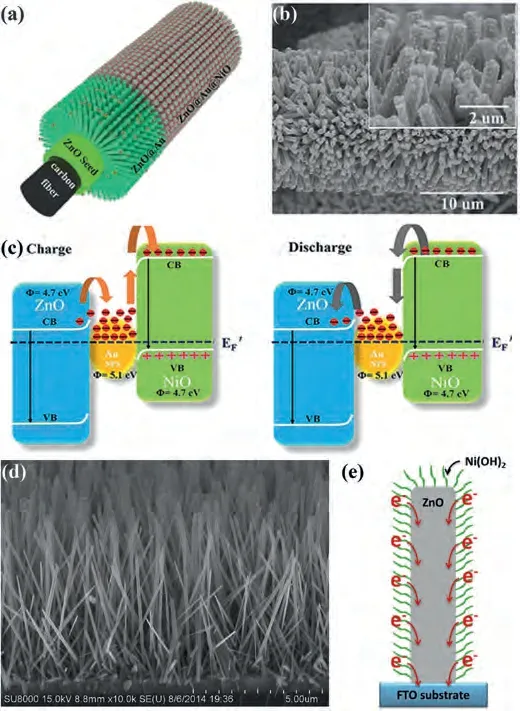
Fig.5.(a) preparation process, (b) SEM image and (c) energy level diagram at the interface during charging/discharging for 3D NiO@Au@ZnO hybrid nanostructure.Reproduced with permission [116].Copyright 2015, American Chemical Society.(d) SEM images and (e) schematic illustration of the electrical pathway of ZnO nanowires.Reproduced with permission [121].Copyright 2016, Elsevier.
In the last few years, the Nickel hydroxide (Ni(OH)2) nanomaterials were widely concerned that combined other electrode materials for supercapacitors and batteries, because Ni(OH)2nanomaterials had a high theoretical capacitance.However, this material also possessed some shortcomings including poor conductivity and low cycle performance.Therefore, it could be restricted at practical application [117].To develop the advantage of the high capacitance of Ni(OH)2, Shiet al.[118] compounded Ni(OH)2with NiCo2S4to improve the specific capacitance of raw materials.Puet al.[119] prepared Ni(OH)2nanoparticles on ZnO array through electrodeposition approach.The ZnO@Ni(OH)2array showed the specific capacitance of 2028 F/g at 10 A/g.Even at 100 A/g, the ZnO@Ni(OH)2array still had a large specific capacitance of 1059 F/g.Additionally, after 500 cycles, the specific capacitance of ZnO@Ni(OH)2array decreased to 1308 F/g at 10 A/g.Shakiret al.[120] also utilized a facile electrodeposition approach to generate Ni(OH)2on the surface of ZnO nanowires.The specific capacitance of Ni(OH)2-coated ZnO nanowires were 3150 F/g at 10 A/g in 1 mol/L LiOH electrolyte.The asymmetric electrochemical capacitors were fabricated that the multiwall carbon nanotubes-textile and Ni(OH)2-coated ZnO nanowires as negative and positive electrode, respectively.In addition, the asymmetric electrochemical capacitors possess an excellent power density of 110 kW/kg at energy density of 54 Wh/kg, and remarkable rate property of 80% initial capacitance retention at current density from 10 A/g to 50 A/g.And the 96% specific capacitance were remained after 5000 cycles.
Loet al.[121] prepared the Ni(OH)2nanoflakes modified ZnO nanowires for supercapacitors through two-step deposition including chemical bath deposition and cathodic deposition as Figs.5d and e shown.The ZnO/Ni(OH)2nanohybrids exhibit an ultrahigh specific capacitance of 1830 F/g at 2 A/g.As electrode materials,the ZnO/Ni(OH)2nanocomposites possessed an energy density of 51.5 Wh/kg at power density of 9 kW/kg.At different current density, the electrode materials always remained the high capacitances of 1830, 1831, 1751 and 1283 F/g at various current density 2, 5,10, 40 A/g.In addition, the cycle performance of the ZnO/Ni(OH)2nanohybrids was reflected that the specific capacitance loss of 20% at 10 A/g after 1000 cycles.Niuet al.[122] adopted a new approach of electrospinning method together with a hydrothermal to fabricate the three-dimensional ZnO@Ni(OH)2core–shell heterostructure.Additionally, they also fabricated the asymmetric supercapacitor with the ZnO@Ni(OH)2and porous carbon microfibers as cathode and anode electrode, respectively.The asymmetric supercapacitor gains a large energy density of 57.6 Wh/kg at 129.7 W/kg.The ZnO@Ni(OH)2electrode materials exhibited the specific capacitance of 2218 F/g at 2 mV/s.Even at 50 mV/s, the capacitance remained 1092 F/g.Moreover, the specific capacitance only loss 6% of original capacitance at 50 mV/s after 2000 cycles.
Ni-based materials including NiO and Ni(OH)2both possess high theoretical capacity.Moreover, compositing Ni-based nanoflakes and nanoparticles could enhance electrolyte permeability.There are more active sites, higher specific capacity and higher rate performance.Therefore, the electrochemistry performances of the ZnO nanomaterials were significantly improved through combined NiO or Ni(OH)2nanomaterials.
As electrode materials, the MnO2nanomaterials were widely applied to energy storage devices account for its advantages such as low cost, abundant reserves, wide operating potential window,high theoretical capacitance (1370 F/g), environmental friendliness.Nevertheless, the actual capacitance of MnO2far from the theoretical value because of its poor conductivity (10-5–10-6S/cm)restricts its electrochemistry performances.The nanocomposites of ZnO with MnO2could perfect reflect the advantages of both.Therefore, MnO2has been studied to increase the specific capacitance of ZnO [123].Huanget al.[124] successfully synthesized the hierarchical ZnO@MnO2core-shell pillar arrays by an easy hydrothermal method (Figs.6a–c).And the supercapacitors were fabricated that the ZnO@MnO2as the binder-free electrode.The binder-free electrodes exhibit the specific capacitance of 423.5 F/g at 500 mA/g.Even at a 10 A/g, the specific capacitance still remained 152 F/g.Additionally, it only losses 8% of the original capacitance at 5.0 A/g after 3000 cycles (Figs.6d and e).
Sunet al.[125] synthesized 3D ZnO@MnO2core@shell branched nanowire arrays through hydrothermal and redox processes.The areal capacitance of 3D ZnO@MnO2core@shell nanocomposites electrode is possessed 31.30 mF/cm2far from other structure.Furthermore, the electrode still possesses 87% capacitance of initial capacitance at 0.02–0.2 mA/cm2.The three-dimensional (3D)MnO2nanowire/ZnO nanorod array composites were successfully synthesizedviaa low temperature solution approach.The MnO2nanowire/ZnO nanowire array nanohybrid has a cap shape at the top compared to acicular ZnO nanowires.This may be formed by MnO2nanowire covering ZnO nanorod.The threedimensional MnO2nanowire/ZnO nanorod array composites electrode exhibited the specific capacitance of 746.7 F/g (areal capacitance 41.5 mF/cm2) at 2 mV/s.And the electrode also displays the specific capacitance that remains 93.5% of the original capacitance at 100 mV/s after 1000 cycles.In addition, the energy density of supercapacitor is 63.1 Wh/kg at power density of 950 W/kg.
Wanget al.[126] have designed the MnO2/ZnO core-shell nanorod array and used it as the anode material to make asymmetric supercapacitors.The volumetric capacitance of the MnO2/ZnO electrode was 0.52 F/cm3at 10 mV/s.When the scan rate increasing 400 mV/s, the volumetric capacitance still was remained more than 70% of the initial capacitance.Additionally, the capacitance only loss 1.5% of the original capacitance in the potential window 0–1.8 V after 5000 cycles.The supercapacitors exhibit the volumetric energy density of 0.234 mWh/cm3at volumetric power density of 0.133 W/cm3.The CNT@ZnO@MnO2nanohybrids were successfully synthesized by Liet al.[127].The fiber-shaped asymmetric supercapacitor (FASC) was fabricated that the CNT@ZnO@MnO2nanohybrids, the CNT fibers and the H2SO4-PVA gel as positive, negative and electrolyte, respectively.The FASC exhibits the energy density of 13.25 μWh/cm2.At different current densities from 0.1 μA to 2 μA, the areal capacities of the CNT@ZnO@MnO2electrode were remained with 386.1, 356.5, 311.7, 285.4 and 253.7 mF/cm3.The capacitance of the CNT@ZnO@MnO2electrode only loss 3.3% of the original capacitance over 1000 cycles.Even after 2000 cycles the capacitance still remained 87.1% of the initial capacitance.After a long charge and discharge test, the charge and discharge curves have no obvious change.Zhaoet al.[128] have synthesized the core–shell ZnO@Au@MnO2nanohybrids on carbon fiber paper (CFP) through a facile approach.As shown in Fig.6f, the fabrication process of the ZnO@Au@MnO2is distinctly displayed.The TEM images of the Au@MnO2nanotube and the ZnO@Au@MnO2nanosheet nanowire were exhibited respectively.The illustrations in these two images correspond to the selected area electron diffraction (SAED) patterns of the composite structures.The specific capacitance of the CFP@ZnO@Au@MnO2electrode is computed about of 623 F/g at 1.1 A/g.Additionally, more than 80% of the original capacitance was retained at scan rate of 50 mV/s over 2500 cycles.Yanget al.[129] successfully synthesized a specific structure of MnO2/ZnO/carbon-fabric (CF)viaa simple three-step-hydrothermal approach (Figs.6g and h).The electrochemistry performances of MnO2/ZnO/CF electrode were investigated that including the specific capacitance of 886 F/g at 2 mA/cm2, even at 100 mA/cm2still possess 50% retention.And the specific capacitance of this electrode was retained about 88.6% of the original capacitance at 10 mA/cm2after 3000 cycles.In addition, the supercapacitor delivered the energy density of 16 Wh/kg at the power density of 27 kW/kg.
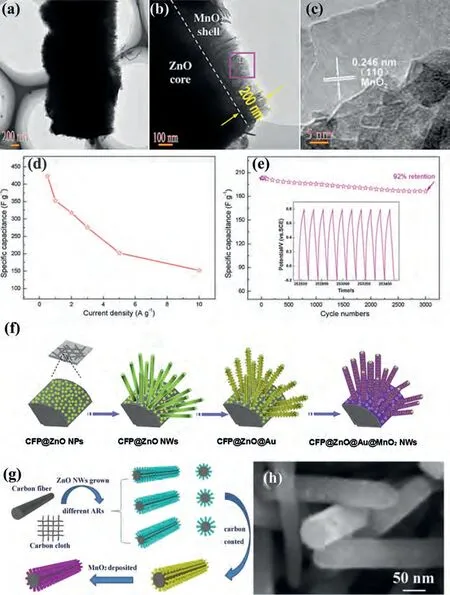
Fig.6.(a, b) TEM, (c) HRTEM image, (d) specific capacitance measured at different current densities and (e) cycling performance of the ZnO@MnO2 core-shell.Reproduced with permission [124].Copyright 2015, Elsevier.(f) The fabrication process for ZnO@Au@MnO2 nanosheet core–shell nanowires on CFP.Reproduced with permission [128].Copyright 2014, Elsevier.(g) The prepared procedure and (h) SEM image of MnO2/ZnO/CF composite nanostructure.Reproduced with permission [129].Copyright 2014,Elsevier.
The Co3O4possess many advantages such as high specific capacitance, environmentally friendliness and low-cost.Therefore,this electrode material has caused extensive attention from all circles.For instance, Donget al.have synthesized 3D graphene/Co3O4as well as exhibited high specific capacitance of 1100 F/g at 10 A/g and outstanding cycling stability.Rajet al.[130] utilized anin-situreduction process of Co3O4-rGO, which delivered the capacitance of 1328 C/g at 2 A/g.Caiet al.[131] investigated unique ZnO@Co3O4core/shell heterostructures to enhance its electrochemistry performance for supercapacitor (Fig.7a).The specific capacitances of the ZnO@Co3O4electrode were 857.7, 759.6,709.2, 677.9, 647.3, 593.8 and 544.2 F/g at 2, 5, 8, 10, 12, 16 and 20 mA/cm2, respectively.In addition, this electrode still remained 830.8 F/g at 6 A/g after 6000 cycles (Fig.7b).
Gobalet al.[132] synthesized the NiO–ZnO/TiO2NTs through the electrodeposition.The NiO–ZnO/TiO2NTs electrode showed the capacitance of 180 mF/cm2at 0.2 mA/cm2, which was about 130 mF/cm2even at 0.45 mA/cm2.On the side, the electrode still remained 92% of original capacitance after ten days.The ZnO@C@NiCo2O4nanorod sheet arrays (NRSAs) were prepared by a simple hydrothermal and electrodeposition approach by Zenget al.[133].The ZnO@C@NiCo2O4NRSA electrode can offer the specific capacitance of 2650 F/g at 5 A/g.Even at 10 mA/cm2, the electrode remained 76% of its specific capacitance after 4000 cycles.
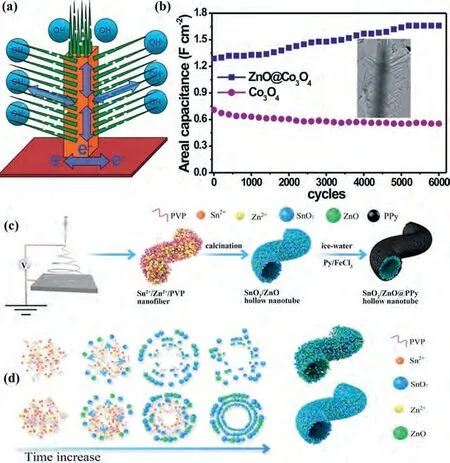
Fig.7.(a) Advantages and (b) cycling performance of the ZnO@Co3O4 electrode for supercapacitors.Reproduced with permission [131].Copyright 2014, American Chemical Society.(c) Prepared process and (d) formation mechanism of SnO2/ZnO hollow nanotubes.Reproduced with permission [139].Copyright 2021, Elsevier.
Overall, TMOs were regarded as promising electrode materials for SCs because of the high theoretical specific capacitance and fast redox reaction.Nonetheless, the inadequate active sites and low electronic conductivity often decrease the actual capacitance of TMOs.Designing heterojunction structures has been regarded as a promising way to resolve these issues because the interface area can be formed by the contact of two (or more) different energy band structures with matching energy band positions [41].ZnO as a fascinating semiconductor material possesses low price, excellent electrical conductivity, good chemical stability and mechanical flexibility.For ZnO-metal oxide composites, ZnO can provide impactful mechanical support and electronic transmission channel.In addition, a favorable synergistic effect and interfacial interaction between ZnO and other components can be formed to generate heterostructures and make the composites possess different functions by an adjustment of physicochemical property of the components.Designing various composites with various architectures between ZnO and other metal oxides can facilitate electrons transfer and ions transport due to the fascinating synergistic effect from multi-phase interface during continuous charge/discharge process of supercapacitors [36].
3.4.Other ZnO-based nanohybrids
3.4.1.Other ZnO-based nanohybrids for supercapacitor
As electrode materials, the conducting polymers possess outstanding conductivity, relatively low cost, and redox properties.So that the electrochemical properties of the composites can be enhanced effectively.The conducting polymers including polypyrrole (PPy), polyaniline (PANI), polythiophene (PTh) and poly(3,4-ethylenedioxythiophene) (PEDOT) were widely utilized in supercapacitors and batteries.
Pandiselviet al.[134] synthesized the ZnO/polyaniline (CSZnO/PANI) ternary nanohybrids through a facile precipitation approach as well asin situpolymerization of aniline.The CSZnO/PANI electrodes exhibit the specific capacitance of 587.15 F/g at 175 mA/cm2.In addition, the specific capacitance of electrode about 80% retention after 1000 cycles.Respectively, the electrode delivered the specific capacitances of 587.15, 541.03 and 421.00 F/g at 150, 175 and 200 mA/cm2.Morenoet al.[135] successfully synthesized the ZnO nanorod arrays@CuS nanocrystalline layer@PEDOT shell layer@MnO2nanoparticles (ZnO NRs@CuS@PEDOT@MnO2) and can deliver the areal capacitance of 19.85 mF/cm2at 5 mV/s.At 50 mA/cm2the specific capacitance was obtained about 12.47 mF/cm2, even at 200 mA/cm2still remaining 11.01 mF/cm2.Additionally, the specific capacitance remains 82% of original capacitance at 100 mV/s after 1500 cycles.
3.4.2.Other ZnO-based nanohybrids for batteries
Yinet al.[136] prepared PPy/ZnO composites through a facile approach.The discharge and charge plateau of the battery with PPy/ZnO interlayer had minor difference.And the ΔEvalue of the cell with PPy/ZnO interlayer was lower than without PPy/ZnO interlayer.In other words, the PPy/ZnO interlayer could decrease the polarization as well as stabilize the discharge plateaus and can deliver the original capacitance of 1194 mAh/g and still remained 579 mAh/g at 0.2 C after 100 cycles.Additionally, the specific capacitances of the battery with PPy/ZnO interlayer were 404 mAh/g at 2 C.When the current density retained 0.2 C, the capacitance remained 770 mAh/g.Then switching back to 0.5 C, the electrode still remained a high specific capacity of 564 mAh/g after 40 cycles.
The conducting polymer of PPy not only possess the advantage of the good electrical conductivity, but also suffer poor cyclic stability due to material expands and contracts seriously during the charging/discharging process for supercapacitors and batteries.As one of the most attractive conducting polymers, the PANI possess some advantages such as high environmental stability, low cost of the monomer, ease of synthesis, good conductivity.Ahmadet al.[137] synthesized hierarchical flower-like ZnO structure with Au nanoparticles.The electrochemistry properties were report that an original discharge capacitance of 1280 mAh/g and a reversible capacity over 392 mAh/g after 50 cycles at 120 mA/g.The Si-Sb-ZnO composites were synthesized through a chemical reduction-mechanical alloying approach by Liet al.[138].They investigated the electrochemical properties of Si-Sb-(ZnO)xcomposite.The results showed that the first charge and discharge capacities were 845.1 and 1301.5 mAh/g, respectively.In addition, the Si-Sb-(ZnO)0.3retained a high specific capacity of 690.6 mAh/g after 200 cycles at 0.1 mA/cm2.After charge and discharge tests at various current densities, the specific capacity of Si-Sb-(ZnO)0.3still remained of 900 mAh/g from 5 mA/cm2to 1 mA/cm2.Zhanget al.[139] using various molecular weight of polyvinylpyrrolidone (PVP) to prepared SnO2/ZnO hollow nanotubesviaelectrospinning followed by calcination process.The higher molecular weight usually caused the smaller diameter and denser structure, which exhibited excellent electrochemical performance.Fig.7c showed in-situ polymerization of PPy on the surface of SnO2/ZnO nanotubes, which enhanced the conductivity and cycling stability.The SnO2/ZnO@PPy anode can deliver a reversible capacity of ~630 mAh/g after 100 cycles at 0.2 C.Fig.7d illuminated the formation mechanism of hollow nanotubes, PVP was resolve caused porous structure and Sn2+/Zn2+transformed oxides with temperature increased.The higher molecular weight of PVP had a lower rate of degradation attribute to denser structure.Li1.4Al0.4Ti1.6(PO4)3is an ideal solid-state electrolyte with high ionic conductivity, low price and excellent safety, while ultrahigh impedance and instability hindered practical application.Haoet al.[140] utilized magnetron sputtering method to prepare a low electronic conductivity solid electrolyte interphase with ZnO layer on the surface of LATP.The ZnO layer cannot only decrease the resistance of interface but also inhibit dendrite growth with enhancing the stability of interface.The Li/ZnO@LATO/LiFePO4full batteries exhibited prominent cycling performance for 200 cycles at 0.1 C.This work provided a reliable way to build a stable interface between solid-state electrolyte and Li metal, which indicated great importance of improving interfacial impedance and stability.
In summary, the ZnO-based composites with core–shell structure need to be constructed, which can be considered as an effective way to improve the electrochemical performance.Both the ZnO core and shell consisted of other TMOs or conducting polymer can participate in electrochemical reaction or enhances the diffusion rate of electrons/ions caused by synergistic effects.Hence, the architecture can offer advantageous synergistic effect due to the combination of the merits of each single component, which decreases the electrochemical polarization and promotes the charge transfer.Nonetheless, the shell thickness often influences the electrochemical performance of ZnO core.Therefore, a suitable thickness of the shell may be further optimized in order to obtain the optimum electrochemical performance.
4.Zn-based bimetallic oxides
As promising candidate anodes, bimetallic oxides possess outstanding theoretical capacity and excellent electrochemistry conductivity, such as Zn2SnO4, Zn2Ti3O8, ZnV2O4, ZnM2O4(M = Co,Fe, Mn), ZnNiSnO4, ZnSnO3.However, there are well known that the large volume changes limit their application.Therefore, these nanomaterials are always combined carbon materials to improve electrochemistry conductivity.Even the electrochemical properties are improved through compounding other superior materials.Doping is another strategy to improve its electrochemical property through enhancing the conductivity of electron and ion in the bimetallic oxide nanoparticles.In SCs system, the charge storage mechanism of Zn-based bimetallic oxides can be ascribed to a chemical adsorption/desorption of OH-ions and redox reaction of other metal ions on the electrode surface.The charge storage mechanism of Zn-based bimetallic oxides can be described the conversion reaction and alloying process.For example, Jinet al.[141] synthesized the Zn2SnO4/activated carbon composites for improving electrochemistry performance through a coprecipitation method.The electrochemistry test demonstrates that the 25% Zn2SnO4(Z-150) possesses a large specific capacitance of 322.6 F/g at 1 A/g.At the current density of 1, 8, 16 A/g, the Z-150 composites retained 96.50%, 91.10% and 81.60% of initial capacitance after 3000 cycles.The pseudocapacitance of Zn2SnO4is ascribed to redox reaction of tin ions and diffusion of OH-, and the electrochemical reaction can be depicted as follows [141].
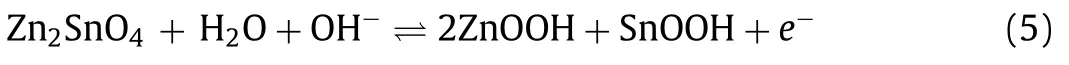
Maggayet al.[142] firstly successfully obtained (Fig.8a) spinel oxide as anode for SIBs by a template-free solvothermal process.The result of XRD revealed the reaction time was critical factor for product acquisition.There were no impurities when the preparation procedure was added to three days.The ZnV2O4anode can cycle at 0.01–3 V and provide an initial capacity of ~540 mAh/g at current density of 50 mA/g (Fig.8b).The reaction mechanism of ZnV2O4can be described as follow [142]:
Discharge process:
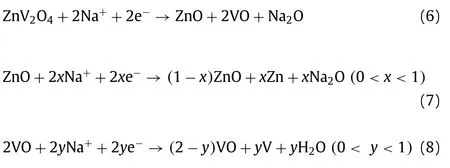
Charge process:

Islamet al.[143] synthesized a novel dandelion-like ZnxCo3-xO4microspheresviacombining co-precipitation method and heat treatment.The structure provided bigger contact area between electrode and electrolyte and ensured the active material was proper utilization.The anode delivered a high discharge of about 610 mAh/g at 0.04 C and retained capacity of~350 mAh/g after 200 cycles.Limet al.[144] prepared high purity Zn2GeO4and Zn2SnO4nanowiresviasimple hydrothermal method, which showed an ultrahigh capacity and good cycling stability.The Zn2GeO4and Zn2SnO4exhibited 1220 and 983 mAh/g after 100 cycles for LIBs and can offer a reversible capacity of about 340 and 310 mAh/g after 100 cycles for SIBs, respectively.When it returned to 0.1 C, the capacity increased to 348 and 340 mAh/g, showing an excellent retention ability for LIBs.The capacity of Zn2GeO4and Zn2SnO4were higher than the theoretical capacity because the conversion reaction attributed a lot.The Li storage mechanisms of Zn2SnO4and Zn2GeO4can be showed as follow [144]:
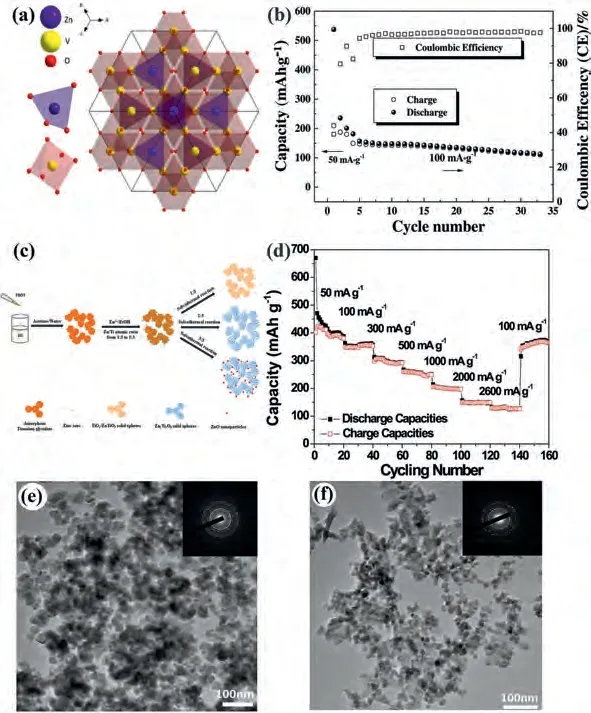
Fig.8.(a) Crystal structure and (b) cycling stability of ZnV2O4.Reproduced with permission [142].Copyright 2018, Elsevier.(c) Prepared process and (d) rate performance of Zn2Ti3O8-x.Reproduced with permission [145].Copyright 2016, Elsevier.(e, f) SEM images of Zn2Ti3O8.Reproduced with permission [146].Copyright 2019, Elsevier.
For Zn2SnO4:
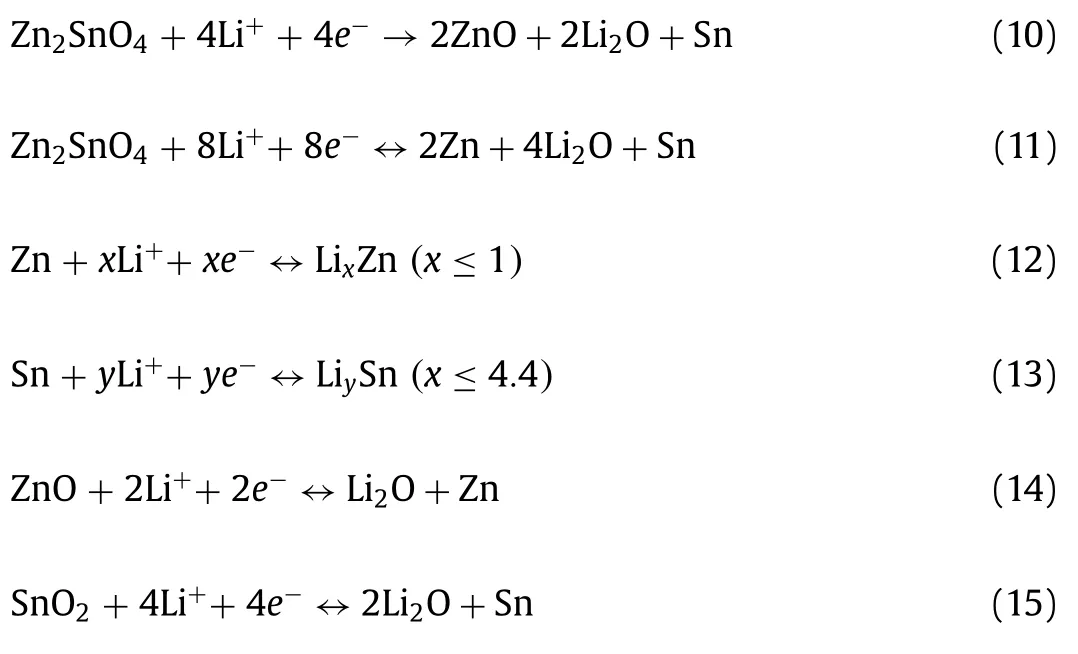
For Zn2GeO4:
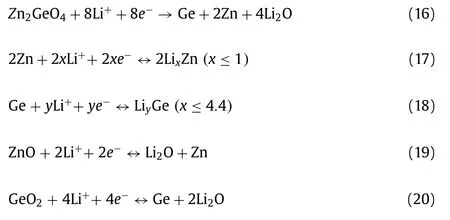
The corresponding Na storage mechanism can be depicted as follows [144]:
For Zn2SnO4:
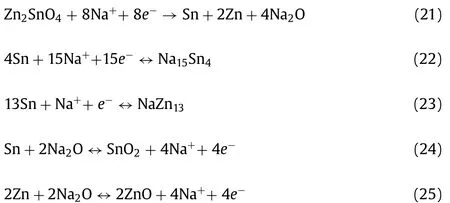
For Zn2GeO4:
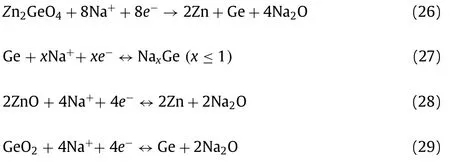
Zinc titanate has received much attention as a feasible material for LIBs anode account for a high theoretical capacity resulting from a high Ti content, good cycling stability and lowcost.Zn2Ti3O8, similar with other Ti-based materials, showed low electronic conductivity and tardy ion diffusion rate.Wanget al.[145] introduced oxygen vacancies to facilitate the establishment of open structure and then improved the Li+kinetics (Fig.8c).The Zn2Ti3O8-xexhibits a better rate capacity and delivers a capacity of 88 mAh/g even at 5000 mA/g and possesses stable cycling performance (Fig.8d).However, Zn2Ti3O8only shows 22 mAh/g at same ultrahigh current density.The oxygen vacancies offer faster transmission channels for Li+, which makes Zn2Ti3O8-xbecome an alternative anode material for LIBs.Liaoet al.[146] prepared Zn2Ti3O8spheres using a facile solvothermal ion exchange method, as shown in Figs.8e and f, which showed a high charge capacity and excellent stability even in full cells.The porous spherical morphology restrains the structure deformation and offers adequate diffusion channel for Li+.The reaction principle of Zn2Ti3O8can be described as follow:
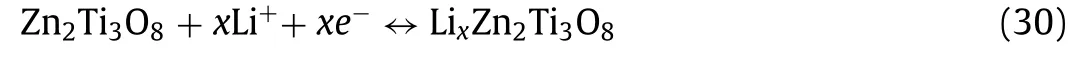
Hanet al.[147,148] prepared ZnTiO3rods and ZnTiO3/multiwalled carbon nanotube, which delivered a high discharge capacity of 166 and 230 mAh/g at 500 mA/g as anode for LIBs, respectively.The lithium storage mechanism can be expounded as [148]:
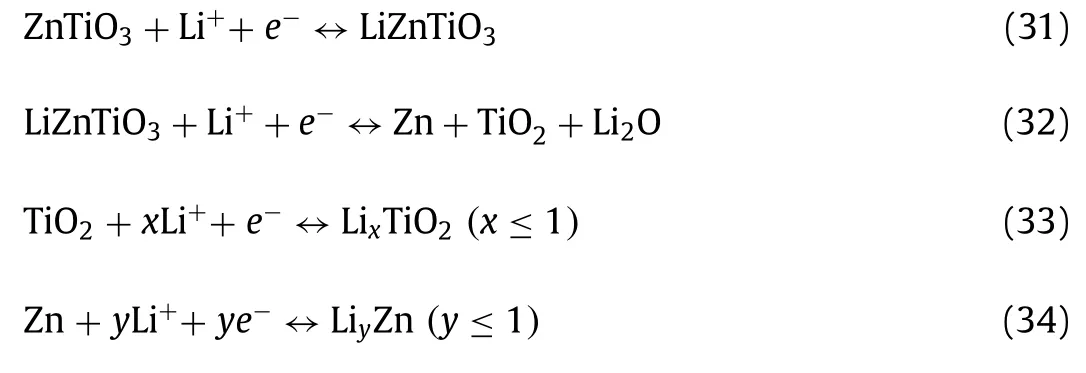
Liuet al.[149] prepared mesoporous ZnCo2O4-ZnO hybrid nanotube with unique hierarchical hollow structure (Fig.9a) and showed an ultrahigh capacity of ~1150 mAh/g at 200 mA/g.Fig.9b compared the rate capacity of ZnCo2O4-ZnO and Co3O4and former was much higher reversible capacity than latter at diverse current densities, demonstrating its excellent rate capability.Manufacturing a compounded amorphous and crystalline hierarchical structure is a promising method to further improve the electrochemical performance of electrode materials, due to the advantages of tuning and manipulating the electrolyte adsorption and ion migratory dynamics during the Li storage process.The reaction process of ZnCo2O4-ZnO can be exhibited as follow [149]:
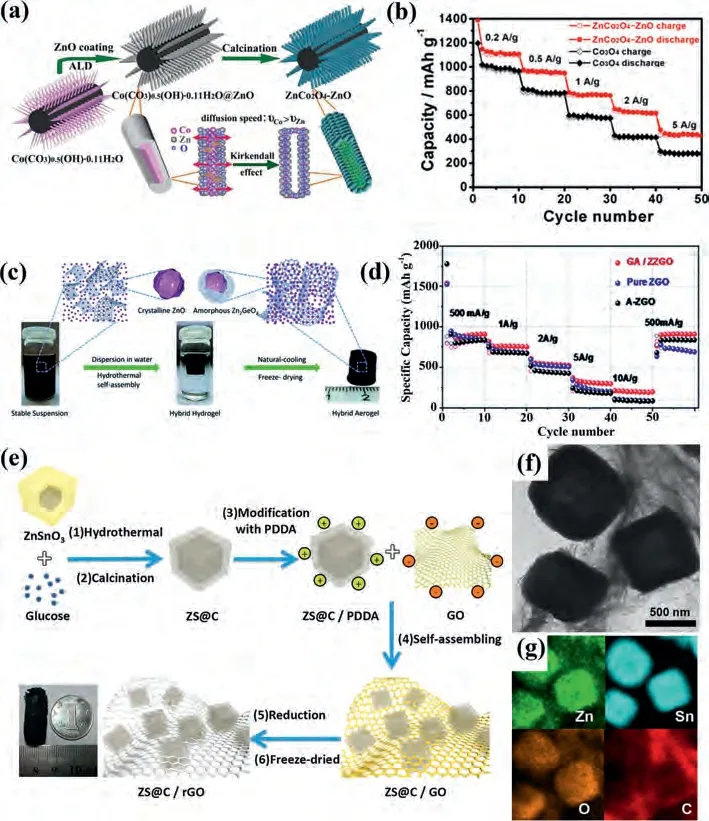
Fig.9.(a) Fabrication and (b) rate performance of ZnCo2O4-ZnO samples.Reproduced with permission [149].Copyright 2016, Elsevier.(c) Preparation process and (d)rate capacity of ZZRO/G.Reproduced with permission [150].Copyright 2017, Royal Society of Chemistry.(e) synthesis, (f) TEM image and (g) mapping of ZS@C/rGO [153].Reproduced with permission [153].Copyright 2017, Elsevier.
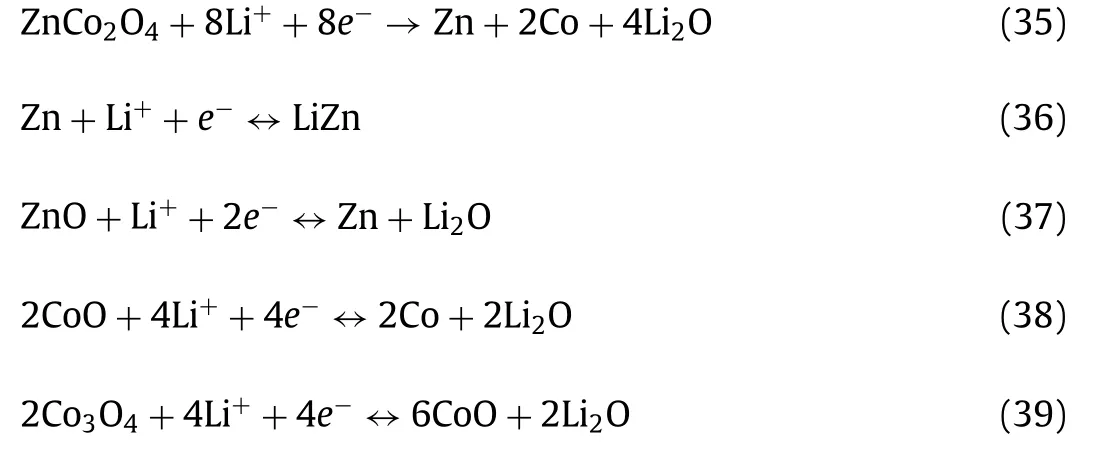
Jianget al.[150] synthesized crystalline–amorphous core–shell ZnO/Zn2GeO4/graphene aerogel (ZZGO/GA) as shown in Fig.9c.The rate capacity of ZZGO/GA at various current density proved its excellent stability, and it can maintain the capacity of 205 mAh/g at 10 A/g (Fig.9d).Lianget al.[151] prepared spinel ZnCo2O4/ZnO/C hierarchically porous structure with a regular cube via two-step annealing.The anode showed an excellent a capacity of ~1100 mAh/g at 100 mA/g and maintained 800 mAh/g after 400 cycles.At same time, ZnCo2O4/ZnO/C also exhibited a pseudocapacitive contribution ratio of 86% at 1 mV/s.Luoet al.[152] used template method to synthesize a sandwich-like ZnSnO3nanoparticles fabricated in carbon nanosheets.The unique structure showed prominent specific capacity and excellent cycling stability with 1107 mAh/g after 200 cycles.Jianget al.[153] utilized coprecipitation and electrostatic self-assembly method to prepare ZnSnO3@C coating rGO sheets (ZS@C/rGO), as Figs.9e–g shown, which delivered an ultrahigh initial discharge capacity of 1984 mAh/g at 100 mA/g and kept 1040 mAh/g after 45 cycles.The Li storage mechanism of ZnSnO3can be represented as follow:
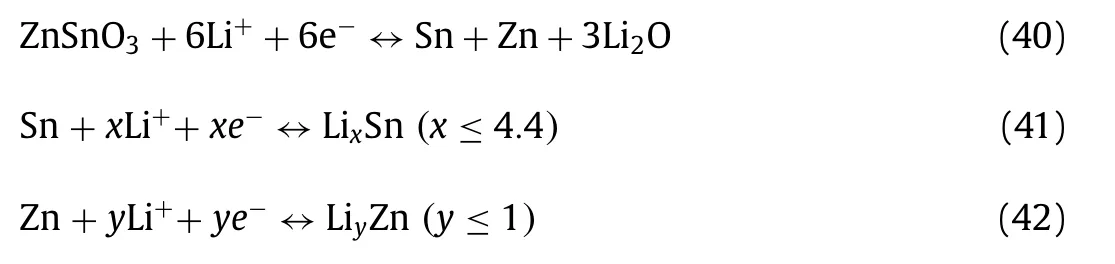
To alleviate the volume expansion of ZnO-based materials, an introduction of another metallic element (such as Ti, Sn, Ge) to establish more stable binary Zn-based oxides is an effective way.The combinations of two metals not only enhance the electrical conductivity, but provide abundant the site of redox reactions.In addition, the zinc oxides-based composites with different morphologies including nanostructures, hollow structures, nano-micro structures, need to be developed.The nanostructure provides larger surface area and more electrochemical active sites and relieves volume change, and thus improves the reversible capacitance for supercapacitors and capacity for batteries.But the remarkable thing is that the large surface area often easily results in detrimental side reactions between electrode and electrolyte in battery system,and decreases reversible capacity.Therefore, constructing threedimensional porous nano-micro hierarchical structures may be an effective strategy to improve the reversible capacitance/capacity and cycle performance due to tight contact between particles, adequate volume of pore to store electrolytes, minimized diffusion path length of ions and abundant electrochemical active sites.
5.Summary and outlook
In this review, we have introduced the most recent progress of zinc oxides-based nanomaterials using in batteries and supercapacitors.There are several modification methods of enhancing the capability for LIBs, SIBs and SCs of zinc oxides-based anodes.Designing the various nanostructures, such as nanotubes, nanosheet and so on, is regard as a potent method.Those structures usually possess more active sites and superior cycling stability, these zinc oxides-based materials exhibited clearly improved electrochemical performance.Coating carbonaceous materials on the surface of zinc oxides-based materials cannot only efficiently enhanced electronic conductivity of the electrodes, but also remit the big volume change during cycling.Recombination other metal oxides with zinc oxides-based materials exhibited prominent electrochemical performance due to the cooperative effect between the various components.On the side, high-molecular polymer also provided plenty of assistance for zinc oxides-based materials by virtue of their excellent conductivity.
In the last decade, various methods have been applied to improve defects and promote the commercialization of zinc oxidesbased, such as nanometerization, doping, and carbon composite modification, etc.However, the enhancements of battery performance of the whole electrode could not merely rely on the modification technology of a single material, the electrochemical characteristics and cycle performance of the electrode material should be improved simultaneously.In general, two or more modification methods will be used in combination to improve the overall performance of the electrode material.In short, the electrode coating layer should have the following characteristics.The materials should provide a physical barrier to prevent side reactions between electrode and electrolyte, high ionic conductivity in order to improve the charge transfer at the interface and good mechanical properties.
The current modification methods focus attention on enhancing the cycling stability of the electrodes.Unfortunately, coating carbon materials according to the predetermined design is difficult to achieve and synthesis process is complicated.This has a huge impact on commercial applications of zinc oxides-based materials.Researching a simple and low-cost way to prepared high-performance zinc oxides-based electrode materials utilizing in large-scale energy storage system is imminently needed.The solidphase method may be a feasible method for the commercialization of zinc oxides-based anodes account for the simple reaction conditions, cheap, and outstanding compatibility with industrial equipment.
The phenomenon of voltage hysteresis is inherent character in all conversion materials including zinc oxide-based anodes, and the value of voltage hysteresis generally is between 0.4 V and 1.2 V.This issue commonly is cause by high resistances resulting from the formation of SEI film and the Schottky-type contact between anode materials and electrolyte addictive.For the latter, zinc oxide-based materials usually are regarded as the semiconductors,while the conductive addictive show the metallic features, which emerges a Schottky-type contact between various types of conductors.Selecting the appropriate electrode design and electrolyte type may be an effective method to resolve the problem of voltage hysteresis, such as coating conductive polymers or Li+-conducting solid electrolyte layers on the surface of anode materials.The study about voltage hysteresis is inadequate, which need more effects in these fields.The low round-trip efficiency of zinc oxide-based anodes is related to the formation of solid electrolyte interphase film,the irreversible oxides decomposition of the, the irreversible adsorption of Li(Na)-ions, electrolyte decomposition and some harmful side reactions.To resolve these problems, some ways, such as electrolyte optimization to decrease side reactions, using prelithiation technology to improve the initial coulombic efficiency, surface engineering to limit the formation of solid electrolyte interphase film, structure or morphology designing to enhance charge transfer capability, can effectively improve the round-trip efficiency of zinc oxide-based anodes.
Zinc oxides-based nanomaterials has attracted considerable attention to be a promising anode material, but more efforts are required to achieve practical application.Before commercialization,a range of defects should be improved to meet the practical requirements, such as high cost, low initial coulombic efficiency,capacity decay and low volumetric energy density,etc.Furthermore, nanometerization of electrode materials depends on the advanced equipment and techniques which leads to a higher cost.Therefore, it is urgently needed to understand the effect mechanism of nanostructure and morphology on electrochemical performance to maximize the battery performance.In the future, continuous efforts should be devoted to achieve the commercialization and widely application of zinc oxides-based electrode materials for new-generation energy storage system.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos.U1960107 and 51774002),the “333′′ Talent Project of Hebei Province (No.A202005018) and the Fundamental Research Funds for the Central Universities (Nos.N2123034 and N2123001).
 Chinese Chemical Letters2022年2期
Chinese Chemical Letters2022年2期
- Chinese Chemical Letters的其它文章
- Comment on “Acid-induced tunable white light emission based on triphenylamine derivatives”
- Strategies for efficient photothermal therapy at mild temperatures:Progresses and challenges
- Liposome-based delivery of biological drugs
- Macrophage-targeted nanomedicine for chronic diseases immunotherapy
- Advances, opportunities, and challenge for full-color emissive carbon dots
- Fluorine-containing agrochemicals in the last decade and approaches for fluorine incorporation
