Small nucleolar RNA host gene 3 functions as a novel biomarker in liver cancer and other tumour progression
Dan-Dan Shan, Qiu-Xian Zheng,Jing Wang, Zhi Chen
Abstract Cancer has become the most life-threatening disease in the world. Mutations in and aberrant expression of genes encoding proteins and mutations in noncoding RNAs, especially long noncoding RNAs (lncRNAs), have significant effects in human cancers. LncRNAs have no protein-coding ability but function extensively in numerous physiological and pathological processes. Small nucleolar RNA host gene 3 (SNHG3 ) is a novel lncRNA and has been reported to be differentially expressed in various tumors, such as liver cancer, gastric cancer, and glioma.However, the interaction mechanisms for the regulation between SNHG3 and tumor progression are poorly understood. In this review, we summarize the results of SNHG3 studies in humans, animal models, and cells to underline the expression and role of SNHG3 in cancer. SNHG3 expression is upregulated in most tumors and is detrimental to patient prognosis. SNHG3 expression in lung adenocarcinoma remains controversial. Concurrently, SNHG3 affects oncogenes and tumor suppressor genes through various mechanisms, including competing endogenous RNA effects. A deeper understanding of the contribution of SNHG3 in clinical applications and tumor development may provide a new target for cancer diagnosis and treatment.
Key Words: Small nucleolar RNA host gene 3 ; Long noncoding RNAs, Biomarker;Clinical characters; Molecular mechanism; Competing endogenous RNA; Liver cancer
INTRODUCTION
Cancer has become one of the significant sources of mortality worldwide. Approximately 14 .1 million individuals are newly diagnosed each year, and approximately 8 .2 million deaths occurred in 2021 based on GLOBOCAN[1 ]. Despite considerable achievements and advances in clinical practices, cancer remains a dreadful illness with a poor prognosis and high health burden, which presents a global threat to health[2 ]. Cancer is a heterogeneous disease with substantial genotypic and phenotypic diversity[3 ].Genomic regulation of the cancer genome plays a vital role in cancer initiation, progression, and metastasis[4 ,5 ]. It is of most importance to explore the intricate link underlying cancer development and progression.
Long noncoding RNAs (lncRNAs) are a class of noncoding RNAs (ncRNAs) with no protein-coding ability[6 ]. Evidence has shown that lncRNAs participate in genomic regulation at the transcriptional,translational, and epigenetic levels[6 ,7 ]. The regulatory functions of lncRNAs include activation and silencing of genes[8 ,9 ], recruitment of epigenetic regulator[10 ], modification of RNA interactions[11 ],transcriptional and posttranscriptional processes[12 ], mRNA decay[13 ], and protein recruitment[14 ].LncRNA-associated regulatory functions are dynamically regulated in a cell-, tissue-, development- and distribution-specific manner[15 ]. LncRNAs in the cytoplasm may act as sponges, stabilizing mRNAs and regulating the mRNA translation process, thus modifying downstream target gene expression[16 ].LncRNAs located in the nucleus may play “cis-acting” or “trans-acting” functions[17 -19 ]. Recently,growing evidence demonstrates that lncRNAs are involved in various cancer functions and pathological processes, particularly in the initiation and progression of tumors[20 ]. LncRNAs regulate various malignant activities, including tumor progression, proliferation, apoptosis, migration, invasion,chromatin remodeling, metabolism[21 -23 ].
More recently, small nucleolar RNAs can be encoded by several lncRNAs called small RNA host genes, which are significantly differentially expressed in many diseases, including cancers[24 ]. Studies have unveiled that small nucleolar RNA host gene 3 (SNHG3 ), which is affiliated with the lncRNA class,plays an influential regulatory role in cancer initiation, development, progression, and tumor-associated microenvironment formation. LncRNA SNHG3 is located in 1 p35 .3 , as shown in Figure 1 (GeneCards,http://www.genecards.org). SNHG3 is mainly localized to the nucleus, mitochondrion, and extracellular space. Although many studies on SNHG3 and tumors have been published, clinical studies and research remains limited. This paper reviews the upregulation of SNHG3 expression in most human tumors and its relationship with negative prognosis as well as the role of SNHG3 in the mechanism of tumorigenesis. Finally, it was concluded that SNHG3 can function as a biomarker in the diagnosis and prognosis of different cancers.
CLINICAL CHARACTERS OF LNCRNA SNHG3
Growing evidence has demonstrated that SNHG3 acts as an oncogene and plays a critical regulatory role in the initiation and development of various cancers. SNHG3 exhibits characteristic oncogenic properties in multiple cancers. Studies have indicated that SNHG3 is significantly upregulated in hepatocellular carcinoma (HCC)[25 ,26 ] and other cancers, such as cervical cancer (CC)[27 ,28 ], and breast cancer (BC)[29 -34 ]. Clear cell renal cell carcinoma (ccRCC)[35 ], colorectal cancer (CRC)[36 -38 ], glioma[39 ,40 ], HCC[25 ,41 ,42 ], non-small cell lung cancer (NSCLC)[43 -47 ], ovarian cancer (OC)[48 -51 ], and papillary thyroid carcinoma (PTC)[52 ]. Different views exist on SNHG3 expression in lung adenocarcinoma, as shown in Table 1 . Aberrant SNHG3 expression might represent a prognostic prediction value and therapeutic target in various cancers.

Table 1 The expression of small nucleolar RNA host gene 3 and its clinical significance in liver cancer and other cancers
HCC
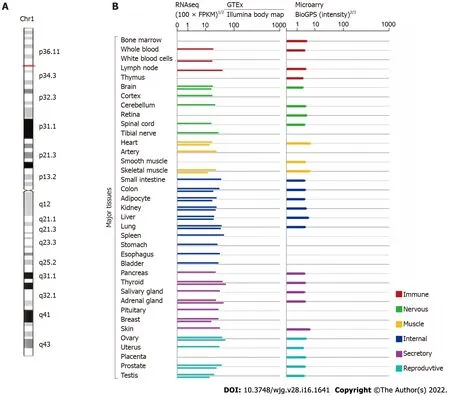
Figure 1 Small nucleolar RNA host gene 3 genomic information (GeneCards, http://www.genecards.org). A: The chromosomal localization of small nucleolar RNA host gene 3 (SNHG3 ); B: The expression level of SNHG3 in normal human tissues.
HCC is one of the most widespread cancerous malignancies, ranking third in cancer-related deaths[53 ].Cures for HCC tend to be more effective when used at an early stage[54 ]. However, patients at this stage do not have specific symptoms or lack biomarkers for early diagnosis, which usually delays diagnosis.Accordingly, the development of biomarkers for early diagnosis and prognosis is urgent. Recent studies have shown that SNHG3 expression is upregulated in HCC tissues compared with normal tissues and positively correlates with tumor stage (P< 0 .001 ), tumor size (P = 0 .003 ), lymph node metastasis (P<0 .001 ), distant metastasis (P < 0 .001 ), portal vein tumor thrombosis (P = 0 .014 ), and relapse (P = 0 .038 )[25 ,26 ]. Based on the above evidence that SNHG3 influences HCC metastasis and growth, SNHG3 has the potential to be a reliable biomarker for the diagnosis and treatment of HCC.
BC
BC accounts for 30 % of all female tumors and is the second leading contributor to cancer deaths in women[55 ]. Early detection of BC can reduce mortality and improve patient survival[56 -58 ]. The discovery of a new biomarker appears critical. Maet al[31 ] reported elevated SNHG3 expression in BC tissues and correlated it with tumor malignancy. They also reported that SNHG3 expression was associated with clinicopathological features, such as histological grade (P= 0 .016 ), tumor-nodemetastasis (TNM) stage (P= 0 .001 ), lymph node metastasis (P < 0 .001 ), estrogen receptor (P = 0 .009 ), and Her-2 (P = 0 .001 )[31 ]. ER and Her-2 are major molecular targets in the pathogenesis of BC and are associated with prognosis and treatment options for BC[59 ]. Therefore, SNHG3 plays a carcinogenic role in the progression and prognosis of BC. Therefore, SNHG3 may play an oncogenic role in the progression and prognosis of BC.
CC
CC is the fourth most prevalent cancer among women worldwide in terms of incidence and mortality[53 ]. The survivability of patients with advanced CC is not desirable, and early diagnosis and treatment of CC improve its prognosis[60 ]. It is important to further explore the mechanisms of CC development to develop new therapeutic approaches[61 ]. The function of SNHG3 in CC has been recognized in several studies. Zhuet al[27 ] published that SNHG3 expression was obviously upregulated in CC tissues, and high SNHG3 expression affected International Federation of Gynecology and Obstetrics(FIGO) stage (P= 0 .011 ) and metastasis (P = 0 .018 ), implying that SNHG3 is a possible prognostic biomarker and a target for treatment in CC.
ccRCC
With high morbidity and mortality, ccRCC is the most common and serious type of renal cell carcinoma[62 ]. Using TCGA and GEO databases, Zhang et al[35 ] found that lncRNA SNHG3 expression was elevated in ccRCC and positively correlated with many clinicopathological parameters. They further quantified the relative expression of SNHG3 in ccRCC tissues compared with normal tissues using qRT–PCR and discovered that the relative expression of SNHG3 was increased in ccRCC tissues[35 ].These results revealed that upregulated SNHG3 expression may play an essential role in the ccRCC pathway.
CRC
CRC is a dominant reason for morbidity and mortality worldwide, yet greater than half of patients have progressed to stage II/III at the time of diagnosis. The treatment of CRC typically consists of curative resectionviasurgery followed by adjuvant chemotherapy to reduce the risk of recurrence[63 ]. Therefore,it is critical to further elucidate the mechanisms of CRC and to pursue the development of effective targeted therapies. SNHG3 expression was remarkably upregulated in CRC tissues compared to nearby normal tissues and correlated with poor outcomes in CRC[36 -38 ]. Moreover, Wen et al[36 ] explored the connection between SNHG3 and clinicopathological parameters, and the results reflected a close association with tumor stage (P= 0 .006 ) and distant metastasis (P = 0 .0033 ). Overall, SNHG3 promotes the progression of CRC, leading to an unfavorable prognosis.
Glioma
Glioma is a fatal brain tumor that can be treated with radiation and surgery, but the median survival of patients is only approximately 14 -17 mo[64 ]. Effective curative treatments for glioma are lacking. Thus,it is especially vital to determine the molecular mechanisms of glioma and identify novel strategies for treatment[65 ]. SNHG3 is highly expressed in glioma tissues compared to normal tissues[39 ,40 ]. Zhanget al[39 ] explored the relationship between SNHG3 expression and the clinicopathological features of GM patients, including tumor size (P= 0 .0300 ) and World Health Organization classification (P = 0 .0278 ).These studies indicated that SNHG3 offer new insights for the future treatment of glioma.
Lung cancer
Lung cancer is one of the cancers with the highest mortality and morbidity rates worldwide[66 ], with NSCLC accounting for 85 % of lung cancer cases[67 ]. Shi et al[44 ] revealed that high expression of lncRNA SNHG3 in NSCLC tissues was associated with TNM stage (P = 0 .0053 ), lymph node metastasis(P= 0 .0006 ), and tumor size (P = 0 .0005 ). Thus, elevated SNHG3 expression is relevant to the lower overall survival (OS) rate of NSCLC patients. Liuet al[67 ] analyzed the TCGA database as well as the GSE19804 public database and found high SNHG3 expression in lung adenocarcinoma. These researchers used Kaplan–Meier plotter database analysis to determine that high SNHG3 expression decreases the OS time of lung adenocarcinoma patients. However, Kanget al[68 ] used quantitative polymerase chain reaction to show that SNHG3 expression levels were decreased in lung adenocarcinoma tissues, and Kaplan–Meier analysis demonstrated that patients with low SNHG3 expression levels had a short OS. In conclusion, SNHG3 is associated with unfavorable prognosis in lung cancer patients, but the expression and function of SNHG3 in lung adenocarcinoma still requires further research.
Ovarian cancer
OC claims the lives of 151900 women worldwide each year and is one of the leading contributors to death in women[69 ]. Because there are no specific symptoms in the early stages, greater than 79 % of OC patients reach stage III or IV[70 ]. The lack of effective biomarkers translates to a poor prognosis in OC patients. Recently, Honget al[50 ] described high SNHG3 expression in OC tissues compared with normal tissues adjacent to cancer, and a high SNHG3 expression level was positively correlated with the FIGO stage (P= 0 .007 ) and lymph node metastasis (P = 0 .001 ) of OC patients. This finding suggests that high SNHG3 expression may be an individual prognostic factor affecting OS in OC patients.
Papillary thyroid carcinoma
PTC is the most common type of thyroid cancer, and the incidence of PTC is increasing[71 ]. The high rate of metastases in the cervical lymph nodes of PTC patients is closely related to the recurrence of PTC[72 ,73 ]. It seems relevant to study the mechanism of metastasis in PTC. Sui et al[52 ] analyzed 42 pairs of tissues and determined that SNHG3 expression was higher in PTC tissues compared with controls. High SNHG3 expression was positively correlated with TNM stage (P = 0 .014 ) and lymph node metastasis (P= 0 .0292 ) in advanced PTC. The above results suggest that SNHG3 could be a carcinogenic lncRNA in PTC.
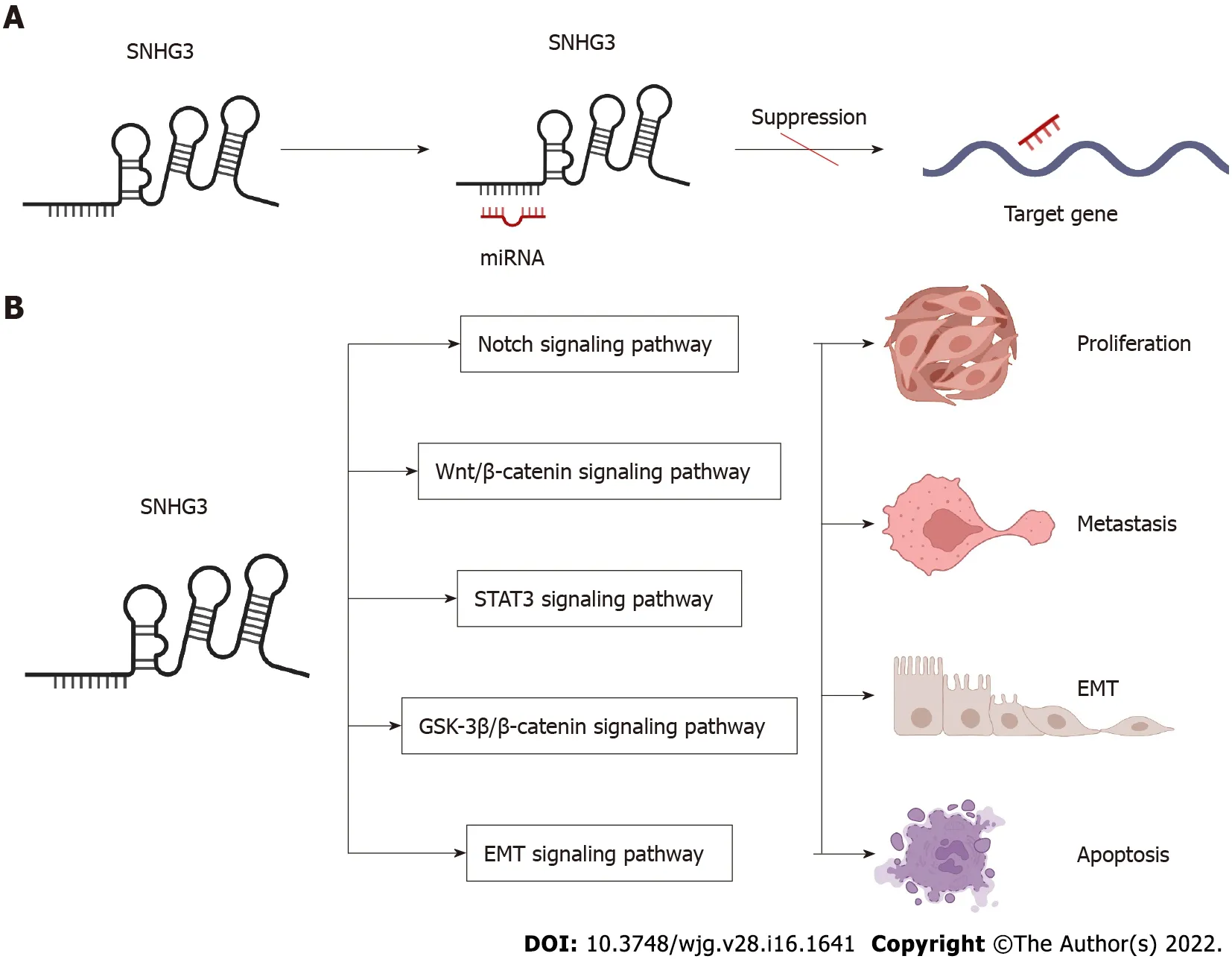
Figure 2 Small nucleolar RNA host gene 3 -mediated mechanisms regulating cancer progression. A: Small nucleolar RNA host gene 3 (SNHG3 )as a ceRNA; B: SNHG3 is involved in signaling pathways. SNHG3 : Small nucleolar RNA host gene 3 ; EMT: Epithelial–mesenchymal transition.
Researchers have elucidated the high expression of SNHG3 in acute myeloid leukemia (AML)[74 ],esophageal cancer[75 ], gastric cancer (GC)[76 -78 ], laryngeal carcinoma[79 ,80 ], oral squamous cell carcinoma[81 ,82 ], osteosarcoma[83 ,84 ], and prostate cancer[85 ,86 ], but have not yet explored the relationship between SNHG3 and clinicopathological features. Subsequent investigations into the biological mechanisms of SNHG3 in other diseases will further support the potential of SNHG3 as a new diagnostic and therapeutic biomarker.
SNHG3 IN VIVO STUDIES
SNHG3 may function as an oncogene and has the potential to become a novel potential therapeutic target for many cancers. Several studies have used animal models to explore the effect of SNHG3in vivo.Zhaoet al[25 ] assessed the role of SNHG3 in HCC growth in vivo. They injected stable SNHG3 -depleted HepG2 cells subcutaneously into the right dorsum of female BALB/c nude mice and measured the volume of tumor. The results showed that SNHG3 depleted tumors grew significantly slower than that of controls. HCC growth was inhibitedin vivodue to the knockdown of the SNHG3 gene[25 ]. Similarly,SNHG3 gene knockdown inhibited the growth of other tumors, such as BC[34 ], CC[27 ], ccRCC[35 ], CRC[36 ,37 ], GC[77 ], glioma[40 ], laryngeal carcinoma[80 ], NSCLC[45 ,46 ], OC[49 ], PTC[52 ], prostate cancer[85 ]. Overall, SNHG3 promoted the progression of the abovementioned tumors.
BIOLOGICAL FUNCTION OF SNHG3 IN CANCER
In the following section, we will explore the biological function of SNHG3 in different cell lines. Many works have shown that SNHG3 affects cancer cell proliferation, epithelial-mesenchymal transition(EMT)[25 ,41 ,44 ,47 ,85 -87 ], apoptosis, invasion, migration, and metabolism[29 ,48 ] specifically through the action of different molecular mechanisms. In HCC, SNHG3 affects cell proliferation, apoptosis,metastasis and invasion mainly by means of competing endogenous RNA (ceRNA) and EMT signaling pathway, as shown in Figure 2 and Table 2 .

Table 2 Function and mechanism of small nucleolar RNA host gene 3 in liver cancer and other cancers
Non-small-cell lung cancer CMT-167 , LLC, CMT-170 , CMT-181 Up TGF-β pathway, IL-6 ,JAK2 , STAT3 pathway Proliferation, migration, EMT 31602642 Non-small-cell lung cancer SPC-A1 , A549 , NCI-H23 , NCI-H460 Up miR-340 -5 p Lymph node infiltration, distant metastases 33225676 Non-small-cell lung cancer A549 , H322 , H1299 , GLC-82 , SPC-A1 Up miR-216 a, ZEB1 Proliferation, migration, invasion 33177840 Non-small-cell lung cancer A549 , HCC827 , H2170 , H520 Up miR-515 -5 p, SUMO2 Proliferation, migration, invasion,EMT 34032148 Oral squamous cell carcinoma SCC4 , CAL-27 Up miR-2682 -5 p, HOXB8 Proliferation, migration 32989886 Oral squamous cell carcinoma SCC-15 , SCC-9 , CAL-27 , HN5 Up Wnt/β-catenin, NFYC Proliferation, migration 31762107 Osteosarcoma Saos2 , MG63 , U2 OS, HOS Up miR-151 a-3 p, RAB22 A Proliferation, migration, invasion 30797154 Osteosarcoma MG-63 , 143 B, HOS, SW-1353 , Saos-2 ,U-2 OS Up miR-196 a-5 p Proliferation, colony formation 30791797 Ovarian cancer SK-OV3 , TOV-21 G, and OVCAR-3 Up Energy metabolism 29921511 Ovarian cancer SKOV3 , HeyA8 , A2780 Up miR-339 -5 p, TRPC3 Proliferation, anti-apoptosis 33149611 Ovarian cancer SKOV3 , 60 OVCAR3 , A2780 , ES2 Up GSK3 β/β-catenin Proliferation, migration 29758922 Ovarian cancer A2780 , SKOV3 , OVCAR3 , OV90 Up miR-139 -5 p, Notch1 Proliferation, migration 33552243 Papillary thyroid carcinoma BCPAP, TPC-1 Up miR-214 -3 p, PSMD10 Migration, invasion, proliferation,and colony formation 32048306 Prostate cancer PC3 , DU145 , 22 RV1 , NCaP Up miR-577 , SMURF1 Proliferation, migration, EMT, antiapoptosis 32248648 Prostate cancer PC3 , 22 RV1 , DU145 , LNCaP Up miR-487 a-3 p,TRIM25 Viability, migration, invasion, EMT 33416420 EMT: Epithelial–mesenchymal transition.
SNHG3 function as a ceRNAs in cancers
LncRNAs and microRNAs (miRNAs) are ncRNAs. LncRNAs compete with miRNAs by acting as sponges for miRNAs to reduce the activity or expression of miRNAs[88 ]. These lncRNAs are called ceRNAs. Numerous studies have found that SNHG3 can function as a ceRNA by competitively binding to various miRNAs, including miR-758 -3 p[74 ], miR-515 -5 p[87 ], miR-330 [29 ], miR-326 [25 ,30 ,34 ,78 ], miR-384 [31 ,39 ,79 ], miR-154 -3 p[32 ], miR-186 -5 p[33 ,75 ], miR3173 –5 p[89 ], miR-139 -5 p[35 ], miR-539 [36 ], miR-182 -5 p[37 ], miR-370 -5 p[38 ], miR-3619 -5 p[76 ], miR-485 -5 p[40 ], miR-128 [41 ], miR-214 -3 p[42 ,52 ], miR-340 -5 p[45 ,80 ], miR-890 [68 ], miR-1343 -3 p[43 ], miR-216 a[46 ], miR-515 -5 p[47 ], miR-2682 -5 p[81 ], miRNA-151 a-3 p[83 ], miR-196 a-5 p[84 ], miR-339 -5 p[49 ], miR-139 -5 p[51 ], miR-577 [85 ], and miR-487 a-3 p[86 ]. In HCC SNHG3 plays its role as a ceRNA mainly by binding to miR-128 [41 ], miR-326 [25 ] and miR-214 -3 p[42 ].SNHG3 acts as a sponge to bind to miRNAs, subsequently blocking the effects of miRNAs on their downstream target mRNAs. Thus, SNHG3 regulates the expression of oncogenes or tumor suppressor genes, such asSRGN[74 ], PKM[29 ], HDGF[31 ], and c-Myc[37 ], ultimately affecting cancer cell proliferation, apoptosis, metastasis, metabolism and EMT, as shown in Figures 3 and 4 .
EMT signaling pathway
The EMT process has been shown to be critical in cancer[90 ,91 ]. Shi et al[44 ] showed that human lung cancer cells overexpressing the SNHG3 gene exhibited increased expression of mesenchymal markers(N-calmodulin and waveform protein) and reduced expression of epithelial cell markers (E-calmodulin)while also promoting cancer cell proliferation and metastasis through the TGF-β pathway. In addition,Zhaoet al[25 ] learned that SNHG3 promotes cancer cell migration by upregulating the expression of ZEB1 , a key transcription factor of EMT in HCC cells. The same conclusion was reached in BC[33 ] and NSCLC[46 ]. The above achievements point to the potential application of SNHG3 in the tumor EMT signaling pathway.
Notch signaling pathway
The Notch signaling pathway is a strongly conserved cellular signaling system in most multicellular organisms and is required for a variety of cellular processes, including stem cell functions, cell proliferation, differentiation, and cell death. Several lncRNAs have been proven to participate in the Notch signaling pathway. Zhanget al[51 ] demonstrated that SNHG3 promotes OC proliferation and migration by regulating Notch1 . In addition, Jiang et al[32 ], suggested that SNHG3 promotes BC cell proliferation and metastasis through upregulation of the Notch signaling pathway. These studies indicate that SNHG3 plays a crucial part in Notch pathways, which have the potential to develop novel therapeutic targets for cancer therapy.

Figure 3 Small nucleolar RNA host gene 3 functions as a competing endogenous RNA in digestive system tumors.
Wnt/β-catenin signaling pathway
Aberrant activation of Wnt/β-catenin signaling was found in some tumors, and Wnt/β-catenin signaling can regulate oncogenes, such asc-MycandBcl-2, leading to tumorigenesis and cell proliferation. SNHG3 may upregulate the Wnt/β-catenin pathway by regulating miR-340 -5 p and YAP1 in laryngeal cancer cells[80 ]. In addition, SNHG3 may also promote the proliferation and metastasis of oral squamous carcinoma cells by upregulating the NFYC and Wnt/β-catenin pathways[82 ]. SNHG3 is involved in the Wnt/β-catenin signaling pathway that mediates tumor development and could represent a new tool for tumor therapy.
STAT3 signaling pathway
Tyrosine kinase signaling delivered by STAT3 is frequently activated in cancer cells, and the STAT3 signaling pathway plays an important role in cancer progression, where it can be activated by cytokines,such as IL-6 [92 ], and growth factors[93 ,94 ]. STAT3 is phosphorylated by receptor-associated JAK and thus enters the nucleus to act as a transcriptional activator, regulating oncogene expression[95 ]. The impacts of STAT3 signaling in suppressing tumor immune surveillance have also been reported[96 ].
Sunet al[76 ] demonstrated that SNHG3 was overexpressed in GC tissues, and cellular experiments revealed that IL-6 -activated STAT3 positively regulates SNHG3 and that SNHG3 promotes stem celllike properties in GC cells. Shiet al[44 ] found that the IL-6 /JAK2 /STAT3 pathway activated SNHG3 in NSCLC and promoted cell proliferation and migration. This finding leads to the conclusion that the STAT3 signaling pathway involved in SNHG3 is a novel mechanism of carcinogenesis, suggesting that SNHG3 may represent a biomarker for the treatment of these carcinomas.
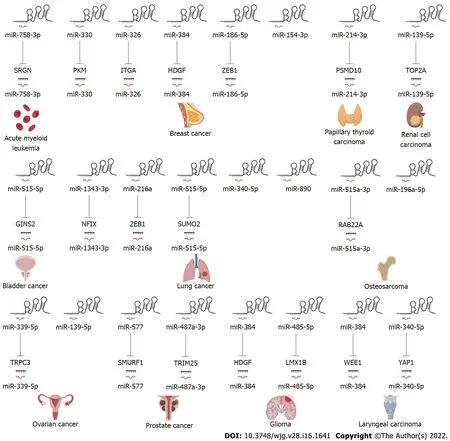
Figure 4 Small nucleolar RNA host gene 3 functions as a competing endogenous RNA in other systemic tumors.
GSK-3 β/β-catenin signaling pathway
GSK-3 β can negatively regulate β-catenin signaling, which is also implicated in cell proliferation[97 ].Research has illustrated that GSK-3 β/β-catenin signaling is, for example, one of the downstream drivers of SNHG3 in OC[50 ], and high expression of SNHG3 fosters cell proliferation and invasion via GSK-3 β/β-catenin.
SNHG3 can revitalize multiple signaling pathways to promote human tumorigenesis, such as the Notch signaling pathway, Wnt/β-catenin signaling pathway, STAT3 signaling pathway, GSK-3 β/βcatenin signaling pathway, and EMT signaling pathway. Thus, SNHG3 may become a target in the treatment of tumors.
CONCLUSION
LncRNAs function as multifunctional signaling modulators, facilitating tumor initiation, progression,and metastasis by regulating tumor cell proliferation, migration, apoptosis, cell cycle, drug resistance,epithelial-mesenchymal transition, metabolic reprogramming, and immune response[98 -100 ].Compelling studies have suggested that lncRNAs can act as diagnostic indices, prognostic biomarkers,and therapeutic targets for diseases[101 ,102 ]. These reports indicate that SNHG3 expression is upregulated in tumor tissues, such as HCC, GC, CC, PTC, and AML, compared to adjacent normal tissues. However, the expression of SNHG3 in lung adenocarcinoma has shown different results and needs to be further explored. At the same time, SNHG3 has been associated with various clinicopathological parameters such as staging and distant metastasis. In addition, SNHG3 has been shown to promote tumour development inin vivoexperiments, and at the cellular level, SNHG3 plays a significant role in promoting tumour cell proliferation, migration and metastasis, disrupting the cell cycle and inhibiting apoptosis in a variety of cancers through a variety of signalling pathways, resulting in a poor prognosis for patients.In short, these findings implied that SNHG3 might function as a new target in the diagnosis and treatment of tumours.
In summary, a better understanding of the function of SNHG3 in the clinicopathological features and mechanisms of tumor development may help to improve the efficiency and targeting of treatment.Further studies on SNHG3 and its regulatory mechanism may pave the way to improve prevention,cancer diagnosis, and treatment based on patients' biological and pathological characteristics.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge all the people that have made this study.
FOOTNOTES
Author contributions:Chen Z carried out the concepts and designed the definition of intellectual content; Shan DD and Zheng QX carried out the literature search and manuscript editing; Wang J performed manuscript review; All authors have read and approved the content of the manuscript.
Supported bythe National Science and Technology Major Project of China, No. 2018 ZX10302206 and 2017 ZX10202203 .
Conflict-of-interest statement:The authors declare that there is no conflict of interests regarding the publication of this paper.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4 .0 ) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4 .0 /
Country/Territory of origin:China
ORCID number:Dan-Dan Shan 0000 -0003 -4761 -7385 ; Qiu-Xian Zheng 0000 -0001 -5609 -7380 ; Jing Wang 0000 -0001 -6652 -2854 ; Zhi Chen 0000 -0002 -0848 -1502 .
S-Editor:Zhang H
L-Editor:A
P-Editor:Zhang H
 World Journal of Gastroenterology2022年16期
World Journal of Gastroenterology2022年16期
- World Journal of Gastroenterology的其它文章
- Comment on “Artificial intelligence in gastroenterology: A state-of-the-art review”
- Viral hepatitis: A global burden needs future directions for the management
- Evaluating the accuracy of American Society for Gastrointestinal Endoscopy guidelines in patients with acute gallstone pancreatitis with choledocholithiasis
- Aspartate transferase-to-platelet ratio index-plus: A new simplified model for predicting the risk of mortality among patients with COVID-19
- Noninvasive imaging of hepatic dysfunction: A state-of-the-art review
- Risk of venous thromboembolism in children and adolescents with inflammatory bowel disease: A systematic review and meta-analysis
