Effect of cholesterol-loaded cyclodextrin enriched extenders on the quality of prefrozen and frozen buffalo semen
Asmaa A. Mostafa, Mohamed S. El-Belely, Sayed T. Ismail, Reda I. El-Sheshtawy, Mohamed I. Shahba
1Abassia Frozen Semen Center, General Organization for Veterinary Services, Cairo, Egypt
2Department of Theriogenology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt
3Department of Animal Reproduction and Artificial Insemination, National Research Centre, Dokki, Giza, Egypt
ABSTRACT
Objective: To investigate the effects of non-permeable cryoprotectant, cholesterol-loaded cyclodextrin, when added at different concentrations into cooled and frozen-thawed semen extended with Tris-citrate-fructose egg yolk glycerol and lecithin-based extenders.
Methods: A total of 40 ejaculates from four buffalo bulls were collected using artificial vagina. Ejaculates were extended with one of Tris-citrate-fructose egg yolk glycerol and lecithinbased extenders which contained different concentrations [0(control), 0.75, 1.50, 2.25 and 3.00 mg/mL] of cholesterolloaded cyclodextrin. The extended semen samples were cooled to 5 ℃ and then frozen slowly to -196 ℃ in 0.25 mL ministraws before being stored in liquid nitrogen pending its evaluation.Sperm motility, live sperm, normal sperm morphology, sperm membrane integrity and acrosome morphology were measured.
Results: Supplementation of cholesterol-loaded cyclodextrin improved progressive motility, viability, morphology and acrosome as well as plasma membrane integrities at 1.50-2.25 mg/mL depending upon types of used extenders and stages of preand post-freezing process (P<0.01). The best concentration was 1.50 mg/mL at pre-freeze stage and 2.25 mg/mL at postfreezing. However, greater concentration (3.00 mg/mL) of cholesterol-loaded cyclodextrin had a detrimental effect compared to the control group with the two evaluated extenders(P<0.01).
Conclusions: Cholesterol-loaded cyclodextrin supplementation at 1.50-2.25 mg/mL concentration could improve pre-frozen and post-thawed buffalo sperm quality. The most suitable concentration is 1.50 mg/mL at pre-freeze stage and 2.25 mg/mL at post-freezing.
KEYWORDS: Buffalo; Semen; Tris; Cooling;Cryopreservation; Cholesterol-loaded cyclodextrin; Semen evaluation
Significance
Cholesterol : phospholipid ratio of the sperm membrane is essential for proper sperm cryopreservation. Cholesterolloaded cyclodextrin improves the permeation of cholesterol into the sperm membrane to preserve its normal fluidity and permeability and protects it from damage, reducing the hazardous premature sperm capacitation with subsequent success of artificial insemination.
1. Introduction
It is known that buffalo spermatozoa are very susceptible to hazards during freezing and thawing processes with considerable damage to motility apparatus, plasma membrane and acrosomal cap[1] as well as leakage of intracellular enzymes[2]. Recently, a very high incidence of sperm capacitation-like changes was also observed following cryopreservation[3]. Differences among species in sperm sensitivity to cold shock damage are primarily due to phospholipid concentration as well as membrane cholesterol :phospholipid ratio[4], as the susceptibility of plasma membrane to undergo lipid phase transition during cooling is inversely related to the proportion of cholesterol[5].
In buffalo, the cholesterol : phospholipid ratio of fresh semen is 0.34±0.05[6], an intermediate value between bull and boar spermatozoa[7,8] and a significant cholesterol loss occurs following cryopreservation[6]. Cholesterol plays multiple roles as regulator of membrane function[9], affecting membrane fluidity[10] and permeability[11]. It also reduces the phase transition temperature of membrane, maintaining it in a fluid state, thereby reducing the damages that can occur at low temperatures[12]. It has been reported that high membrane cholesterol levels reduce membrane crystallization at low temperature and thereby inhibit indirectly premature capacitation by diminishing the biological activity of sperm surface proteins[13]. Due to the critical role of cholesterol: phospholipid ratio in sperm cryopreservation, a strategy has been developed to modify the cholesterol membrane prior cryopreservation to improve sperm cryosurvival.
As cholesterol is a hydrophobic molecule, not soluble in aqueous semen diluents, it has been successfully inserted in cell membrane through cyclodextrins (cholesterol-loaded cyclodextrins). Treating sperm with cholesterol-loaded cyclodextrins before freezing has improved cryosurvival and sperm quality after thawing in several cold shock-sensitive species including buffalo bulls[14,6,15], bovine bulls[16-21] stallions[22-26], rams[27-31] and goats[32-35]. Therefore,the present study was implemented to explore the effects of nonpermeable cryoprotectants, cholesterol-loaded cyclodextrins,when added at different concentrations into cooled and frozenthawed semen extended with Tris-citrate-fructose egg yolk glycerol(TCFYG) and lecithin-based extenders (TCFGL).
2. Materials and methods
2.1. Buffalo bulls
Four buffalo bulls (aged 3.5-5.0 years) maintained at the Abassia Buffalo Semen Freezing Center, Central Organization for Veterinary Services, Ministry of Agriculture, Egypt, were selected to be the source of semen. The buffalo bulls were maintained under uniform standard nutrition and managerial practices. They were in good general health conditions (600-800 kg body weight), free from general and genital diseases. During summer, breeding bulls were kept cool and comfortable by splashing water at least 3-4 times a day, protected from direct wind blasts, housed in a place with comfortable microenvironment with least humidity, fed during cool hours, and had a free access to cool drinking water. They were fed 6 kg dry matter+2 kg tibn and 3.5 kg dried barseem/animal/day in summer and 6 kg dry matter+2 kg tibn and 28 kg barseem/animal/day in winter. Temperature humidity index was 72-78.
2.2. Semen collection and initial evaluation
The four bulls were used weekly for semen early collection program using an artificial vagina. Semen collections were made in the early morning. Two successive semen samples were collected by means of an artificial vagina attempts with 15 min interval. The ejaculates were pooled to eliminate variability among the collected samples. The collected ejaculates (10 per bull, total 40 in each experiment) were immediately put into a water bath at 35 ℃for 10 min and evaluated for visual motility using a high power ordinary microscope (Leica DM750 at 400×) with closed circuit television, sperm concentration using Neubauer haemocytometer and abnormality % using eosin-nigrosin stain. The heterogenic semen samples quantifying a minimum standard of 1 mL volume, 70% motility, total sperm defects lower than 20% and with concentration of 600×10⁶ spermatozoa/mL of the ejaculate were selected for further processing.
2.3. Experimental design
The pooled semen was divided into two parts diluted with one of the two different extenders: TCFYG and TCFGL. The TCFYG extender was prepared by dissolving 3.028 g Tris, 1.678 g citric acid and 2.000 g fructose in 100 mL bi-distilled water and then adding 20% egg yolk and 7% glycerol with a penicillin-streptomycin mixture as antibiotic at 0.01 mL/mL of the extender according to Ijaz et al[36]. As we had already documented the 1.0% soya lecithin,enriched in egg yolk-free extender, as the optimal concentration providing cryopreservation of sperm quality variables in buffalo bulls[37]. The semen samples diluted with one of the two extenders were poured into pre-warmed dried test tubes containing different concentration of cholesterol-loaded cyclodextrin: 0 (control), 0.75,1.50, 2.25 and 3.00 mg/mL[6,15]. All diluents were adjusted to a pH of 6.7 using a pH meter (HANNA instrument, HI 8314). Semen was extended to adjust the concentration of sperm to 30×10⁶ cells in a 0.25 mL ministraws (IMV, France), and then slowly cooled to 5 ℃for a 2-hour period. Straws were packed with the extended semen (at 5 ℃ working environment) and kept at the same temperature for 4 h to equilibrate. Packed straws of extended semen were processed for freezing[38].
2.4. Evaluation of semen quality parameters
The evaluation was performed after cooling and freezing of bull spermatozoa. Frozen straws were thawed at 37 ℃ for 1 min. The characteristics examined were motility, liveability, normal sperm(morphology), sperm membrane and acrosome integrities.
2.4.1. Motility
Progressive motility was estimated subjectively in a drop of diluted semen using a pre-warmed 2.9% sodium citrate dehydrate solution.The drop was placed on a glass slide covered by a clean cover slip,and then was examined under the microscope (Leica DM750 ×400).At least 200 spermatozoa from a minimum of four microscopic fields were examined. Motility was estimated on a continuous scale of 0% to 100%[39].
2.4.2. Live sperm percentage and abnormality
Live spermatozoa percentage was evaluated using eosin-nigrosine stain in uniform smears using bright field optics (Leica DM750×400). Abnormal spermatozoa was counted in the same smear; at least 200 sperms were counted in 5 microscopic fields[40].
2.4.3. Membrane integrity [hypo-osmotic swelling test (HOST)]
The hypo-osmotic solution (125 mOsm/L) was prepared by dissolving 6.25 g of sodium citrate dihydrate and 11.25 g of fructose in 1 000 mL distilled water. A volume of 10 μL semen was gently mixed with 1 mL of solution and incubated for 60 min at 37 ℃.After incubation, a drop of the solution containing semen was placed on a glass slide, covered with a cover slip and examined under the microscope (×400). A total of 200 spermatozoa were counted; the percentage of spermatozoa positive to HOST (having swelled or curled tail) was determined[41].
2.4.4. Acrosome morphology
It was assessed using Giemsa stain according to method of Watson[42]. A drop of diluted semen was smeared on a pre-warmed slide and air dried. The smears were fixed by immersion in 10% buffered formal saline for 15 min, and then washed in running tape water. The smears were air dried and then immersed in buffered Giemsa solution in coplin jar for 90 min, after which they were rinsed briefly in distilled water and dried. The dried smears were studied under light microscope at magnification of 1 000× using oil immersion lens. The percentage of normal acrosome was calculated for about 200 spermatozoa. The acrosome was considered to be normal when the stain was clearly and evenly distributed over the spermatozoa anterior to equatorial segment.
2.5. Statistical analysis
Data were analyzed using the SPSS (2005) computerized program v. 14.0. All data were subjected to one-way analysis of variance by using computerized statistical analysis. The data were expressed as mean±standard deviation (mean±SD). Significant difference between means was calculated using Duncan test at P<0.05. The analytical design was factorial design (general linear model) to clarify the effect of cholesterol-loaded cyclodextrin concentrations and extenders on the pre- and post-freezing semen variables. Treated means were compared by the least significant difference (LSD) test at 5% levels of probability. Five replicates were carried out for each treatment. For each replicate, four straws were used for assessment of sperm characteristics. All statistical methods were done according to Snedecor and Cochran[43].
2.6. Ethics statement
The study was approved by the Medical Research Ethics Committee of the National Research Centre, Dokki, Egypt. The study registration number was 19/104 and its date was 10th October 2019.
3. Results
3.1. Effects of cholesterol-loaded cyclodextrin concentrations on pre-freezing semen quality variables with use of TCFYG and TCFGL extenders in buffalo bulls
Table 1 shows effects of cholesterol-loaded cyclodextrin on buffalo semen characteristics after equilibration at 5 ℃ in TCFYG and TCFGL extenders. There was an interaction between semen extenders and different concentrations of cholesterol-loaded cyclodextrin on pre-freeze semen quality variables. All studied parameters in semen containing different concentrations of cholesterol-loaded cyclodextrin were better in lecithin (TCFGL)-than in egg yolk (TCFYG)- based extenders.

Table 1. Effects of cholesterol-loaded cyclodextrin concentrations on pre-freezing semen quality variables with use of TCFYG and TCFGL extenders in buffalo bulls (%).
The interaction between cholesterol-loaded cyclodextrin and egg yolk revealed that compared to the control group, progressive motility, viability, normal morphology, acrosome integrity and membrane integrity were significantly higher in samples containing 1.50 mg/mL cholesterol-loaded cyclodextrin (P<0.05).
Concerning the interaction between cholesterol-loaded cyclodextrin and lecithin, results revealed that compared to the control group,individual motility, viability, morphology, acrosome integrity and membrane integrity were significantly improved in samples containing 1.50 mg/mL cholesterol-loaded cyclodextrin (P<0.01).
The interaction between extenders and concentration of cholesterolloaded cyclodextrin on semen quality parameters revealed that the best (1.50 mg/mL) at pre-freeze stage, about 10% higher motility and membrane integrity as well as about 6% higher viability,morphology and acrosome integrity were recorded in TCFGL as compared to TCFYG extender groups.
3.2. Effects of cholesterol-loaded cyclodextrin concentrations on post-thawed semen quality variables with use of TCFYG and TCFGL extenders in buffalo bulls
Table 2 shows the effects of cholesterol-loaded cyclodextrin on post freezing semen variables after one week storage in TCFYG and TCFGL diluents. All studied parameters in semen containing different concentrations of cholesterol-loaded cyclodextrin were higher in lecithin (TCFGL) as compared to egg yolk (TCFYG)-based extenders.
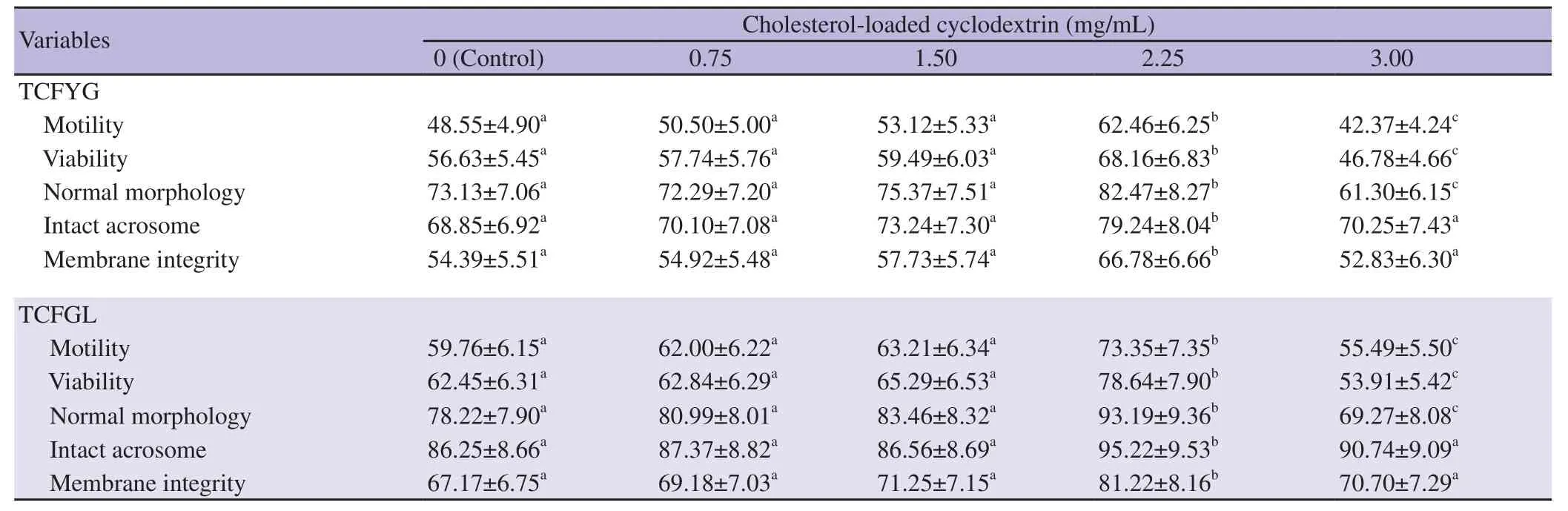
Table 2. Effects of cholesterol-loaded cyclodextrin concentrations on post-thawed semen quality variables with use of TCFYG and TCFGL extenders in buffalo bulls (%).
The interaction between cholesterol-loaded cyclodextrin and egg yolk demonstrated that in comparison with the control group,progressive motility, viability, morphology, acrosome integrity and membrane integrity were significantly ameliorated by the addition of 2.25 mg/mL cholesterol-loaded cyclodextrin (P<0.01).
With respect to the interaction between cholesterol-loaded cyclodextrin and lecithin, the lecithin-based extender containing 2.25 mg/mL cholesterol-loaded cyclodextrin exerted a significant improvement concerning progressive motility, viability, morphology,acrosome integrity and membrane integrity as compared to the control samples (P<0.01).
The interaction between extenders and concentration of cholesterolloaded cyclodextrin on semen quality parameters revealed that the best (2.25 mg/mL) at post-thaw stage, about 10% higher motility, viability and normal morphology as well as about 15% higher acrosome integrity and membrane integrity were recorded in lecithin-based extender group as compared to egg yolk-based extender group.
It is noteworthy to mention that the higher level of cholesterolloaded cyclodextrin (3.00 mg/mL) at the pre- and post- freeze stages deteriorated significantly progressive motility, viability and morphology percentages of spermatozoa in both TCFYG and TCFGL extenders as compared to the control group (P<0.01). On the other hand, this higher cholesterol-loaded cyclodextrin level did not affect both acrosome and membrane integrities compared to the control group in the two extenders during pre- and postfreezing stages.
4. Discussion
In this study, we investigated the beneficial effects of cholesterolloaded cyclodextrin enrichment in egg yolk- and lecithin- based extenders on the pre-freeze and post-thaw semen quality variables.Concerning combination effects of cholesterol-loaded cyclodextrin with both extenders, Moce and Graham[17] suggested that cholesterol and sperm need to co-incubate in a medium which is lipid-free.Otherwise, most of the cholesterol added to sperm in the presence of egg yolk or lecithin will be transferred to both extenders’ lipid droplets rather than the sperm, and thereby cannot benefit sperm viability after cooling and freezing. Based on this suggestion, sperm cells were incubated at 37 ℃ for 15 min in 0.75, 1.50, 2.25 and 3.00 mg/mL cholesterol-loaded cyclodextrin before being processed in TCFYG or TCFGL extenders. Concerning progressive motility,viability, morphology, intact acrosome and membrane integrity of cooled and post-thaw sperm, the results of the present study showed that samples incubated in extender containing lecithin were better than that containing egg yolk. This result indicates that lecithin could act more synergistically with cholesterol-loaded cyclodextrin at low temperature than did egg yolk. To our best knowledge, this is the first study on the effect of different concentrations of cholesterolloaded cyclodextrin on quality parameters of semen extended in soya lecithin. This finding re-confirmed the substitution of egg yolk with lecithin in semen extenders to achieve optimal semen quality results. However, combination effects of egg yolk and cholesterolloaded cyclodextrin revealed significant improvement of sperm motility, viability and integrities of acrosome and plasma membrane in buffalo bulls[1,14,6,15,44,45], stallion[25,26,46], bovine bulls[16,18,47],rams[27-31] and goats[32-35].
The interaction between different concentrations of cholesterolloaded cyclodextrin, used extenders and stage of freezing process advocated that 1.50 mg/mL cholesterol-loaded cyclodextrin concentration improved pre-freeze semen characteristics in the two used extenders. In addition, increasing cholesterol-loaded cyclodextrin concentrations to 2.25 mg/mL in both extenders significantly and positively affected the post-thaw semen traits. In agreement with our observations, Rajoriya et al[6] and Longobardi et al[15] found that buffalo semen extended in egg yolk-based extender with 1.50 and 2.25 mg/mL cholesterol-loaded cyclodextrin were effective in increasing percentage of intact (non-capacitated)acrosome and percentage of plasma membrane integrity. The improved membrane stability and hence increased sperm motility and viability percentages as well as reduced sperm capacitation(increased acrosome integrity) are likely related to a direct effect of cholesterol-loaded cyclodextrin on the sperm membrane cholesterol: phospholipid ratio. This is particularly important because the percentage of sperm undergoing cryocapacitation in buffalo bulls is much higher than in other male domestic species[3]. Efflux of sperm membrane cholesterol during cryopreservation may occur due to prolonged exposure of cells with detrimental proteins of seminal plasma[45,47]. Bellin et al[48] have shown that most abundant proteins from bovine seminal plasma bind to sperm membrane cholesterol,resulting in latter efflux, an important step of sperm capacitation to occur prior to fertilization[49].
Studies have shown that the greater the cholesterol content of plasma membrane, the less flexible and fluid it is[7]. The cholesterol content and cholesterol : phospholipid ratio of fresh sperm membranes plays an important role in the resistance of the sperm to cold shock damage[50]. Rajoriya et al[6] found that the cholesterol and phospholipid contents of fresh buffalo sperm plasma membrane were 18.9% and 57.2%, respectively, and the cholesterol : phospholipid ratio was found to be 0.34, which is an intermediate value between cattle bull and boar spermatozoa[8]. In confirmation to the current results, Rajoriya et al[6] observed that although a significant loss of cholesterol from sperm membranes after cryopreservation was intended, the incubation of cholesterol-loaded cyclodextrin to buffalo sperm prior to processing maintained significantly higher cholesterol levels and cholesterol : phospholipid ratio in the sperm after freezing and thawing across different cholesterol-loaded cyclodextrin treatment concentrations than the control sperm. It has been shown that cholesterol has a stabilizing effect on sperm plasma membrane[9], and its efflux is expected to cause destabilization of membrane[51].
Spermatozoa plasma membrane integrity and plasma membrane fluidity is a prerequisite for maintenance of optimal fertility. In the present study, proportional increase in the plasma membrane and acrosome integrities of cholesterol-loaded cyclodextrin-incubated spermatozoa than those of the control spermatozoa was observed during pre-freeze and post-thaw stages. This may be attributed to the fact that lipid phase transition is eliminated or the temperature at which it occurs is lower for cholesterol-loaded cyclodextrin incubated spermatozoa than for control sperm. In agreement to this view point, Moce and Graham[17] mentioned greater plasma membrane fluidity at lower temperature for bull sperm with higher cholesterol : phospholipid ratio than for un-treated sperm. At the same time, greater cholesterol : phospholipid ratio of cholesterolloaded cyclodextrin-incubated sperm cells results in increasing sperm membrane permeability to cryoprotectants and lessening osmotic stress, as it was shown that cholesterol-loaded cyclodextrinincubation increases the osmotic tolerance of stallion sperm[24].
Our results showed that the decreased plasma membrane integrity(high plasma membrane fluidity) in frozen-thawed than that in prefreeze spermatozoa in the control group might be due to efflux of cholesterol from plasma membrane and consequently low cholesterol: phospholipid ratio. This increased fluidity and permeability results in increased influx of extra-cellular calcium into the spermatozoa.Calcium plays an important role in premature capacitation and acrosome reaction during cryopreservation, consequently affecting sperm surviving cryoprotection[52,28]. However, when sperm cells were incubated in 1.50 and 2.25 mg/mL cholesterol-loaded cyclodextrin during pre-and post- freezing stages, respectively, in lecithin-based extender and, with a lesser magnitude, in egg yolkbased extender, the acrosome and plasma membrane integrities were improved and motility and viability of cryopreserved spermatozoa were ameliorated.
In the present work, percentages of acrosome and plasma membrane integrities containing relatively very high cholesterol(3.00 mg/mL cholesterol-loaded cyclodextrin group vs. the control,0.75, 1.50, 2.25 mg/mL cholesterol-loaded cyclodextrin groups)were significantly deteriorated, suggesting inhibitory action of cholesterol addition above threshold level (3.00 mg/mL) on acrosome and membrane integrities. This finding confirms studies of other workers in buffalo bulls[53,15] and stallion[54] observing that relatively very high levels of cholesterol in the membrane interfere with the physiological process of sperm capacitation through increased stability of acrosome and plasma membrane. Furthermore,it has been shown that higher cholesterol-loaded cyclodextrin levels increased not only membrane rigidity, which decreased the motility and survival rates[55], but also the oxidation process of cholesterol which produced oxysterols that are toxic to spermatozoa[56].
There are some limitations in the study. The use of cholesterolloaded cyclodextrins enriched extenders in buffalo in vivo fertility test (female insemination) to get the conception rate is needed.
In conclusion, cholesterol-loaded cyclodextrin supplementation at 1.50-2.25 mg/mL concentration could improve pre-frozen and postthawed buffalo sperm quality. The most suitable is 1.50 mg/mL at pre-freeze stage and 2.25 mg/mL post-freezing.
Conflict of interest statement
The authors declare that they do not have any conflict of interest and all persons gave their informed consent prior to their inclusion in the study.
Funding
The study received no extramural funding.
Authors’ contributions
The authors had performed all the items of the experimental design,the collection of semen, the diluting concentrations, the freezing process, semen evaluation and the preparing of the manuscript.
 Asian Pacific Journal of Reproduction2022年3期
Asian Pacific Journal of Reproduction2022年3期
- Asian Pacific Journal of Reproduction的其它文章
- Sjögren’s syndrome and reproductive outcomes
- Antioxidant potential of pentoxifylline on spermatozoa of small ruminants
- Placental pathologies and fetal outcome in pregnant women with COVID-19: A retrospective study
- Sperm DNA fragmentation does not affect the clinical outcomes in the cumulative transfers of an ICSI cycle along with blastocyst transfers in couples with normozoospermic male patients
- Investigation of FOXP3 (rs3761548) polymorphism with the risk of preeclampsia and recurrent spontaneous abortion: A systemic review and meta-analysis
- Oxidative stress and female reproductive disorder: A review
