Effect of Zhiyang Pingfu Liquid on epidermal growth factor receptor inhibitor-related skin lesion
Xu Zhang, Ke-Xin Tan, Jia Li, Chong-Xiang Xue, Xing-Yu Lu, Hui-Jing Dong, Yi-Xuan Yu, Zi-Xin Hu, Hui-Juan Cui
1. Beijing University of Chinese Medicine, Beijing 100029, China
2. Integrative Oncology Department, China-Japan Friendship Hospital, Beijing 100029, China
ABSTRACT Objective: To verify the efficacy of the Chinese medicine “Zhiyang Pingfu Liquid” on the lesion associated with Epidermal Growth Factor Receptor Inhibitors (EGFRIs). Methods:Female BN rats were divided into Control group and Gefitinib group randomly. The Gefitinib group was administered gefitinib for 21 days. After 21 days, the rats in the Gefitinib group were grouped again and randomly divided into Model group, Gefitinib + ZY group, and Gefitinib+ NS group. Starting from day 22, rats in Gefitinib+ZY or NS were given different drugs for 7 days besides the other conditions are as the same as before. Observe the morphological changes and histopathological changes of the skin during the research. The changes of inflammatory factors such as TNF-α and IL-6 in the serum of were detected by ELISA.Results: The application of “Zhiyang Pingfu Liquid” for 7 days could significantly reduce the skin inflammation whether in gross or pathological view. The concentration of TNF-α and IL-6 in Gefitinib+ZY is significantly lower than those in the Model group (P=0.002, P=0.002)and there is no significant changes compared with the Control group (P=0.279, P=0.165).Conclusion: Chinese herbal “Zhiyang Pingfu Liquid” can reduce the lesion and inflammatory caused by EGFRIs.✉Corresponding author: CUI Hui-juan, M.D., Chief Physician.E-mail:
Keywords:Zhiyang Pingfu Liquid Epidermal growth factor receptor inhibitors Skin lesion Efficacy
1. Introduction
EGFRIs have become the standard first-line treatment for a variety of malignancies such as advanced lung cancer with positive EGFR gene mutation and advanced colorectal cancer with wild-type KRAS gene. Among the adverse reactions related to EGFRIs, with an incidence rate of up to 100% [1], of which rash is the most common[2], which has become the most important factor affecting the quality of life of patients with advanced tumors. [3].
Regarding the occurrence of EGFRIs-related rash, it is currently believed that there is a clear relationship with the inflammatory response of keratinocytes [4, 5], and the main mechanisms include abnormal secretion of pro-inflammatory factors and chemokines[3, 6], keratinocytes abnormal proliferation and differentiation [7].In terms of treatment, domestic and foreign clinical guidelines all use tacrolimus, doxycycline, minocycline and other antibiotics or hormones for topical or oral use as the recommended treatment plan[8-12], but the clinical effect is not satisfactory.
Previous literature and studies [13, 14] have found that traditional Chinese medicine has good efficacy and high safety in the treatment of targeted drug-related rashes, and has been recommended by domestic guidelines [12]. It is believed that adverse reactions such as rashes caused by targeted drugs can be attributed to the categories of “acne”, “drug poison eruption” and “pulmonary wind acne”in traditional Chinese medicine. Its etiology and pathogenesis are nothing more than two aspects: internal and external factors. The internal factors are mainly related to the lack of endowment, and the external factors are mainly related to drugs and toxins and the obstruction of the skin and other factors such as “wind, dampness,heat, poison and blood stasis” [12].
Professor Cui Huijuan [15] believed that although the EGFRIsrelated rash is on the skin, the root is the disease of the lung meridian, because “the skin is dominated by the lung”. If windheat attacks the lung, rashes, itching, pustules, etc. may be seen.The long-term course of the disease leads to the injury of both qi and yin, which can lead to dry skin, scaling and other symptoms.Therefore, in the treatment of rashes, it is emphasized that “clearing heat and detoxifying, drying dampness and swelling, and expelling wind and relieving itching” are the basic principles, so that “the heat can be eliminated, the dampness can be removed, and the wind can be stopped”, then the disease can be cured. Based on rich clinical experience, Professor Cui created a pure Chinese medicine external preparation “Zhiyang Pingfu Liquid”.
“Zhiyang Pingfu Liquid” is mainly composed of Scutellaria baicalensis Georgi (Huangqin), Sophora flavescens Ait (Kushen),Dictamnus dasycarpus Turcz (Baixianpi), Portulaca oleracea L(Machixian). Among them, Scutellaria baicalensis Georgi is the monarch medicine in the prescription. Its nature and taste are bitter and cold. It enters the lung meridian and the large intestine meridian,etc., and exerts the effects of clearing away heat and dampness,purging fire and detoxifying. Sophora flavescens Ait and Portulaca oleracea L are both minister medicines, assisting the monarch medicine to strengthen its heat-clearing and detoxifying power.Dictamnus dasycarpus Turcz used as a medicine to assist the envoys,helping the monarchs and ministers to enhance the effect of clearing away heat and detoxification, and at the same time, it can also exert its curative effect of dispelling wind and relieving itching. The whole formula is rigorous and exquisite, and it has the functions of clearing away heat and detoxifying, drying dampness and reducing swelling,dispelling wind and relieving itching.
“Zhiyang Pingfu Liquid” has been used in clinical practice for more than 10 years as a clinical experience formula. Its curative effect on EGFRIs-related rash is clear [16-18], and the effective rate is over 90%. It has been included in the expert consensus [12]. Based on the basis of previous clinical research, our research group conducted the following basic experiments to verify its efficacy, provide more sufficient evidence for further clinical application and research, and lay a foundation for further mechanism research.
2. Materials and methods
2.1 Animals
BN rats, female, weighing in the range of 100-120 g. The animals were purchased from Beijing Weitong Lihua Laboratory Animal Technology Co., Ltd. The rearing environment is the SPF animal room of the Medical Laboratory Animal Platform of the Institute of Clinical Medicine of the China-Japan Friendship Hospital (SYXK(Beijing) 2016-0043).
2.2 Drugs
Gefitinib tablets: Trade named Iressa, purchased from AstraZeneca.“Zhiyang Pingfu Liquid”: Which is composed of Scutellaria baicalensis Georgi (Huangqin), Sophora flavescens Ait (Kushen),Dictamnus dasycarpus Turcz (Baixianpi), Portulaca oleracea L(Machixian) was supplied by China-Japan Friendship Hospital Pharmacy Department.
2.3 ELISA kit
Rat TNF-α ELISA kit and rat IL-6 ELISA kit were purchased from Raybio Company.
2.4 Establishment of animal model
BN rats were randomly divided into Control group and Gefitinib group. The control group did not do any intervention; the rats in the gefitinib group were given gefitinib by gavage. Gefitinib was administered at a dose of 37.5 mg/kg qd for 21 days.
2.5 Groups and interventions
On the 22nd day of the experiment, the rats in the gefitinib group were divided into groups again, and they were randomly divided into 6 rats in the model group, 9 rats in the gefitinib + “Zhiyang Pingfu Liquid” group (Gefitinib + ZY), 6 rats in the Gefitinib +saline group (Gefitinib + NS), and the back hair of the rats was shaved. The rats in each experimental group continued to be given gefitinib, and the dosage was as the same as before. At the same time, the Geftininb+ZY group began to use the traditional Chinese medicine, and the Gefitinib+NS group received the same dose of saline. The dosage of external drug in each experimental group was 1.4ml per animal, once a day. After 30 minutes of administration, the residual drug on the skin was wiped off with water, and the drug was administered continuously for 7 days.
2.6 Monitoring indicators
2.6.1 Grades of rash
Before the topical drug intervention and 7 days after the intervention, the rashes on the shaved parts of the back of the rats were recorded in detail and graded strictly according to the standard[19]. (Table 1)
2.6.2 Skin inflammation status score
Before and 7 days after the topical drug intervention, the inflammatory state of the back skin of the rats was recorded in detail and scored strictly according to the scoring standard [20]. (Table 2)
2.6.3 Morphological changes of skin
During the experiment, a digital camera was used to photograph and record the skin on the back of the rat, and the morphological changes of the skin of the rat were dynamically observed during the experiment.
2.6.4 Pathological changes of skin
The skin samples were fixed with 10% formalin solution,embedded in paraffin, and then sliced. After sectioning, standard treatments such as dewaxing and ethanol dehydration with different concentration gradients were performed, and hematoxylin-eosin(HE) staining was performed.2.6.5 Inflammatory factors in serum
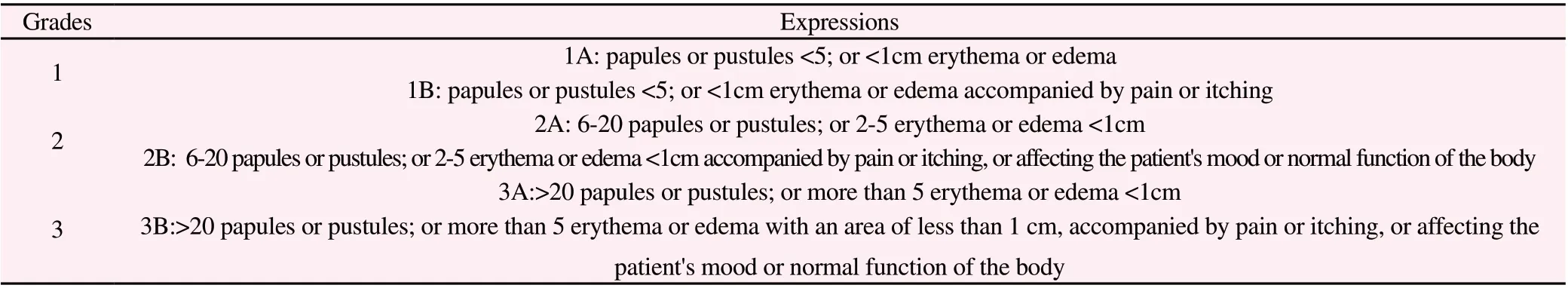
Table 1 MASCC Grading Standards
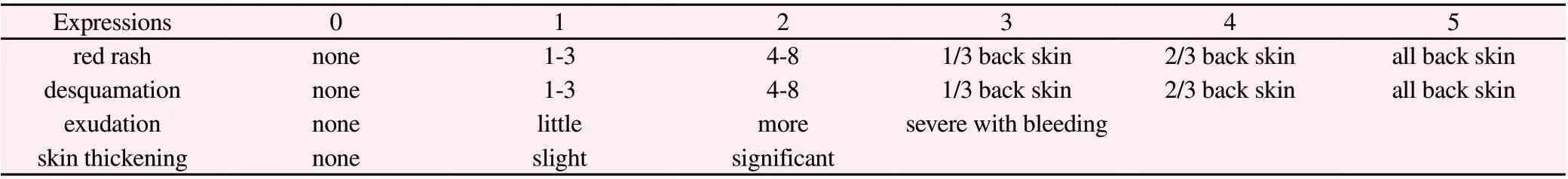
Table 2 Inflammation status score of skin
The contents of inflammatory factors TNF-α and IL-6 in serum of rats were determined by ELISA. BN rats were anesthetized, 5ml of blood from the apex of the heart was taken, left standing for 1-2 hours, centrifuged with a centrifuge, after centrifugation, about 1ml of supernatant was drawn with a pipette, stored in a 2ml EP tube,and stored in - 80 ℃ refrigerator to be tested. The experimental operation was carried out according to the kit instructions, and the concentration of the target inflammatory factor in the serum of the sample was finally measured and recorded.
2.7 Statistical methods
SPSS 20 statistical software was used for statistical analysis,and the measurement data in the data were expressed as mean ±standard deviation. The overall data obeyed the normal distribution or approximately normal distribution, and the t test was used for analysis; if the data did not conform to the normal distribution,the nonparametric test was used. The multi-sample means were compared using analysis of variance. Fisher's exact probability method was used for comparison of drug efficacy.
2.8 Ethical Approval
This study was approved by the Ethics Committee of the Medical Laboratory Animal Platform, Institute of Clinical Medicine, China-Japan Friendship Hospital (No. 190114).
3. Results
3.1 Reducing the grade of the rash
Except for the rats in the control group, the rats in each group developed rashes of different degrees on the back of the neck and behind the ears; during this experiment, the rash on the back and behind the ears of the rats was mainly concerned. Through the analysis of the experimental results, it was found that the rash on the back of the rats in the Model group gradually aggravated with the prolongation of the medication time; the rash of the rats in the Gefitinib+ZY group began to alleviate after 3 days of treatment with traditional Chinese medicine, and a total of 8 rats had more obvious rash after 7 days of treatment than before treatment. Only 1 rat in the Gefitinib+NS group had better skin rash than before treatment.In terms of effective rate, the effective rate (88.89%) of " Zhiyang Pingfu Liquid " was significantly higher than that of normal saline(16.67%) (P=0.001). (Table 3)
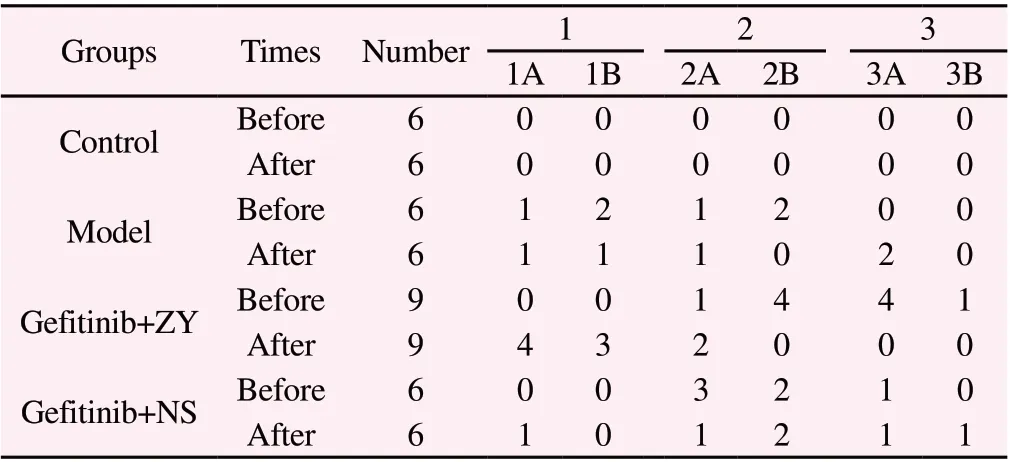
Table 3 Grades of rash
3.2 Decreasing skin inflammation score and reduce inflammatory response
Inflammation scores were scored and recorded on the dorsal skin of rats before and after topical drug intervention (Table 4).Through statistical analysis, it was found that after the application of traditional Chinese medicine, the inflammation score in the Gefitinib+ZY group was significantly lower than that in the Model group and Gefitinib+NS group (P=0.001). (Figure 1)

Table 4 Skin inflammation status score (mean ± standard deviation)

Figure 1 The inflammatory status scores of the rats before and after the experiment.(Remarks: **p<0.01)
3.3 Skin morphological changes
3.3.1 Stage of model establishment
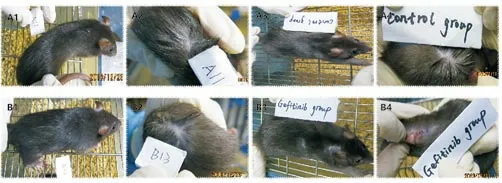
Figure 2 The different skin expressions before experiment and after 21days.
It can be seen from Figure 2 that the skin of the control group did not change significantly before and after the experiment, while the rats in the gefitinib group had typical red papules. The manifestations of skin inflammation such as crusting are consistent with the clinical and literature reports.
3.3.2 Before and after topical drug intervention
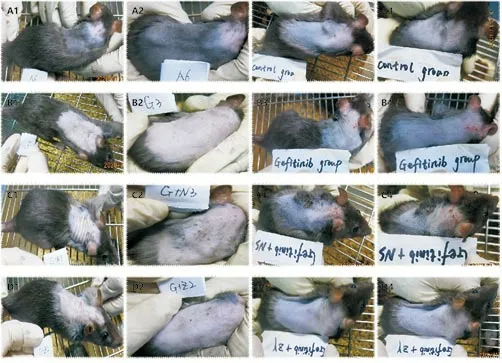
Figure 3 The different expressions before and after topical drug intervention.
It can be seen from Figure 3 that there was no significant change in the skin manifestations in control group; the skin manifestations of the rats in the Model group and Gefitinib+NS group were significantly worse than those before 7 days, especially the skin behind the ears of the rats. Inflammatory manifestations such as crusting and exudation were observed; while in Gefitinib+ZY group,the rash on the back of the rats subsided significantly after 7 days of application of “Zhiyang Pingfu Liquid”.
3.4 Skin pathology
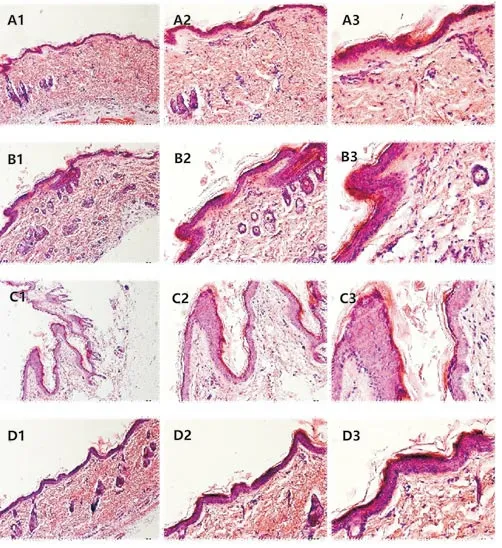
Figure 4 The pathological expressions of rats in different groups.
Compared with the control group, both the Model group and the Gefitinib+NS group had a large number of inflammatory cell infiltration, epidermal thickening, acantholysis, parakeratosis and other manifestations of skin inflammation. While the rats in the Gefitinib+ZY group showed better performance same as the control group. There was no significant difference, and the inflammation was not obvious.
3.5 Inflammatory factors
3.5.1 TNF-α concentration (Table 5)
In terms of TNF-α, the concentration of Model group was significantly higher than that of control group (p<0.001). The TNF-α concentration in the Gefitinib+ZY group was significantly lower than that in the Model group (p=0.002), and there was no significant difference compared with the control group (p=0.279).Compared with the control group, the concentration of TNF-α in the Gefitinib+NS group was significantly higher (p=0.002), but not significantly lower than that in the Model group (p=0.210). (Figure 5.A)
3.5.2 IL-6 concentration (Table 5)
In terms of IL-6, the concentration of Model group was significantly higher than that of control group (p<0.001). The concentration of IL-6 in the Gefitinib+ZY group was significantly lower than that in the Model group (p=0.002), but had no significant change compared with the control group (p=0.165). The concentration of IL-6 in the Gefitinib+NS group was significantly lower than that in the Model group (p=0.029), and there was no significant change compared with the control group (p=0.064). (Fig. 5.B)
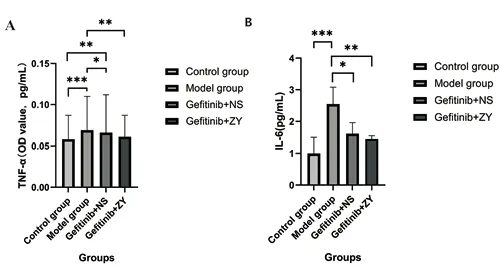
Figure 5 TNF-α and IL-6 concentration in the groups.(Remarks: *p<0.05,**p<0.01,***p<0.001.)

Table 5 The concentration of TNF-α IL-6 (Mean ± standard deviation, pg/mL)
4. Discussion
According to previous research [21], after 21 days of gefitinib application, the EGFRIs-related rash model can be successfully established, and the animal skin will have obvious red papules,crusting, exudation, etc., and obvious inflammation can be seen in the skin pathology. In addition, the concentration of inflammatory factors such as TNF-α and IL-6 in serum will be significantly higher than normal. In this study, the rats in the gefitinib group also showed the same performance after the application of gefitinib,and the blood indexes were significantly improved compared with the control group, which was similar to the previous research basis and literature reports [22-25]. Thus we could verify the successful establishment of the rash animal model.
Starting from the 22nd day of the experiment, except for the control group and the Model group, each study group started to use different drugs externally on the basis of oral gefitinib. In the study, we found that in terms of the general skin phenotype, the red papules and scabs of the rats in the Model group gradually increased with the prolongation of gefitinib administration time. The scabs and exudation were the most serious in each group, and the inflammation score was higher. The rats in the Gefitinib+NS group were treated with normal saline, the red papules, scabs, and exudation in the rats were not significantly relieved compared with before, and even gradually. In the Gefitinib+ZY group, the red papules, scabs,exudation and other manifestations of the rats began to gradually reduce after 3 days of application of " Zhiyang Pingfu Liquid ".After 7 days of application of traditional Chinese medicine, the inflammatory manifestations such as exudation were significantly relieved compared with those before treatment and those in the Model group, and some rats' body surface crusting and exudation even disappeared completely. The inflammation score was the lowest among the experimental groups.
From the skin pathological observation, the HE staining of the skin of the rats in the Model group and Gefitinib+NS group showed obvious inflammatory manifestations such as epidermal thickening and neutrophil infiltration, which were similar to previous reports[22, 26, 27]. Compared with the control group, there was no significant difference in skin HE staining in the Gefitinib+ZY group, and there was no significant difference in epidermal thickness and infiltration of neutrophils. Therefore, in terms of skin manifestations alone, "Zhiyang Pingfu Liquid " has a significant effect in treating rashes related to target drugs. The results of this study are consistent with previous clinical studies.
As far as hematological indicators are concerned, the concentration of inflammatory factors such as TNF-α and IL-6 is closely related to the degree of inflammation. [29]. Mechanistically, TNF-α can activate the EGFR signaling pathway in keratinocytes and promote the phosphorylation of EGFR, but if it is overexpressed, it will promote the expression of inflammatory mediators in keratinocytes,leading to the occurrence of inflammatory responses [30]. IL-6 as the core mediator of inflammation [31], has the effect of catalyzing and amplifying immune inflammation [29, 32, 33]. In the blood of patients with target drug-related rash, the expression of cytokines such as TNF-α and IL-6 will be significantly increased [3], which may be an important factor leading to EGFRIs-related rash [6]. Similarly,in this study, the serum concentrations of TNF-α and IL-6 in the Model group were significantly higher than those in the normal rat,which was consistent with previous literature and so on, maybe the important reasons for the inflammatory reaction such as obvious red papules and skin scabs appeared in rats.
Studies have confirmed that if TNF-α, IL-1 and other antagonists are used for treatment, the adverse reactions such as rash will be significantly alleviated [23, 24]. In this study, after the Gefitinib+ZY group applied " Zhiyang Pingfu Liquid ", the serum levels of inflammatory factors TNF-α and IL-6 were significantly lower than those of Model, and had no significant difference with the control group. Compared with the Model group, the inflammatory factor TNF-α concentration of the rats in the Gefitinib+NS group was not different but was still significantly higher than that of the control group. Therefore, in terms of serological indicators, " Zhiyang Pingfu Liquid " can reduce the levels of inflammatory factors such as TNF-α and IL-6, and alleviate skin rash and other reactions in rats, and its effectiveness has been further confirmed.
In addition, modern pharmacological studies have found that the main drug components of " Zhiyang Pingfu Liquid " include Sophora flavescens Ait [34, 35], Scutellaria baicalensis Georgi [36],Dictamnus dasycarpus Turcz [37], Portulaca oleracea L [38, 39] can down-regulate the expression of cytokines such as TNF-αand IL-6 in peripheral blood, and exert an anti-inflammatory effect. Therefore,based on the above research results, we can confirm that "Zhiyang Pingfu Liquid" can down-regulate the levels of inflammatory factors such as TNF-α and IL-6 in the blood, and significantly reduce the skin lesions caused by EGFRIs.
Conflict of Interest: No conflict of interest.
Author contributions: ZHANG Xu for the conception, writing and revision, experimental design and operation of the article. CUI Huijuan for the experimental design, article conception, manuscript revision and proofreading. TAN Ke-xin for the article revision and data analysis. LI Jia and XUE Chong-xiang for the experimental operation and picture information Collection. LU Xing-yu, DONG Hui-jing, YU Yi-xuan, HU Zi-xin proofread the content of the article and collect and check the data.
 Journal of Hainan Medical College2022年7期
Journal of Hainan Medical College2022年7期
- Journal of Hainan Medical College的其它文章
- Analysis on medication rule of traditional Chinese medicine treating chemotherapy-induced diarrhea based on traditional Chinese medicine(TCM) inheritance computing platform system
- Study on medication rules of traditional Chinese medicine for Meniere's disease based on data analysis
- Efficacy and safety of traditional Chinese medicine in the treatment of coronary heart disease complicated with anxiety and/or depression after PCI: A systematic review and meta-analysis
- Effect of Xifeng Capsule on blood stasis in patients with rheumatoid arthritis by regulating miR-126-VEGF/PI3K/AKT signaling pathway
- Design and characterization of a bifunctional bybrid antibacterial peptide LLH for bactericidal/endotoxin neutralization effects
- Box-Behnken response surface method combined with fingerprint to optimize the extraction process of total anthraquinone from Cassia seeds
