Application of nanomaterial for enhanced oil recovery
Tuo Ling ,Ji-Rui Hou ,Ming Qu ,*,Ji-Xin Xi ,Infnt Rj
a Unconventional Oil and Gas Research Institute,China University of Petroleum,Beijing,PR China
b Oil and Gas Technology Research,Changqing Oilfield Branch Company,Xi'an,Shanxi,710018,PR China
Keywords:Nanoparticle Preparation method of amphiphilic nanomaterial Stability Mechanism N-EOR
ABSTRACT Nanofluid offers more opportunities and challenges over the traditional surfactant and polymer solutions during enhanced oil recovery (commonly referred to as tertiary oil recovery) due to its remarkable properties.This review mainly discusses the preparation methods of amphiphilic nanoparticles due to their higher interface activity than sole hydrophilic or hydrophobic nanoparticles (SHNPs).The nanofluids' stability is reviewed in this work.Moreover,the mechanisms of nanofluids in enhancing oil recovery (N-EOR) in terms of interfacial tension reduction,wettability alteration,foam stabilization,emulsion stabilization,structural disjoining pressure,and depressurization-increasing injection are mainly summarized and reviewed.Also,the synergistic effects of nanofluids and traditional surfactants and polymers are discussed.Finally,nanofluids’ challenges and prospects are also outlined.The nanofluids can still be regarded as an outstanding candidate for enhancing oil recovery significantly in the future although there are limitations on their applications from laboratory scale to field scale.
1.Introduction
Over recent decades,there has been a distinct contradiction that the amount of produced oil cannot meet the social development needs.Even though renewable energy has grabbed the global market's attention,the demand for crude oil still exists(Aftab et al.,2017;Rezk and Allam,2019).Hence,crude oil will act as a primary energy source for the development of human society.However,the current methods for recovering oil have been facing severe challenges.Thereby,developing novel technologies or materials for extracting crude oil significantly in the future is essential.
Generally,the crude oil extraction process can be categorized into primary,secondary,and tertiary oil recovery stages.Usually,40-50%of crude oil can be recovered through the primary(natural energy-driven) and secondary (water-driven) stages,respectively(Ahmed,2010).After those two stages,tertiary oil recovery techniques are crucial to further extract residual crude oil trapped within reservoir pores and channels.Traditional tertiary oil recovery technologies can be classified into chemical flooding methods,thermal recovery methods,and miscible flooding methods.Miscible flooding refers to the technique that the displacing phase(such as CO2,flue gas,liquefied petroleum gas,methane,etc.) can mix up with crude oil above the minimum miscibility pressure(MMP) to enhance oil recovery (EOR) due to the disappearance of interfaces.However,miscible flooding also has certain limitations.For example,it is difficult to achieve the required pressure from 610 to 1524 m (Alnarabiji and Husein,2020).Another obstacle is the formation of gas channeling during the high viscosity oil displacement process.The thermal recovery methods such as steam flooding,huff and puff,and fire reservoir technologies utilize the high temperature to decrease highly viscous oil viscosity.The realistic challenges for widely applying thermal methods are the lower thermal efficiency and higher construction costs(Shah et al.,2010).In terms of chemical flooding processes,injection of surfactant or polymer or alkali solutions into the reservoir helps to reduce the interfacial tension (IFT) of the oil-water interface,alter the wettability of rock (oil-wet to water-wet or neutral-wet) and improve the micro or macro sweep efficiency (Tod et al.,2020;Esfandyari et al.,2020;Klemm et al.,2018;Seetharaman et al.,2020;Shaker Shiran and Skauge,2013;Tackie-Otoo and Ayoub Mohammed,2020).However,the degradation of polymers and adsorption of surfactants during the migration forward at harsh reservoir conditions (high temperature,high pressure,and high salinity) limit the usage of chemical agents.Also,chemical agents are not environmentally friendly and economical as the oil price is depressed(Abidin et al.,2012;Negin et al.,2017;Shah et al.,2010).Hence,there is an urgent for new EOR technologies or novel materials to substitute the traditional EOR technologies or agents for enhancing oil recovery.
Nanotechnology has been widely implemented in various sectors like medical,food,electronics,oil,and gas(Konefal et al.,2020;Lee et al.,2018;Li et al.,2017b;Morozovska et al.,2020;Singh et al.,2021;Xiong et al.,2020).Nanotechnologies are commonly referred to as the applications of nanomaterials possessing nano-scale size(1-100 nm) at least in one dimension.Nanomaterials have distinctive properties such as nano-scale size,quantum effects,and massive surface area (AfzaliTabar et al.,2017;Kazemzadeh et al.,2018;Lau et al.,2017).According to their shapes,nanomaterials can be classified into spherical-like shape,rod-like shape,and sheet-like shape(Hong et al.,2006;Qu et al.,2020;Raj et al.,2019;Yan et al.,2013).Over the decades,nanomaterials enhancing oil recovery(N-EOR)technologies have shown significant successes in overcoming the limitations faced by traditional enhancing oil recovery technologies (Foroozesh and Kumar 2020).Nanomaterials are commonly dispersed into specific fluids such as water,ethanol,or other dispersants (forming nanofluids) for improving oil recovery during N-EOR processes.The improvement in oil recovery is due to the massive surface area and dangling bonds of nanomaterials,making them easier to interact with the surrounding materials (AfzaliTabar et al.,2017;Kazemzadeh et al.,2018;Lau et al.,2017;Sofla et al.,2018).
Applications of various nanomaterials for N-EOR have gained considerable attention worldwide.Reported nanomaterials for NEOR include SiO2(Lu et al.,2017),TiO2(Sohel et al.,2008),MoS2(Qu et al.2020,2021),Al2O3(Rezvani et al.,2020),CuO (Bahraminejad et al.,2019),ZnO(Alnarabiji et al.,2018)and graphene(AfzaliTabar et al.,2017).The nanofluids,injected into the oil-bearing area,can reduce IFT of the oil-water interface (Esmaeilzadeh et al.,2014),change the rock surface's wettability from oil-wet to water-wet(Hill et al.,2020),generate structural disjoining pressure at the three-phase contact region(Liang et al.,2020b;Zhang et al.,2016)and reduce oil viscosity(Patel et al.,2018).Although nanomaterials'dosage concentration is lesser than conventional surfactants or polymers,they promise to enhance oil recovery significantly in the future.The prerequisite for broad applications of nanofluids is to guarantee their stability before they are injected into core samples or actual reservoirs with harsh conditions.Unstable nanofluids can reduce reservoir permeability and damage pore-throat structures due to nanomaterials'aggregation and sedimentation behaviors at harsh reservoir conditions (Chakraborty and Panigrahi,2020).The addition of surfactants or polymers into nanofluids has been conducive to improving nanofluids'stability(Rezk and Allam,2019;ShamsiJazeyi et al.,2014).Stable nanofluids can migrate deep into the reservoirs and play a significant role in extracting crude oil trapped within reservoir pores and channels.
So far,many articles have reviewed the applications of nanomaterials to improve oil recovery due to their promising prospects(Ali et al.,2020;Hajiabadi et al.,2020;Khalil et al.,2017;Li et al.,2021).Yakasai et al.(2021) has mainly reviewed the effects of various nanomaterial parameters(size,morphology,concentration,temperature,pH value,salinity,etc.) on enhancing oil recovery during flooding processes.Besides,the applications of nanomaterials have also been comprehensively discussed in the IFT reduction,wettability alteration,asphaltene precipitation,and emulsion stability.The manufacturing of bionanomaterials for enhancing oil recovery has been reviewed and discussed by Agi(Agi et al.,2021).However,the large-scale production of bionanomaterials is the major hindrance faced by these materials.Carbon-based nanomaterials have also gained more considerable attention due to their unexpected properties and efficiencies(Sikiru et al.,2021).Hajiabadi et al.(2020) has shown a comprehensive review of the carbon-based nanomaterials on EOR,drilling,formation evaluation and seismic characterization.In addition to study nanofluids for enhancing oil recovery by using physical simulation experiments,the numerical simulation methods are also conducive to facilitating the development of nanofluids in improving oil recovery.Aliu et al.(2020)has reviewed the latest achievements of Lattice Boltzmann methods on studying nanofluids in heat and mass transfer applications.Some other reviews have also discussed the applications of various nanomaterials in EOR,drilling,fracturing and reservoir sensing,etc (Ko and Huh,2019;Rattanaudom et al.,2021;Zhou et al.,2020).However,there are not many reviews focusing on the synthesis of amphiphilic nanomaterials for the N-EOR.Amphiphilic nanomaterials with hydrophilic and lipophilic nature have more significant advantages in enhancing oil recovery compared to sole hydrophilic or lipophilic nanomaterials (SHNPs).Inspired by this philosophy,this review mainly discusses the preparation methods of amphiphilic nanomaterials.Besides,techniques for evaluating nanofluids’ stability and mechanisms about N-EOR (IFT reduction,wettability alteration,foam stability improvement,structural disjoining pressure,etc.) have been also reviewed.We also present both our perspectives and achievements on N-EOR in this work.Moreover,the synergistic effects between nanofluids and chemical agents are also addressed.Finally,the further prospects and challenges faced by nanofluids for further improving oil recovery are also discussed.
2.Synthesis of amphiphilic nanomaterials
Owing to the fascinating and promising properties such as small size effects,quantum size effects and surface effects,nanomaterials have been proved to be excellent materials for EOR.Compared to SHNPs,amphiphilic nanomaterials with hydrophilic and hydrophobic nature have gained more attention across the fields of particulate surfactants,environmental protection,food safety,and energy.That is due to their distinct physicochemical properties like stronger interfacial activity (Aveyard et al.,2003;Lattuada and Hatton,2011;Lv et al.,2018;Walther et al.,2008;Wan et al.2017,2018).In the view of amphiphilic nanomaterials synthesis,plenty of methods have been proposed over the recent decades,including the template masking method (Liu et al.,2013;Wu et al.,2015;Zhang et al.,2013),utilization of specific surface functional groups(Ji et al.,2014) and selective modification via manipulation of π-π stacking interactions(Yang et al.,2016).Generally,the preparation methods of amphiphilic nanomaterials can be categorized into two ways,namely direct preparation and indirect preparation,as shown in Table 1.
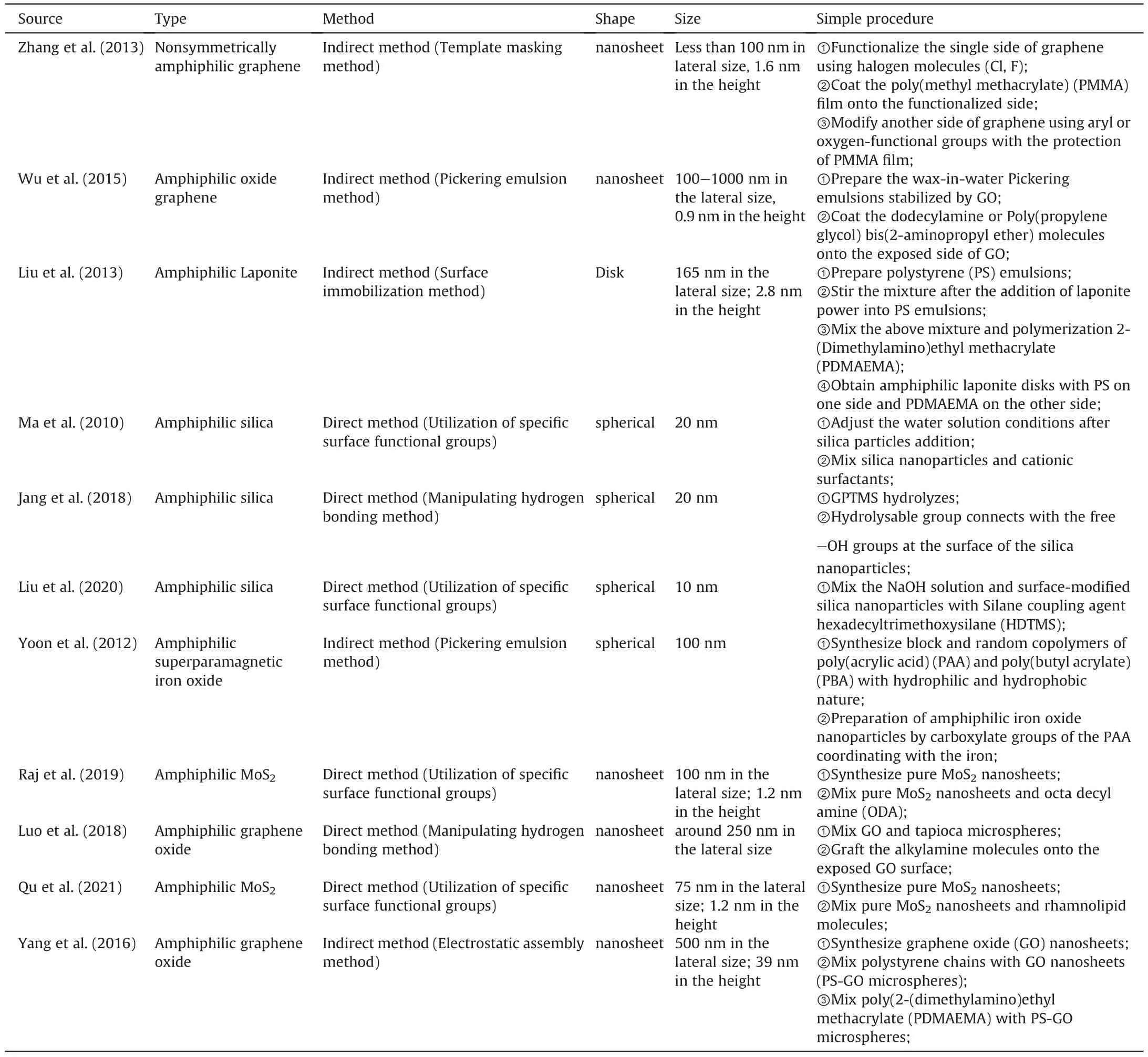
Table 1Summary of amphiphilic nanomaterials preparation.
Pickering emulsion is one of the most commonly used indirect preparation approaches.It plays a vital role in controlling the shape and morphology of nanomaterials.De Folter et al.(2014) reported Pickering emulsions with cubic and peanut-shaped particles achieved 90% surface coverage,higher than that achieved with ordinary spherical particles.Gao et al.(2014) numerically studied the surface activity of different shapes of amphiphilic nanomaterials and found sphere and rod shapes have only one equilibrium state,but the discotic shape has another metastable state:reverse orientation.The SHNPs of SiO2nanoparticles were modified using organic hydrophobic chains through a Pickering emulsion method(Wu et al.,2015).Wu et al.(2020) has also prepared amphiphilic silica nanoparticles using a Pickering emulsion method.Briefly,the SiO2nanoparticles were mixed with paraffin wax and water phase to form a Pickering emulsion via stirring.Then,KH550 silanecoupling agent was grafted onto the exposed surface of SiO2nanoparticles adsorbed onto Pickering emulsions.After that,lauric acid was added to react with the amino group via the amidation reaction.Finally,the amphiphilic SiO2-C12nanoparticles wereobtained after centrifugation.Besides,amphiphilic graphene oxide nanosheets can be prepared using a template polymer or be defunctionalized by altering the ionic strength based on electrostatic assembly.Yang et al.(2016) had successfully prepared amphiphilic graphene oxide nanosheets possessing hydrophobic polystyrene chains on one side and hydrophilic poly(2-(dimethylamino)ethyl methacrylate)chains on the other side.This method is first to protect one side of nanoparticles and then functionalize another side.Although this method is popular because of its mild and straightforward,the graft effect depends on the preparation of individual nanoparticle,which is difficult to control.
There are two ways for direct preparation based on the sol-gel method:emulsion interface self-assembled sol-gel and templateassisted sol-gel.Silica amphiphilic nanosheets were prepared by crushing Janus hollow spheres synthesized at the self-assembled materialization of an amphiphilic emulsion interface (Liang et al.,2011).The synthesized Janus nanosheets,as particulate surfactants,can be used to reduce oil-water interfacial tension and then collect oil drops.The flexible amphiphilic nanosheets (3-butyldianhydride mercaptopropyltrimethoxysilane (BDMPS)) can be prepared through a self-assembled monolayer of an amphiphilic silane on a template (Liu et al.,2015).They are used as solid emulsifiers.The template-assisted sol-gel method can be used to prepare smaller thickness amphiphilic nanosheets over the emulsion interface self-assembled sol-gel approach.Graphene oxide(GO)-based amphiphilic nanosheets have been synthesized via manipulation of hydrogen bonding.The GO and tapioca starch microspheres were evenly dispersed in the water phase.Then,GO was adsorbed onto the surface of tapioca starch microspheres via hydrogen bonds.Finally,the single-side surface hydrophilization of GO transformed into a hydrophobization surface by functionalizing alkylamine molecules,as shown in Fig.1.The amphiphilic nanosheets were released from the starch microspheres by sonication and heating in ethanol (Luo et al.,2018).Another direct approach for preparing amphiphilic nanomaterials is the hydrothermal reaction process.The SHNPs of MoS2nanosheets were synthesized through a simple hydrothermal method firstly,and then the surfaces of pure MoS2nanosheets were functionalized using CTAB or ODA molecules (Qu et al.,2021;Raj et al.,2019).
Amphiphilic nanomaterials can also be classified into spheres,nanosheets,and rods according to their shapes.The interfacial activity of amphiphilic nanosheets is more significant than that of amphiphilic spheres or rods,resulting in more considerable desorption energy from the interface to the bulk phase (Jia et al.,2016;Wei et al.,2018).As a result,amphiphilic nanosheets can be employed to effectively improve emulsion and foam stability once adsorbed onto interfaces,eventually improving oil recovery (Jia et al.,2016).
3.Nanofluid stability
Nanofluid is defined as the dispersion of nanomaterials in a specific dispersant (water,ethanol,etc.) at a particular concentration.It is crucial to evaluate the stability of nanofluids before they are injected into reservoirs.They are thermodynamically unstable.Nanomaterials in the bulk phase tend to aggregate to decrease the system energy due to the high surface energy.Nanofluid stability evaluation can be done using the solid sedimentation method,Zeta potential measurement,and spectral analysis.
3.1.Sedimentation method
The sedimentation method for assessing nanofluid stability is the most intuitive and pervasive technique.This method measures the weight or volume of sediment with time at the bottom of the liquid column.Meanwhile,photographs of the dynamic deposition process can be recorded as an essential indication to determine nanofluids' stability qualitatively,as shown in Fig.2.Chakraborty et al.(2017,2018) took sedimentation photographs to evaluate the stability of Cu-Al LDH and TiO2nanofluids kept in a glass vial over time.The results showed that the stability of nanofluids was directly related to particle size.The phenomenon of poor stability and faster sedimentation rate of nanofluids is likely to be occurred in a specific fluid due to larger particle size.Besides,copper and Al2O3nanofluids' stability was also determined using the sedimentation method(Li et al.,2007;Zhu et al.,2009).However,this method requires more time to observe nanofluids’ change trends and capture high-quality photographs as an intuitive indication.
3.2.Zeta potential measurement
Zeta potential measurement has also been commonly used to determine suspension stability(Chakraborty and Panigrahi,2020).Electrostatic repulsion exists between adjacent nanoparticles in the nanofluids due to the same charge on nanoparticles'surfaces.This phenomenon positively inhibits particle coalescence and then improves nanofluid stability (Ismay et al.,2013).On the other hand,van der Waals force,widely existing among particles,is an attractive force that is not conducive to nanofluids' stability.The Zeta potential,consisting of electrostatic repulsion force and van der Waals force,is a comprehensive reflection index.The higher value of Zeta potential represents higher repulsive force and better nanofluid stability (Cacua et al.,2019).The Au nanofluids with excellent stability were prepared by Kim (Kim et al.,2009).The outstanding stability of Au nanofluids was ascribed to the larger negative Zeta potential values among Au nanoparticles in the water phase.In general,stable suspensions signify that bulk suspensions’absolute Zeta potential value is greater than 30 mV (Esfandyari et al.,2015;Fatehah et al.,2014).In other words,nanofluids with a lower absolute Zeta potential value tend to agglomerate faster than the nanoparticles with a higher absolute Zeta potential value.Cacua et al.(2019) studied the effects of surfactant concentrations and pH values on Zeta potential values of alumina nanofluids.The results revealed that higher absolute Zeta potential values represent the smaller average diameter of alumina nanoparticles.Also,the higher stability of magnetic graphite nanofluid can be obtained when the Zeta potential was around 41.3 mV(Souza et al.,2012).In general,the Zeta potential value of suspensions can be affected by ionic strength (ion type),pH value,temperature,etc.
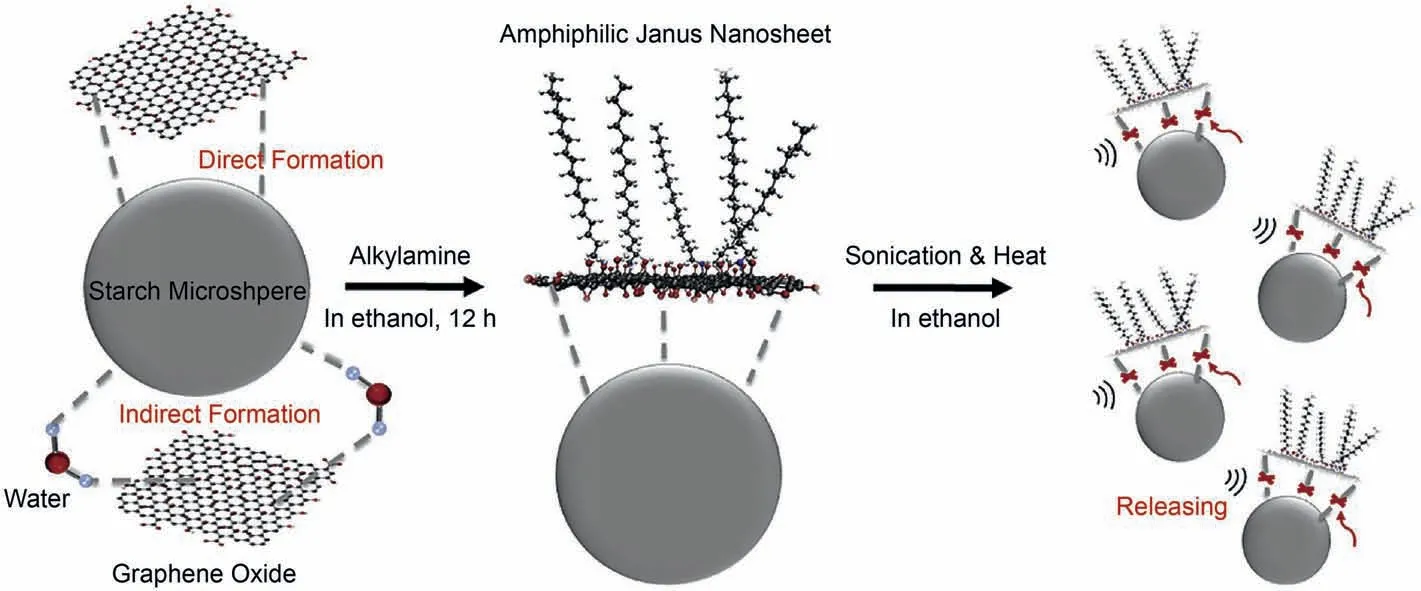
Fig.1.Synthesis of graphene-based amphiphilic nanosheets via hydrogen bonding (Luo et al.,2018).
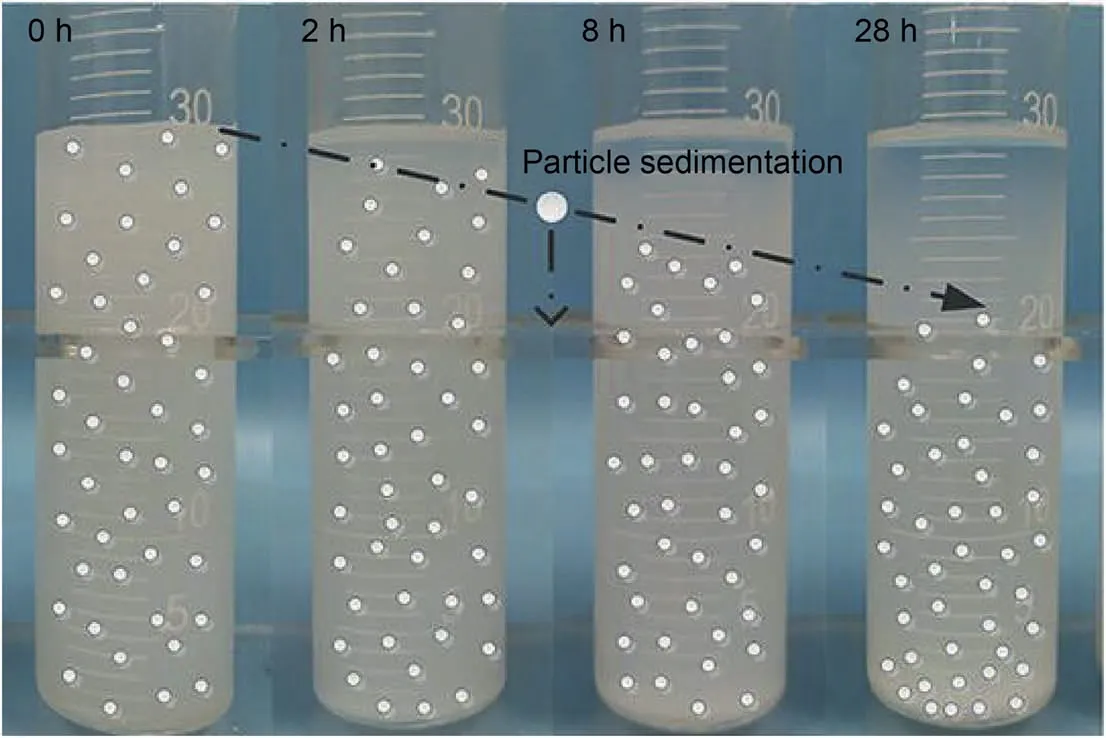
Fig.2.Time-lapse images of nanofluid destabilizing over time (Sun et al.,2020).
Known from DLVO theory,nanoparticles possessing surface charge in the nanofluids can attract opposite charges around them after those nanoparticles are evenly dispersed in a specific fluid.Adsorbed opposite ions can form a diffused electric double-layer,including a dense layer (Stern layer) and a loose layer (Diffuse layer)(Gomez-Flores et al.,2020).For instance,the diffused electric double-layer structure around negatively charged nanoparticles is exhibited in Fig.3.It can be seen that the oppositely charged ions distribute around the nanoparticle.Zeta potential(repulsion force)formed between diffuse layers (at the slipping plane) effectively prevents nanoparticles from contacting and agglomerating.The thickness of the diffused electric double-layer,referring to as“Debye length”,can also limit these nanoparticles’ trajectory.Therefore,a stable dispersion can be formed at a higher repulsion force and longer Debye length among nanoparticles (Bukar et al.,2014;French et al.,2009;Lombardo et al.,2015).
The addition of opposite charge ions can compress the thickness of the diffused electric double-layer and reduce the value of Zeta potential at the slipping plane.In other words,the addition of opposite charge ions can lead to an unstable dispersion(Saleh et al.,2008).The effects of different valent ions on both the thickness of the diffused electric double-layer and Zeta potential values are significantly different (Ji,2014).At the same concentration,the effects of different valent ions on Zeta potential value have the following logical relationship:trivalent >bivalent >monovalent.In addition to ion strength,pH value also has a pronounced effect on nanofluids' stability,which is commonly related to another indicator (isoelectric point).The isoelectric point is defined as the pH value of a dispersion system when the Zeta potential of dispersion is equal to zero.The dispersion is the least stable at the isoelectric point(Keller et al.,2010).Fig.4 shows the effects of pH value on the nanofluid stability and the isoelectric point for a specific nanofluid.It is essential to evaluate the nanofluids' electrification performance to avoid nanoparticles’ adsorption on the rock surface and accumulation among nanoparticles at the harsh reservoir conditions during N-EOR processes.
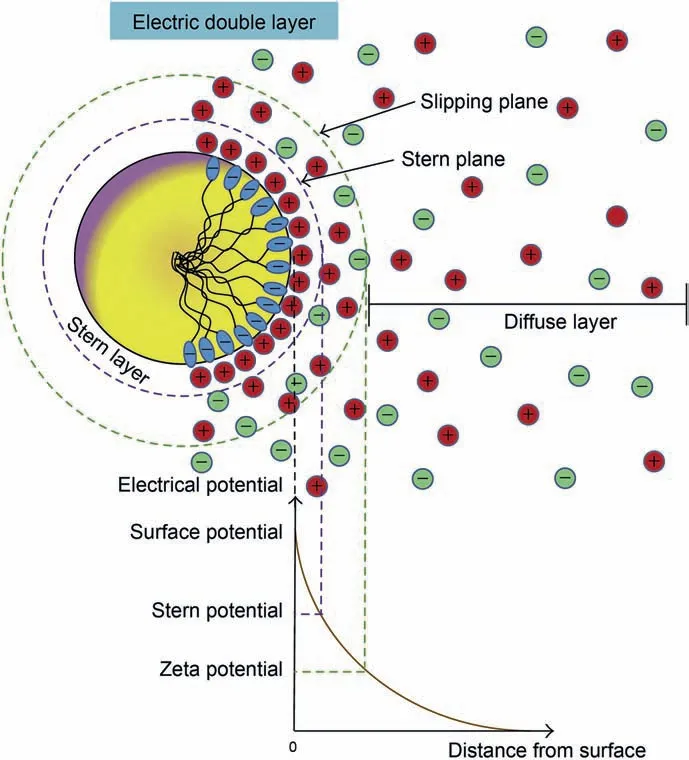
Fig.3.Schematic representation of diffused electric double-layer around charged nanoparticles (Lombardo et al.,2015).
3.3.Spectral method
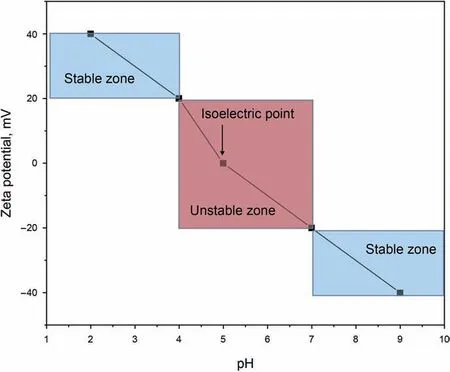
Fig.4.Effect of pH values on the stability of nanofluids (Pate and Safier,2016).
Another method to determine nanofluid stability is spectral analysis.Here,we mainly discuss the spectral analysis methods:spectral absorbance analysis method and multiple-light scattering method.Based on the Beer-Lambert Law,the spectral absorbance analysis method detects the absorption of incident light with a specific wavelength by nanoparticles using a UV-vis spectrophotometer.The absorbance of nanoparticles can be significantly affected by nanoparticle concentration,and there is a relationship between the absorbance and concentration of nanoparticles (Eq.(1)).Therefore,nanoparticle concentration can be calculated by detecting nanofluid absorbance in the same position over time.Finally,the stability of nanofluids can be determined by nanoparticle concentration over time.For example,the UV-vis spectrophotometer was introduced to investigate the relationships between MWNT concentrations and MWNT nanofluids'stability at different sediment times(Chen and Xie,2010).The purpose can be achieved by detecting the UV-vis absorbance value at various MWNT concentrations and different sediment times.The alumina and copper nanofluids’ stability was also evaluated using a spectrophotometer after the nanofluids were deposited for 24 h(Huang et al.,2009).Besides,the stability of FePt nanofluids was investigated and measured by using spectrophotometer analysis(Farahmandjou et al.,2009).

Where A is the absorbance,I0represents incident light intensity,I represents transmission light intensity,a represents molar absorption coefficient,b is liquid height,and c is the concentration of nanoparticles.
The Multiple-Light Scattering method detects the intensity change of transmittance light and back-scattering light using the Turbiscan Lab Expert Stabilizer when a pulsed near-infrared light(λ=880 nm) source passes through the sample pool.The micromigration behaviors (agglomeration and sedimentation behaviors) of nanoparticles in the bulk phase can be characterized by analyzing the spectrum changes(transmission light spectrogram or back-scattering light spectrogram)with the time and height of the sample.A detailed analysis of the spectrogram can be found in our previous work (Liang et al.,2020a).The schematic diagram of the multiple-light scattering method is shown in Fig.5.Apart from spectral analysis,nanofluid's stability can be quantitatively described using TSI,short for the Turbiscan Stability Index.The lower the TSI value is,the more stable the nanofluid is.Eq.(2) is used to calculate the TSI value.

Fig.5.The principle of TURBISCAN Lab Expert stability analyzer.
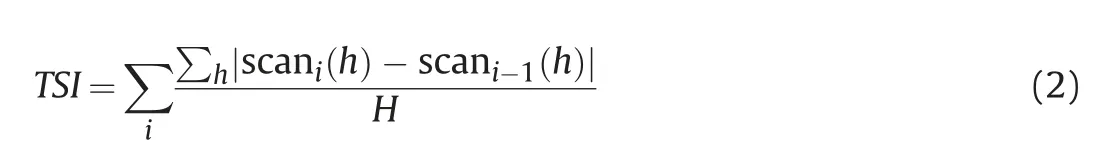
Where i is scanning number;h is scanning point height;scani(h)is the average light intensity;H represents the number of sample scanning data points.
Apart from the above three main methods,there are several other ways to evaluate nanofluid stability.According to the Stokes Eq.(3),nanoparticles’settling velocity is proportional to the square of nanoparticle diameter.Hence,nanoparticles with smaller sizes result in more stable dispersion.The diameter distribution of nanoparticles can be measured using a dynamic light scattering(DLS) technique.

where v is the settling velocity,Δρ represents the density difference of the solid phase (nanoparticles) and dispersed medium (water phase),g is the acceleration of gravity,d is the nanoparticle diameter,and η represents the viscosity of the dispersed medium.
Except for the previously mentioned factors affecting nanofluid stability,nanofluid stability can also be affected by dispersant types,temperature,and surface groups.For instance,nanomaterials’surface modification has been commonly implemented to improve nanofluid stability (Kamiya and Iijima,2010;ShamsiJazeyi et al.,2014).The surface-modified agents include surfactants,polymers,silane coupling agents,and other organic or inorganic materials.The surface-modified agents coating on the nanomaterial surface can improve repulsion force and steric hindrance among nanoparticles to keep them dispersed well (Kamiya and Iijima,2010;Li et al.,2020;Wei et al.,2018).The silica nanoparticles were modified using zwitterionic and hydrophilic silanes by Hadia (Hadia et al.,2021).The results found that the coated silica nanoparticles can remain stable for at least 6 months.Extensive discussions on how to improve nanofluid stability can be seen in other reviews focusing on nanofluid stability (Chakraborty and Panigrahi,2020;Heinz et al.,2017).
4.Mechanisms of nanofluids on N-EOR
4.1.Interfacial tension
Meniscus interface locating at the two immiscible phases contact region is formed due to interfacial tension,resulting in an uneven displacement front and lower oil recovery during flooding.Therefore,it is crucial to improve oil displacement efficiency by reducing the interfacial tension at the microscopic level(Zhao and Wen,2017).Many studies confirmed that nanomaterials' addition to the displacing fluid can reduce the IFT,which leads to the oil recovery improvement (Hendraningrat and Torsaeter,2015;Roustaei et al.,2013;Zaid et al.,2013).For instance,γ-Al2O3,MgO,and TiO2nanoparticles showed great IFT reduction ability(Nowrouzi et al.,2019).The IFT value of the nanofluids-crude oil interface is inversely proportional to the temperature and salinity and directly proportional to the nanoparticle concentration and pressure(Nowrouzi et al.,2019).Nanofluids can decrease IFT of the oil-water interface because of the formation of nano-layers at the oil-water interface.After nanomaterials’adsorption at the oil-water interface,the interfacial properties (interface energy) can be changed.Du et al.(2010) found that the binding energy (ΔE) of nanomaterials at the oil-water interface can be determined by oilwater interfacial tension,according to Eq.(4):

Where γ0represents the interfacial tension of the system in the absence of nanomaterials,γ represents the interfacial tension of the system in the presence of nanomaterials,η is the fraction of area occupied by nanomaterials at the oil-water interface,and R is the hydrodynamic radius of nanomaterials.
However,some researchers also demonstrated that SHNPs have no contributions to IFT reduction (Biswal and Singh,2016;Fereidooni Moghadam and Azizian,2014;Ma et al.,2008;Pichot et al.,2012).Metin et al.(2012) found that SHNPs (silica nanoparticles) cannot adsorb onto the decane-water interface and did not significantly affect the IFT of the decane-water interface because they are not amphiphiles.Moreover,the results showed that IFT of the decane-water interface in the presence of SHNPs was not sensitive to their particle size and concentration.
Amphiphilic (Janus) nanomaterials were identified to reduce interfacial tension.Amphiphilic nanomaterials can promote the adsorption of amphiphilic nanomaterials onto the oil-water interface,resulting in IFT reduction significantly.Li et al.(2018a)revealed that the adsorption arrangement of amphiphilic nanomaterials (active nanoparticles) and SHNPs were different due to surface functional groups (Fig.6).SHNPs distribute in one side of the bulk phase at the oil-water interface.This adsorption equilibrium of SHNPs at the oil-water interface can be easily broken under external force.On the contrary,the amphiphilic nanoparticles can adsorb at the oil-water interface and evenly distribute in both the water and oil phases.Those adsorption behaviors significantly reduce interfacial tension and improve interfacial viscoelasticity due to forming a stable and stronger nanometer interfacial film over SHNPs(Qu et al.,2020).Qu et al.(2020)reported that the IFT between simulated oil consisting of kerosene and paraffin and CTAB-MoS2nanofluid could be decreased to 14.9 mN/m compared to DI water-simulated oil system(IFT~36 mN/m).Besides,the IFT of the decane-water interface in the presence of silica nanoparticles modified with PEG showed a dramatic decrease trend as nanoparticle concentration increased or nanoparticle diameter decreased (Metin et al.,2012).As a result,the excellent interfacial activity of amphiphilic nanoparticles is beneficial for improving oil recovery during N-EOR by reducing IFT.
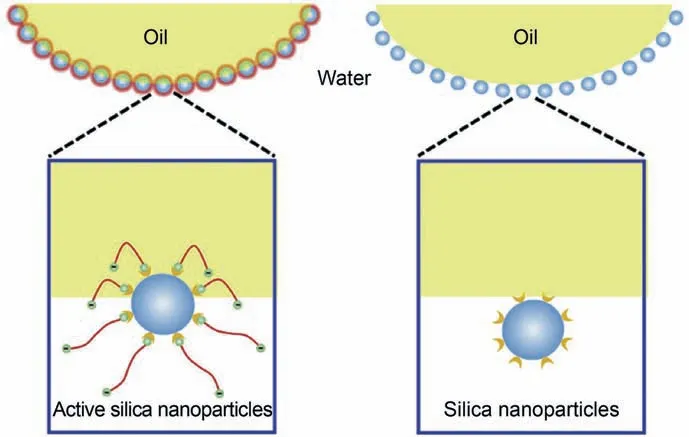
Fig.6.Schematic illustration of amphiphilic silica nanoparticles (left) and SHNPs(right) at the oil-water interface (Li et al.,2018a).
IFT of the oil-water interface is an indicator that strongly influences oil recovery.Capillary number theory is proposed based on C-EOR technologies,such as surfactant and polymer flooding.It can also be introduced to explain the displacement mechanisms of nanofluids to some extent.Known from capillary number theory(Nc),Eq.(5),IFT reduction results in a decreased capillary force,thereby improving the capillary number.The larger the capillary number is,the more favorable it is for oil displacement.
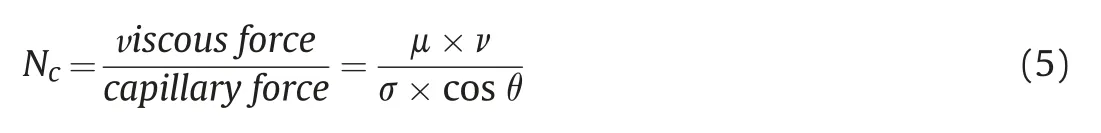
where μ represents dynamic viscosity of displacing fluid,ν is Darcy velocity displacing fluid,σ is interfacial tension between the displaced and displacing fluid,and θ is the contact angle.
However,it should be noted that nanomaterials have many unique properties and interfacial behaviors over traditional surfactants.For instance,surfactants can adsorb at the oil-water interfaces in the form of a single layer according to monolayer adsorption theory (Zhou et al.,2018).After surfactants reach saturated adsorption at the oil-water interface,surfactants preferentially form micelles in the bulk phase.However,nanomaterials can adsorb at the oil-water interface and form multilayer adsorption (Qu et al.,2020;Raj et al.,2019).Compared to surfactant adsorption,nanomaterials’ adsorption behaviors may lead to distinct interfacial properties,significantly contributing to oil recovery.Therefore,an urgent need for theory to better understand nanofluid flooding mechanisms during the N-EOR process is necessary.
4.2.Wettability alteration
Most of the reservoir matrix possesses oil-wet (hydrophobic)nature because of the crude oil environment around its surface.Oilwet rock surface indicates the firm adhesion of crude oil onto the rock surface,leading to an oil film's formation.Thus,more energy is required to exfoliate oil film from the rock surface.Besides,the pressure difference (capillary force) at the curved oil-water interface is pointed into the displacing phase (water phase) due to the oil-wet rock surface.The capillary force is resistant to crude oil's flow,resulting in low oil recovery.Therefore,wettability alteration of rock surface from oil to water-wet or oil to neutral-wet is favorable for enhancing oil recovery.The wettability of the rock surface can be determined by measuring its contact angle (CTA).The CTA for an oil-wet surface is greater than 105°,while for a neutral-wet surface,CTA ranges from 75°to 105°.When the CTA is lower than 75°,the rock surface denotes water-wet characteristics.
Recently,it is proved that nanoparticles can effectively spread onto the solid surface to change the rock surface's wettability.SiO2nanoparticles with super-hydrophilic surfaces were used to decrease contact angle from 100°to 0°on the aged glass (Maghzi et al.,2012).Karimi et al.(2012) proposed that the change of contact angle of carbonate reservoir rock surface treated with zirconium oxide (ZrO2) nanofluid was ascribed to the formation of nanoparticle structuring (they called “nanotextured surfaces” on the rock surface).According to their theory,nanotextured surfaces'formation changing the wettability of carbonate rock surfaces can take up at least 2 days.Besides,the effects of wettability on oil recovery using polymer-coated silica nanoparticles were comprehensively studied by Omran (Omran et al.,2020).The results revealed that the polymer-coated silica nanoparticles could change the oil-wet glass with a contact angle of 143.30°into a water-wet glass with a contact angle of 48.75°.Some studies suggested that wettability alteration from oil-wet to strongly water-wet showed more fantastic effects on enhancing oil recovery than weakly waterwet.Hadia et al.(2021) evaluated the ability of coated silica nanoparticles to change the solid surface's wettability on aged sandstone and carbonate rock surfaces.The results showed that the coated silica nanoparticles can alter the wettability to water-wet by adsorbing on rock surfaces.Besides,the coated silica nanoparticles can be more effective in changing the wettability of carbonate surface instead of sandstone surface.The synergistic effects of ZrO2nanoparticles and different nonionic surfactants on the wettability alteration of carbonate samples were investigated by contact angle measurement (Karimi et al.,2012).Results showed that the strongly oil-wet rock surface could transform into a strongly waterwet surface after treatments using different nanofluids.Moreover,three types of Fe3O4nanoparticles (SHNPs,coated with EDTA,coated with SLS) were systematically studied to change the wettability of a carbonate rock surface substrate from oil-wet to water-wet (Shalbafan et al.,2019).Findings revealed that Fe3O4nanoparticles coated with EDTA/SLS can dramatically decrease the carbonate substrate contact angle of 140°(strongly oil-wet)to 27°and 22°(strongly water-wet),respectively.Furthermore,they also demonstrated that the increase in aging temperature positively affected wettability alteration,while increasing pressure had a negligible effect.The electro-kinetic method was also proposed to study the wettability alteration (Dehghan Monfared and Ghazanfari,2019).The rock surface wettability can be determined by detecting electro-kinetic parameters change (streaming potential coupling coefficient and Zeta potential) during the wettability alteration processes.The most significant advantage of this method considers the pore-throat structure during wettability alteration processes compared to the contact angle measurement.
The water-wet surface represents that water can adhere to rock surfaces to form a water film.The thickness of water film increases with the increase of water-wet degree (He and Hua,1998).As a result,the flow channel's diameter for crude oil is also relatively decreased,especially in a low permeability reservoir.The decrease in the flow channel's diameter leads to high injection pressure and low oil recovery.In the case of a neutral wet surface,the piston displacement mode is expected to be achieved.Owing to the reduction of the core sample's hydrophilicity,the improvement of oil recovery efficiency was achieved as functionalized silica nanoparticles were injected into the water-wet core (Roustaei et al.,2013).An investigation of nanofluid with respect to its ion strength on wettability alteration of carbonate surface was performed(Hou et al.,2019).Results showed that the presence of Na+can promote the adsorption of nanoparticles on the rock surface by neutralizing the negatively charged portion that existed on the carbonate surface.
4.3.Foam stabilization improvement
Foam flooding is one of the promising techniques,which has been widely used to recover crude oil from reservoirs.The oil recovery efficiency of foam flooding is improved due to the delay of gas channeling,improvement of sweep efficiency (Li et al.,2016),increase of oil displacement efficiency (Li et al.,2019a),and adjustment of mobility ratio (Sun et al.,2015).Yang et al.(2021)reported the synergy of hydrophilic nanoparticles (T40) and nonionic surfactants (C12E23) on CO2foam stability at elevated temperatures and extreme salinities.Sandpack experimental results indicate that the C12E23/T40 foam can enhance the oil recovery 20.1% after water flooding by increasing the sweep area and flooding efficiency.However,foam is generally considered a thermodynamically and kinetically unstable system.Foam can be destroyed by an external force,especially under harsh reservoir conditions (high temperature,high salinity,high crude oil saturation).To overcome those barriers,foam stabilizers such as polymer,solid particles,and gels can be introduced to improve foam stability.The primary mechanism for polymer and gel additions is improving the viscosity of interfacial film,leading to a lower gas flow speed between adjacent bubbles.However,improved viscosity of foam leads to the difficulty of foam injection into the reservoirs.It is observed that the nanoparticles’ addition into foam liquids can improve foam stability and foamability by adsorbing onto the gasliquid(AlYousef et al.,2017).Yin et al.(2018)showed that cellulose and surfactant synergism can significantly improve foam stability by increasing the interfacial film thickness and delaying the liquid drainage.Yang et al.(2017) reported that modified AlOOH nanoparticles can be utilized to stabilize foam generated by SC(Sodium cumene sulfonate)at a broad range of concentrations.Nitrogen and methane foam were also generated using surfactant-nanoparticlebased fluid,and the properties of foam at the presence or absence of nanoparticles were also analyzed systematically(Xu et al.,2020).Results showed that the based fluid viscosity and foam stability were significantly improved after the addition of 1.0 wt% SiO2.Besides,more than 30% of oil recovery factors can be enhanced using surfactant-NP foam flooding over surfactant foam flooding.Fig.7 shows the mobilizing oil mechanisms when foam contacts with residual oil during foam migration forward at the presence or absence of nanoparticles.The shape of surfactant foam tends to deform under the action of external force.Meanwhile,foam is inclined towards the flow over the residual oil drop,as shown in Fig.7b.Compared with surfactant foam,surfactant-NP foam exhibits an excellent ability to keep its spherical structure while contacting with residual oil drop,as shown in Fig.7d.The dense arrangement of nanoparticles at the liquid-gas surfaces strongly increases the film thickness and strength,which can inhibit foam deformation and then generate a stable foam.Consequently,an additional force at the contact region of surfactant-NP foam and oil drop is generated to effectively mobilize residual oil drop trapped within reservoir pores and throats.
The type of used surfactants,concentration,the size distribution of nanoparticles,and type of nanoparticles (contact angle) can affect the stability of the surfactant-NP foam.Here,desorption energy theory is mainly employed to interpret the mechanisms of foam stabilized by nanoparticles.
4.3.1.Desorption energy of a single spherical nanoparticle

Fig.7.Schematic of foam at the presence or absence of nanoparticles during mobilizing residual oil drop process,(a) and (c) differences in crude oil adsorption of the surfactant foam and the surfactant-NP foam,(b) and (d) differences in the degree of deformation of the liquid film for the surfactant foam and surfactant-NP foam (Xu et al.,2020).

Fig.8.a A single spherical nanoparticle adsorbed at the gas-water surface,b desorption energy of a specific nanoparticle (R,30 nm).
A single spherical nanoparticle is considered to be adsorbed onto the gas-water interface,as shown in Fig.8a.The following Eqs for desorption energy of spherical particles come from the previously published paper (Binks and Lumsdon,2000).Eq.(6) shows the contributed surface energy of the gas phase.

Area immersed in the gas phase is calculated according to Eq.(7)given below:

Hence,the ΔEsgcan be obtained from Eq.(8):

Similarly,the surface energy contributed by the water phase is calculated from Eq.(9):

The surface energy contributed by the eliminated gas-water interface owing to the presence of spherical nanoparticle is calculated,Eq.(10):
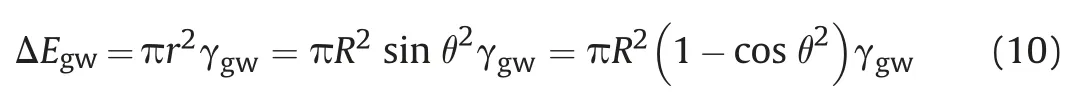
As a result,the required energy to remove the single adsorbed spherical nanoparticle to the water phase can be determined according to Eq.(11):

Known from the Young Eq.(12):

Eq.(11) can be simplified to Eq.(13):

Similarly,the required energy to remove the single adsorbed nanoparticle to the gas phase can be determined according to Eq.(14):

According to Eqs.(13)and(14),the desorption energy of s single spherical nanoparticle from the surface to the bulk phase is calculated and drawn in Fig.8b as a function of the contact angle of the nanoparticle surface.Several parameters of nanoparticle radius(R,30 nm),surface tension (γgw,55 mN/m),and temperature(298 K) are given to calculate a single spherical nanoparticle's desorption energy.It can be concluded that when the contact angle is 90°the maximum desorption energy is achieved.This denotes that the nanoparticles are not easily desorbed from the surface to the bulk phase.From Fig.8b,it is observed that a single nanoparticle needs about dozens of thousand KBT to desorb from the surface to the bulk phase.Compared to surfactant needing several KBT,nanoparticles show a significantly potential ability to stabilize foam.
4.3.2.Desorption energy of a single nanosheet
As mentioned above,nanosheets can also be used to stabilize foam as a stabilizer.Similar to the desorption energy theory of a single spherical nanoparticle,we propose a new desorption energy Eq for single nanosheet desorption from the surface to the bulk phase.The assumptions used to derive the Eq are given below:
1 The thickness is negligible relative to its length and width;②The nanosheet adsorbed at the surface is completely spread out.The adsorption schematic of a single nanosheet is shown in Fig.9a.
The unilateral area of a single nanosheet is recorded as A.Therefore,the surface energy contributed by the area immersed in the gas phase is calculated from Eq.(15):

The surface energy contributed by the area immersed in the water phase is calculated from Eq.(16):

The surface energy contributed by the eliminated gas-water surface owing to the presence of a single nanosheet is obtained from Eq.(17):

Hence,the required energy to remove the adsorbed single nanosheet to the water phase can be determined according to Eq.(18):
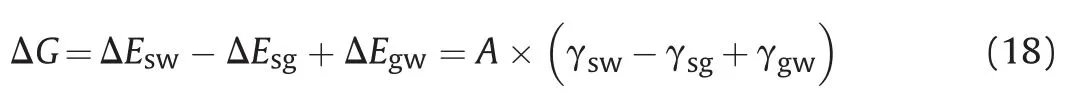
According to Young Eq.(12),Eq.(18) can be simplified to Eq.(19):

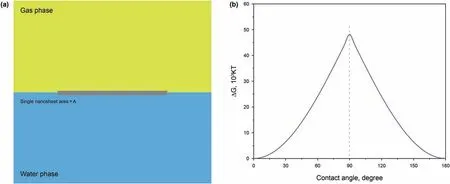
Fig.9.a A single nanosheet adsorbed at the gas-water surface,b desorption energy of a specific nanosheet.
Similarly,the required energy to remove the adsorbed single nanosheet to the gas phase can be determined according to Eq.(20):

According to Eqs.(19)and(20),the desorption energy of s single nanosheet from the surface to the bulk phase is calculated and drawn in Fig.9b as a function of the contact angle of the nanosheet surface.Several parameters of nanosheet size (Length,60 nm),surface tension(γgw,55 mN/m),and temperature(298 K)are given to calculate a single nanosheet's desorption energy.It concludes that the maximum desorption energy is achieved as the contact angle is 90°.Compared to a single spherical nanoparticle(R,30 nm),a single nanosheet requires more desorption energy to get rid of the surface.This result signifies that nanosheets show a promising prospect for improving foam stability.
4.4.Emulsion stabilization improvement
Numerous literature and pilot plant studies confirm that oil recovery can be improved through crude oil emulsification.Several mechanisms,like micro displacement efficiency improvement,macro sweep efficiency improvement,and oil viscosity reduction(oil in water emulsion),are used to verify such phenomenon (Guo et al.,2018;Karambeigi et al.,2015;Li et al.2018b,2019b;Ning et al.,2018).Chen et al.(2013) reported that higher oil recovery can be achieved when improving emulsification ability.Moreover,emulsions were also used to block pores during steam flooding(French et al.,1986).Therefore,improvement of emulsion stability is necessary during the emulsion migration process under harsh reservoir conditions.
Over the decades,Pickering emulsions stabilized by particles have gained considerable attraction for EOR due to their advantages in improving emulsion stability over conventional surfactant emulsion (Tyowua et al.,2017;Zhang et al.,2018).Nanoparticles can improve emulsion stability by forming single or multilayered nanoparticle interfacial films at the oil-water interface (Stancik et al.,2004).The mechanical strength and viscoelasticity of interfacial film can be improved by the adsorption of nanoparticles,preventing oil droplets from deformation and collision coalescence(Li et al.,2019b;Tambe and Sharma,1995).Modified gold nanoparticles and alkane were evenly mixed to form O/W emulsions,which were still stable during performed cooling-heating cycles(Kubowicz et al.,2010).Additionally,adsorption behaviors of soy protein isolate-chitosan nanoparticles (SPI-CS) at Pickering emulsion interfaces were characterized using confocal laser scanning microscopy(CLSM),as shown in Fig.10(Yang et al.,2020).The SPICS nanoparticles and corn oil were dyed with Nile blue (red) and Nile red(green)to enhance the experiments'visual effect.It can be intuitively observed that SPI-CS nanoparticles were tightly adsorbed at the oil-water interfaces,forming nanoparticles interfacial film to prevent coalescence of adjacent oil droplets.Moreover,Koroleva and Yurtow (2020) systematically studied Pickering emulsions’ stability with magnetite/silica and gold/silica nanoparticles using experimental and mathematical modeling.Experimental results demonstrated that Pickering emulsions stabilized by magnetite/silica nanoparticles can prevent emulsions from coalescence.It also confirms that silica and magnetite nanoparticles can be adsorbed onto the oil-water interface to form chain-like heteroaggregates extended into the bulk aqueous phase.The formation of 3-D gel-like network around oil droplets is beneficial to inhibit their demulsification and coalescence.Once the nanoparticles get adsorbed onto the oil-water interfaces,it is difficult to be detached from the interface.The required desorption energy can be calculated based on Eqs.(13),(14),(19) and (20) just using the relevant oil-water interface parameters instead of gas-water parameters.

Fig.10.CLSM image of Pickering emulsions stabilized by SPI-CS nanoparticles (Yang et al.,2020).
4.5.Structural disjoining pressure
Over the decades,nanofluid has been a promising candidate for enhanced oil recovery.The conventional C-EOR mechanisms cannot be used to interpret the faster spreading velocity of nanoparticles on the solid surfaces during the nanofluid flooding process.Such behaviors of nanoparticles occur due to the formation of disjoining pressure in the confinement of the three-phase contact region formed by an oil drop on a solid surface.The disjoining pressure is contained by van der Waals's force (Gennes,1985).Hirasaki (1991) proposed that the disjoining pressure is a combination of electrostatic forces and van der Waals' force.The above two kinds of conventional disjoining pressures(CDP)are generated due to the London-Van der Waals force with a short-range nature.However,the most popular theory is the concept of structural disjoining pressure (SDP).Recently,Wassan and Nikolov (Wasan and Nikolov,2003) proposed the concept of SDP,which is a kind of force normal to the interface with long-range nature.The formation of SDP results from spherical nanoparticles' ordering in a confining region (wedge film).In other words,the origin of SDP is due to the confinement of nanoparticles at the wedge film structure instead of the freedom nanoparticles in the bulk phase.The wedgefilm depth can be extended from one nanoparticle to several nanoparticle diameters along the direction of the opening of the wedge film.An analytical expression(Trokhymchuk et al.,2001)for calculating SDP based on a solution of the Ornstein-Zernike statistical mechanics is showed in the following Eqs:

Where d is the diameter of nanoparticle,h is the wedge film thickness,and all other parameters(Π0,Π1,ω,φ2,κ,δ)in Eq.(22)are fitted as cubic polynomials in terms of the nanofluid volume fraction (φ).P is the osmotic pressure,which is a function of the nanofluid volume fraction,as shown in Eq.(23):

Here,npis the number of particles per unit volume of the system.
Known from Eqs.(21)-(24),the osmotic and structural disjoining pressures at the confinement region increase by increasing the nanoparticles'volume fraction.The volume fraction is inversely proportional to the diameter of a nanoparticle.The SDP depends on the nanoparticle diameter,temperature,volume fraction,and other nanoparticles’ properties.Moreover,the absolute value of structural disjoining pressure is equal to that of osmotic pressure when the wedge film depth is less than the nanoparticle diameter.The higher the osmotic pressure,the higher the SDP.The above results are verified by the mathematical models of Chengara (Chengara et al.,2004).
The SDP presents an oscillatory decay profile with the increase of film thickness from vertex to bulk phase.The highest SDP at the vertex of wedge film reaches 50000 Pa and enhances nanofluid spreading.Meanwhile,the spreading of nanoparticles on the solid surface can change the solid surface's wettability from oil-wet to neutral-wet or water-wet.As a result,the oil drop trapped within reservoir pores and throats can be gradually detached by the wedge film's moving forward.Recently,ShamsiJazeyi et al.(2014)pointed out that the structural disjoining pressure contained four components:SDP,van der Waals,electrostatic,and solvation forces.Those affect the detached process of oil drop from a solid surface to the bulk phase.In particular,the ability of wettability alteration can be virtually affected by the electrostatic force of nanoparticles.An increase in electrostatic repulsive forces on the surface of the nanoparticles can increase the disjoining pressure,which leads to the spreading of nanofluid on the solid surface,followed by detaching the oil drop from the solid surface.
Affected by structural disjoining pressure,the confinement region between oil drop,solid surface,and the water phase is distinct at nanoparticles' presence or absence.Two distinct contact lines(Fig.11):an outer one(among the oil droplet,solid,and water film)and an inner one (among the oil droplet,solid,and a mixed oilwater film) were observed when seen from the top of oil drop(Kondiparty et al.,2012).The outer contact line represents the conventional three-phase contact line,while the inner line is the boundary where the spreading edge of the nanofluid film and the oil-solid interface meet.The inner contact line is formed and gradually moves towards the center of the contact region between the oil drop and solid surface due to nanoparticles' presence.This spreading behavior of nanofluid is driven by the net spreading force,which is defined as the difference between the film tension gradient (structural disjoining pressure gradient) arising from the structuring of the nanoparticles in the wedge confinement and the contribution from the resisting capillary and hydrostatic pressures(the buoyancy because the drop is sessile).Moreover,the nanofluid film's spreading speed on the solid surface increases with an increase in nanoparticle concentration and oil drop volume.

Fig.11.Photomicrograph taken using reflected-light interferometry depicting the inner and outer contact lines and the nanofluid film region (Kondiparty et al.,2012).
Wasan and Nikolov (2003) observed the formation of a twodimensional (2D) colloidal crystal structure in the wedge film when the wedge film thickness was less than twice the nanoparticle's diameter.As the thickness of wedge film is more than three times the nanoparticle's diameter,nanoparticles'arrangement in the wedge-film structure becomes disordered.The vertex of wedge film can generate high disjoining pressure(nearly 50 kPa),promoting the wedge film moving forward and the nanoparticles spreading on the solid surface.With the lapse of time,the oil drop trapped on the solid surface is detached.
Zhang et al.(2014) investigated the dewetting phenomenon of the simulated oil film in a glass capillary in the presence of nanoparticles,as shown in Fig.12.The thick oil film formed after nanofluid driven is unstable and broken to form an oil annular rim.Finally,the annular rim is detached from the solid surface to form a spherical oil droplet due to nanoparticles’ arrangement in the wedge-film region.The observed phenomenon is repeatable.As a result,the nanofluid can be widely applicable in extracting crude oil film from the rock surface during N-EOR.
However,it should be noted that the formation of SDP at the confinement structure (wedge film) can be regarded as one of the mechanisms for N-EOR when the volume fraction of nanoparticles is more than 20 v% (Kondiparty et al.,2012;Wasan and Nikolov,2003).The dewetting phenomenon occurred at the capillary tube when the volume fraction of SiO2was 20 v%.On the other hand,Chengara et al.(2004)found that the contact line's position had no appreciable change when the volume fraction of nanoparticles was less than 20 v%.Those results demonstrate that nanofluids can only play positive effects on removing residual oil from a solid surface when the nanoparticles volume fraction is more than 20 v%.However,many N-EOR experiments were conducted at a very dilute concentration of nanoparticles in the literature (Hu et al.,2016;Li et al.,2018a).Therefore,it is worthy of studying whether the structural disjoining pressure arising from the confinement wedge film region during N-EOR flooding can interpret the residual oil removal process from the rock surface when the concentration of nanoparticles is low.Moreover,the above phenomena and Eqs are investigated and obtained when using spherical nanoparticles.The critical volume fraction (20 v%)may be decreased when using nanosheets due to their flake-like shape (Qu et al.,2021).
4.6.Depressurization and increasing injection
More oil reserve has been stored in low permeability reservoirs(permeability 0.1 to 50 mD).Micron or nano-sized pores and throats are widely distributed in low permeability reservoirs,resulting in the higher injection pressure of displacing fluid and lower oil recovery (Cheng et al.,2006).Hence,it is necessary to reduce injection pressure and increase injection for high-efficiency developing low permeability reservoirs.
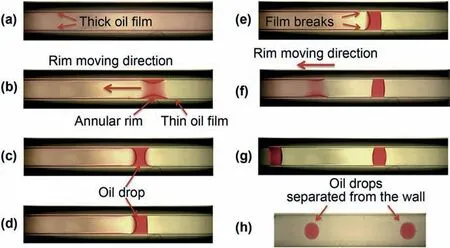
Fig.12.Evolution of hexadecane film inside the glass capillary (ID=282 ± 2 μm),(a)thick film after nanofluid driven,(b) dewetting of the thick film in the form of the annular rim,(c)formation of double-concave meniscus across the capillary,(d)rupture of the thin film on the right side of the meniscus,(e-g)repeats the previous steps and forms a cylindrical drop,(h) separation of cylindrical drops from the capillary wall by the nanofluid (Zhang et al.,2014).
Recently,nanofluids have been considered a kind of drag reduction agent.Polesil,a kind of pressure-decreasing and augmented injection agent consisting of nanoparticles,was firstly applied in China (Lau et al.,2017).However,the effects of nanofluids applied in pilots were not very satisfactory owing to various reasons.Yu et al.(2020) reported that micro-emulsion-based silicon nanofluids can reduce the water injection pressure to more than 40% at the silica concentration of 1 wt%.Modified silicon dioxide(SiO2)was also prepared to achieve the highest pressure drop rate (34%) when the injection volume was less than 0.3 times the pore volume (PV) (Hai et al.,2020).Initially,the mechanism for applying nano-silicon dioxide (SiO2) in the pressure drop and enhancing water injection technology can be expressed as the nano-adsorption layer replaces the hydration layer on the rock surface,and then pore diameter is increased(Li et al.,2017a;Wasan and Nikolov,2003).However,the hydration layer height is found to be around 5-30 nm at driven pressure of 0.01-2 MPa,which is the same order of magnitude as the nanoparticle size (He and Hua,1998).Hence,this interpretation may not be suitable for depressurization and increasing injection technology.Cottin-Bizonne et al.(2003) found that the synergistic effects of wettability and roughness of rock surface can significantly decrease the flow resistance of fluids in pores and throats.Di et al.(2007) proposed another mechanism for interpreting depressurization and increasing injection.He thought that hydrophobic nanoparticles can adsorb onto the rock surface to form nanoparticle layers instead of replacing a hydrated layer.The slip effects of nanoparticles can significantly decrease the flow resistance and then increase injection.The micro-tube was designed to study the impact of nanofluids injection rate and pore size distributions on pressure reduction (Wang et al.,2018).Experimental results exhibited that hydrophobic nanoparticles showed greatly pressure-decreasing ability,first increased and then decreased with the injection rate increases,as shown in Fig.13.The results can be attributed to that the adsorption of hydrophobic nanoparticles improves rock surface roughness.

Fig.13.Effects of injection rate on pressure-decreasing when using microtube (Wang et al.,2018).
5.Nano-assisted C-EOR
Recently,C-EOR and N-EOR technologies are two major branches to enhance oil recovery.Many reviews focus on the synergism of C-EOR and N-EOR technologies (nano-assisted C-EOR)(Cheraghian and Hendraningrat,2016;Kamal et al.,2017;Negin et al.,2016).For instance,combinations of nanoparticles and polymers are manipulated to improve the properties of pure polymer solutions,such as thermal stability,viscoelasticity,and mechanical stability.Taborda et al.(2021) reported that polymer solution's thermal stability and viscosity retention can be significantly improved by introducing nanoparticles,resulting in an excellent mobility ratio during the polymer flooding process.Kang et al.(2019)also had mixed the silica nanoparticles and amphiphilic polymer to form an NP-polymer composite under high temperature and high salinity conditions.Results showed that the polymer solution's apparent viscosity and viscoelasticity were significantly increased after silica NPs addition(Fig.14).The synergistic effect of titanium dioxide NPs (optimal concentration,2.3 wt%) and HPAM polymer solution was also evaluated to mobilize dead oil from sandstone core samples(Cheraghian and Goshtasp,2016a).Results revealed that improved oil recovery was ascribed to the improvement of viscosity after TiO2addition.
Apart from NP-polymer synergism,NP-surfactant synergism has also been widely studied for further changing interfacial properties(Munshi et al.,2008).The mixtures of surfactants and NPs can further lead to IFT reduction,wettability alteration,oil viscosity reduction,foam/emulsion stability improvement,and capillary force decrease (Ravera et al.,2006;Rosen et al.,2005).A novel nanofluid was prepared by combining the positively charged amino-terminated silica nanoparticles (SiNP-NH2) with a negatively charged anionic surfactant (Soloterra 964) via electrostatic force (Zhou et al.,2019).Results proved that oil-water interfacial tension was decreased by 99.85%,and the contact angle was increased by 237.8% over the original value of 13.78 mN/m and 43.4°,respectively.The interfacial tension of the oil-brine system was decreased from 19 to 8 mN/m after introducing SiO2nanoparticles (Hendraningrat et al.,2013).Moreover,more than 13% of heavy oil recovery was achieved using the mixture of SDS/SiO2NPs compared to SDS alone (Cheraghian et al.,2017).The oil recovery improvement can be attributed to the increased viscosity of the displacing phase.Many similar results,including IFT reduction and wettability alteration from oil-wet to water-wet after introducing nanoparticles,were also reported by other published papers(Ahmed et al.,2018;Al-Anssari et al.,2017;Cheraghian and Goshtasp,2016b;Parvazdavani et al.,2014).
6.Current challenges and future prospects of nanoparticles for NEOR
Although many nanoparticles(SiO2,CuO,MoS2,etc.)have been successfully studied to enhance oil recovery in the laboratory,there are still many concerns limiting their applications from laboratory scale to field scale.This section discusses the current challenges and future prospects faced by nanoparticles in EOR.
6.1.Improvement of nanofluid stability
Nanoparticles are often used to enhance oil recovery in the form of nanofluid.The dispersion degree of nanoparticles in a specifically based fluid has been affected by temperature,salinity,based fluid properties (alcohol,water),nanoparticles’ surface properties,etc.Once unstable nanofluid is injected into complex reservoirs,the solid nanoparticles tend to aggregate or deposit to form a large size cluster,resulting in damage to reservoir permeability and pores and throats structures.Hence,nanofluid stability improvement,especially under harsh reservoir conditions (high temperature,high salinity and etc.),should be considered before following properties evaluation.
6.2.Improvement of surface activity of nanoparticles
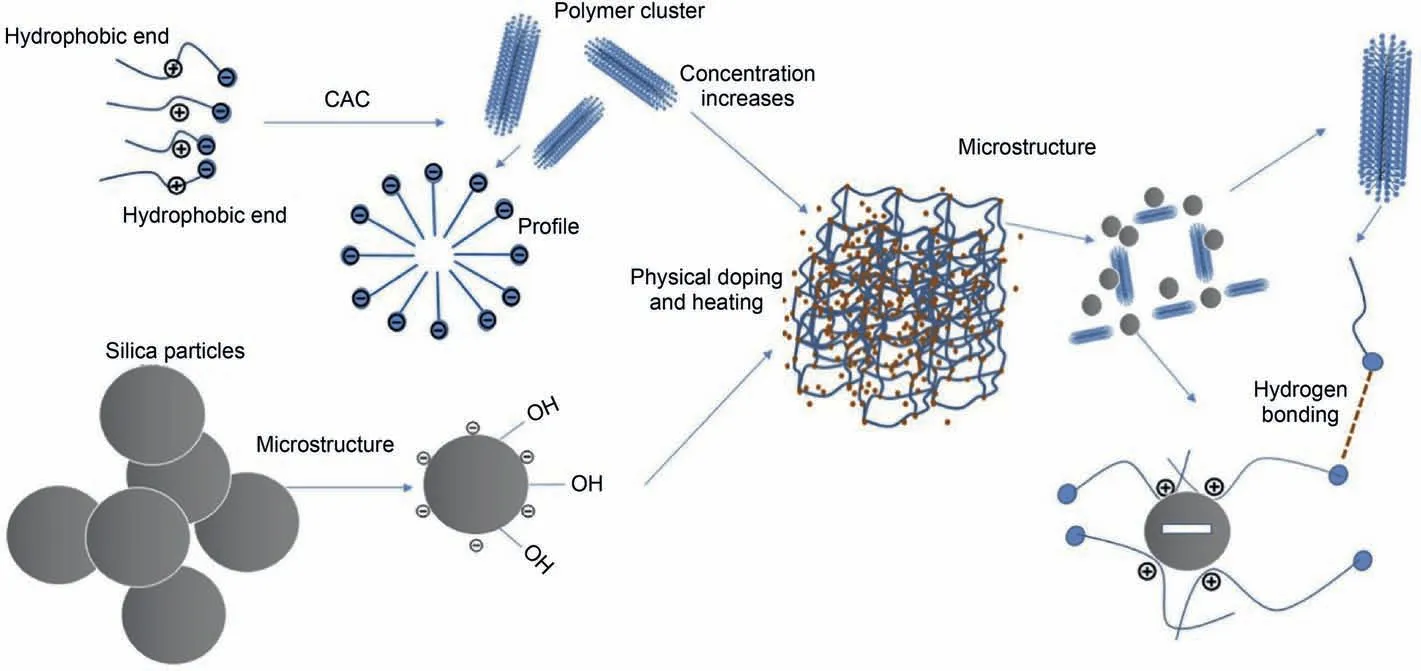
Fig.14.Mechanism of silica NPs to improve amphiphilic polymer viscosity (Kang et al.,2019).
Amphiphilic (Janus) nanoparticles with hydrophilic and lipophilic nature show excellent properties for EOR over SHNPs.As a kind of solid surfactant,amphiphilic nanoparticles can significantly reduce interfacial tension,self-adsorb onto the interface,and change interface properties.Commonly,the SHNPs possessing single wettability (hydrophilic or hydrophobic) tend to disperse into the bulk phase instead of interfaces and have no apparent contributions to interface properties.Therefore,amphiphilic nanoparticles are superior to SHNPs for N-EOR.
6.3.Clarification in EOR mechanisms and flow in porous media of nanofluids
Apart from interfacial tension reduction,wettability alteration,and foam/emulsion stability improvement,nanofluid's unique oil displacement mechanism is the generation of structural disjoining pressure in the confinement structure (wedge film).However,as mentioned above,this structural disjoining pressure occurs when the volume fraction of used spherical nanoparticles is more than 20 v%.Therefore,we should urgently reveal the mechanisms for oil displacement during the N-EOR process while using nanofluid.Moreover,can the critical value for volume fraction (20 v%) be decreased when using nanosheets?
Another concern limiting nanofluid application to field scale is the flow in porous media of nanofluid during N-EOR.There is no definite answer for adding solid nanoparticles to the water phase to affect the flow in porous media of the water phase,such as the relative permeability curve.
6.4.Production cost reduction of nanofluid
Lastly,there are many ways to prepare various nanofluids.However,it is necessary to reduce nanofluids’ production costs as the oil price decreases,which is also the economic premise for the broad application of nanofluids from laboratory scale to field scale.
As the price of chemicals is increasing,nanofluid oil displacement technology (N-EOR) can replace traditional chemical oil displacement technology (C-EOR) in the future due to the numerous advantages of nanoparticles and the relatively low production cost.Over the decades,SiO2nanoparticles are the most widely used nanomaterial to boost EOR.However,using different nanomaterials instead of regular spherical nanoparticles may yield better results in EOR due to their unique physical and chemical properties.
7.Conclusion
This work summarizes the most recent progress of nanoparticles used as nanofluids for enhancing oil recovery.Here,we mainly summarize the preparation methods of amphiphilic nanoparticles for the crude oil development,followed by the evaluation techniques for nanofluid stability.The mechanisms of nanofluids during N-EOR processes in terms of interfacial tension reduction,wettability alteration,foam stabilization,emulsion stabilization,structural disjoining pressure,and depressurization-increasing injection are also discussed and reviewed.Compared to SHNPS,the amphiphilic nanomaterials show great potential in improving oil recovery.However,there is much literature focusing on the applications of nanofluids for oil recovery at the laboratory level,but the successful field applications are few.Besides,we also summarize the limitations on their applications from laboratory scale to field scale in terms of large-scale production,lower production cost,and surface properties improvement.Such difficulties can be overthrown by improving the synthesis techniques.Thereby,the nanofluid can still be regarded as an outstanding candidate for enhancing oil recovery significantly in the future.
Acknowledgments
The authors gratefully appreciate the financial support of the Science Foundation of China University of Petroleum,Beijing(Grant No.2462020XKBH013).Financial supports from the National Natural Science Foundation of China (Grant No.51804316) and the Science Foundation of China University of Petroleum,Beijing(Grant No.2462017YJRC037) are also significantly acknowledged.Finally,Tuo Liang wants to thank the invaluable care and support from Jiaxin Xi (my wife) over the years.You are the world to me.
- Petroleum Science的其它文章
- Retraction notice to“Interactions of ferro-nanoparticles(hematite and magnetite) with reservoir sandstone:Implications for surface adsorption and interfacial tension reduction”[Petrol.Sci 17 (2020)1037-1055]
- Carbon nanotube enhanced water-based drilling fluid for high temperature and high salinity deep resource development
- Impacts of inorganic salts ions on the polar components desorption efficiency from tight sandstone:A molecular dynamics simulation and QCM-D study
- New insights into the mechanism of surfactant enhanced oil recovery:Micellar solubilization and in-situ emulsification
- Ce2(MoO4)3 as an efficient catalyst for aerobic oxidative desulfurization of fuels
- Numerical analysis on the influence of vortex motion in a reverse Stairmand cyclone separator by using LES model

