Effect of AMPS(2-acrylamido-2-methylpropane sulfonic acid) content on the properties of polymer gels
Tin-Ci Zhng ,Ji-Jing Ge ,*,Ho Wu ,Hong-Bin Guo ,Bo-Lei Jio ,Zhen Qin
a School of Petroleum Engineering,China University of Petroleum (East China),Qingdao,266580,Shandong,China
b Petroleum Engineering Institute,Northwest Branch of Sinopec,Urumqi,830011,Xinjiang,China
Keywords:Gels 2-Acrylamido-2-methylpropane sulfonic acid Enhanced oil recovery Hydrolysis Syneresis
ABSTRACT The BST oilfield in the northwestern Taklamakan Desert is a fractured carbonate reservoir,but issues of water breakthrough are becoming increasingly severe with the development of water flooding.Unfortunately,the high-temperature and high-salt conditions (130 °C,71695 mg/L) of the BST oilfield pose challenges for the development of plugging agents.In this study,the effects of 2-acrylamido-2-methylpropane sulfonic acid (AMPS) content on AM/AMPS copolymers and gels were studied through viscosity measurements,nuclear magnetic resonance (NMR),and cryo-scanning electron microscope(Cryo-SEM).Moreover,the AMPS stabilization mechanism of the polymers and gels was explained.Heatresistant and salt-tolerant gel systems were developed,and their gelation properties,thermal stability,injection capacity,and plugging ability were evaluated.Experimental results showed inconsistencies between the effects of AMPS content on the polymers and gels.For the polymers,the thermal stability increased with increased AMPS content in the polymer.However,excessive AMPS content resulted in poor gelation and low strength.The developed gel systems with S30 polymer (AMPS content is approximately 26%) exhibited excellent thermal stability,controllable gelation time,good injection capacity,and plugging ability.The field application results indicated that most production wells had a positive response,with reduced water-cut and increased daily oil production.
1.Introduction
Water flooding is a widely used technique to enhance oil recovery and improve oil production rate (Li et al.,2021).However,permeability heterogeneity,gravity segregation,and viscous fingering are the cause of poor sweep efficiency during water flooding (Sie and Nguyen,2021;Seright and Brattekas,2021).Water channeling can also occur after decades of water flooding,leading to earlier water breakthrough,increased water cut,reduced oil recovery,and increased water handling costs.
Researchers have employed many technologies to reduce water production and improve water displacement recovery,such as polymer flooding (Jouenne,2020;Kang et al.,2015),surfactant flooding (Mohamed et al.,2018;Almahfood and Bai,2021),foam flooding(Zhao et al.,2009;Hu et al.,2020),and gels(Ge et al.,2021;Sun et al.,2020;Elsharafi and Bai,2016).One of the most pervasive technologies is the use of polymer gels due to their low cost,extended shelf life,and simple operation.Under high-temperature and high-salt conditions,polymer gels have better thermal stability and plugging ability.Moradi et al.(1993) developed a gel system consisting of an acrylamide (AM) polymer,formaldehyde,and phenol,which survived 2.5 years of aging at 113°C(or 9 months of aging at 149°C).Dovan et al.(1997) investigated the effects of organic cross-linkers on polymer gels and found that a gel prepared with a polymer,hexamethylenetetramine (HMTA),and hydroquinone (HQ) had excellent thermal stability and a controllable gelation time even under high-temperature conditions.Lashari et al.(2018) prepared a thermally stable gel with PADC,HMTA,and resorcinol,which was stable for 90 days under high-temperature(100°C) and high-salt (51984 mg/L) conditions Liu et al.(2020)also developed a heat-resistant gel that contained a copolymer,bisphenol-A,and HMTA,and displacement experiments showed the plugging ratio of the gel exceeded 95%.
Phenolic compounds and aldehyde compounds can be employed as cross-linkers to prepare polymer gels for hightemperature and high-salt conditions.In addition to the crosslinkers,the polymers are also vital gel components.However,HPAM polymers undergo hydrolysis,forming acrylate groups under high-temperature conditions(Seright,2010),and the complexation of the acrylate groups and divalent cations causes over-crosslinking and syneresis (Zhang et al.,2015).As a result,the processing method is critical for developing heat-resistant and salt-tolerant gels and improving the stability of the polymer.Numri et al.(2018) found that the introduction of 2-acrylamido-2-methylpropane sulfonic acid (AMPS) inhibits AM hydrolysis and improves polymer resistance to temperature and salt.Moreover,AM/AMPS copolymer gel systems had been prepared,which exhibited excellent stability under high-temperature and high-salt conditions.Albonico and Lockhart (1993) developed a gel system containing a PAAm/AMPS copolymer,HMTA,and phenol,and the system was stable for 150 days at 120°C.Unomah et al.(2018)also investigated the effect of polymer type on gels and found that gels prepared with AM/AMPS copolymers had the best thermal stability.Zhu et al.(2017)also prepared a thermally stable gel containing an AM/AMPS/NVP copolymer,HMTA,and resorcinol,and the gel was stable for 5 months at 150°C.
Although some heat-resistant and salt-tolerant gel systems have been developed with an AM/AMPS copolymer,phenolic compounds,and aldehyde cross-linkers,the influence and stabilization mechanisms of AMPS are not clear.Thus,this research investigated the properties of gels prepared with different AM/AMPS copolymers through viscosity measurements,nuclear magnetic resonance (NMR),and cryo-scanning electron microscope (Cryo-SEM).The mechanisms of AMPS polymer and gel stabilization were explained,and a suitable gel system for use in the BST oilfield(130°C,71695 mg/L) was developed.
2.Materials and methods
2.1.Materials
Four polymers used in this study were provided by Qingdao Golden Brick Technology Co.,Ltd,the chemical structure of polymers was identified with a NMR spectrometer and their AMPS percent ratios were determined by the elemental analysis method(see in the supplemental material).The details of the four polymers are listed in Table 1 (the molecular weight refers to the viscosity average molecular weight).Hexamethylenetetramine (HMTA) and hydroquinone (HQ) were employed as cross-linkers,which were purchased from Shanghai Aladdin Biochemical Technology Co.,Ltd.Thiourea (TH) was used as an antioxidant,which was purchased from Sinopharm Chemical Reagent Co.,Ltd.Synthetic brine was used to simulate the high-salt conditions of the BST oilfield,and the brine composition is shown in Table 2.

Table 1Polymer properties.

Table 2Ion composition of BST formed brine.
2.2.Methods
2.2.1.Gelling solution preparation
First,2 wt% polymer stock solution was prepared according to the following procedures.First,(1) a container with a known amount of brine was vigorously stirred to create a deep vortex,then(2) the polymer was slowly added to the shoulder of the vortex toeffectively wet the polymer beads,and finally(3)the container was sealed to minimize evaporation and was continuously stirred for 24 h to ensure complete dissolution of the polymer (Zhang et al.,2015).Then,pre-weighted TH,HMTA,and HQ were diluted into the brine,and the gelling solution was obtained by mixing the previously obtained brine solution and polymer stock solution.Finally,20 g of prepared gelling solution was transferred into an ampoule,which was placed in an oven (130°C) to initiate crosslinking.
2.2.2.Gelation time measurements
Gelation time is an important index that is closely related to operational safety.Sydansk (1990) proposed a method for characterizing the qualitative gel strength (gel strength code method),where the strength of the gel was classified into 10 grades(A-J).In this study,the gels were removed periodically from the oven at set intervals,and the gel strength was determined according to the gel strength code method.Finally,the gelation time was defined as the strength of the gel when it reached grade “F”.
2.2.3.Viscosity and elastic modulus measurements
The thermal stability of the polymers was characterized by the viscosity retention over aging time.First,polymer solutions were prepared at 0.6 wt% concentration,then polymer solutions were placed into the oven (130°C),finally the polymer solutions were taken from the oven and the viscosity was measured with an Anton Paar MCR 92 rheometer equipped with a cup &nob setup (CC39),and the measurement time was 5 min.The viscosity retention can be defined by the following expression:

where Rvis the viscosity retention,η1is the viscosity of the polymer solution after heat treatment,and η0is the initial viscosity.
The gel strength was characterized by quantifying the elastic modulus,which was measured with an Anton Paar MCR 92 rheometer equipped with a 25-mm parallel plate (PP25).For the elastic modulus measurements,the strain range was set to 1%-500% and the frequency was fixed at 10 rad/s.All the rheological experiments were done at 25°C.
2.2.4.Syneresis rate measurements
To evaluate the thermal stability of the gels,the syneresis rate should be measured during gel heat treatment.The syneresis rate can be defined by the following expression:

where Rsis the syneresis rate of the gels,m1is the weight of the water expelled from the gel after aging,and m0is the weight of the gelling solution (20 g in this study).
2.2.5.Cryo-SEM measurements
The gel microstructure was observed by Cryo-SEM,and the samples were prepared according to the following steps.First,(1)the bulk gel was frozen in liquid nitrogen (-160°C),then (2) the water in the gel was sublimed by reducing the pressure,and finally(3) the pre-frozen dried gel was placed onto the conductive adhesive,and the gel surface was sprayed with gold.The gel microstructure was then observed using a scanning electron microscope(SEM,Hitachi SU8010).
2.2.6.NMR measurements
A solid-state NMR sample was first prepared,as gels prepared in the brine (71695 mg/L) solution contained large quantities of inorganic salt,which was removed to prevent any adverse effects on the NMR test.However,dehydration cannot occur under hightemperature conditions,as these conditions can accelerate the hydrolysis of the amide group.Therefore,a solvent extraction method was adopted in this study to remove the inorganic salt and water from the bulk gel,according to the following steps.First,(1)the bulk gels were immersed in deionized water 7-10 times(4 h at a time)to remove the salt,next(2)the bulk gels were immersed in ethanol 4-6 times (4 h at a time) to remove water from the gels,and then (3) the bulk gels were placed in a vacuum drying oven(60°C) for 10 h to remove the ethanol.Afterward,the solid-state NMR sample was prepared by grinding the dried bulk gel into a powder.Finally,13C NMR tests were conducted at 400 MHz using a WB solid-state NMR spectrometer.In the solid-state NMR test,the rotation frequency was 3 kHz,the recycle delay was 3 s,the number of increments was set to 10,000,and a CPTOSS impulse sequence was used.
2.2.7.Flooding test
The plugging ability of the gel was characterized via a breakthrough pressure gradient,which was measured by a flooding test.The following methods were employed.First,(1) the flooding experiment setup was built according to Fig.1,then(2)the gelling solution was injected into a thin metal tube,the thin metal tube was employed to simulate reservoir fracture,with length of 50 cm,and the inner diameters ranging from 0.8 mm to 1.6 mm,next (3)thin tubes containing gelling solution were sealed and placed into an oven(130°C),then(4)the thin tube was placed in the flooding experiment setup,and the brine was injected into the thin tube,while the pressure along the thin tube was recorded,and finally(5)the breakthrough pressure was defined as the pressure when brine drained from the end of the thin tube.The breakthrough pressure gradient was calculated by dividing the breakthrough pressure by the length of the thin tube.
3.Results and discussion
3.1.Polymer screening
Polymers are essential components of gelling systems,and they significantly affect the gel performance.In this section,thermal stability,gelation properties,and gel strength were measured to obtain a suitable polymer.
3.1.1.Thermal stability of polymers
Hydrolysis reactions occur under high-temperature conditions(>75°C),converting AM groups into acrylic acid(AA)and reducing the viscosity of polymer solutions,which is detrimental to gel properties.In this study,the viscosity retention rates of several polymers were adopt to evaluate thermal stability.The mass fraction of the polymer was set to 0.6 wt%,and TH(0.3 wt%)was used as the antioxidant,to prevent oxidative degradation.The viscosity changes of the four polymer types are shown in Fig.2.We found that the viscosity of all the polymers decreased as the aging time increased.However,polymers with more AMPS had a more gradual loss in viscosity compared to polymers with less AMPS.Thus,polymers with higher amounts of AMPS exhibited better stability under high-temperature and high-salt conditions,which was consistent with previous research (Nurmi et al.,2018).The previously described phenomenon occurred due to two reasons:(1)the AMPS group had stronger thermostability than the AM group,and(2) the hydrolysis of the AM group was inhibited by the neighboring AMPS group due to steric and electrostatic repulsion effects.Thus,polymers containing higher amounts of AMPS retain higher thermal stability,which may be best suited for use in developing heat-resistant and salt-tolerant gels.
3.1.2.Gelation properties
To further determine polymer suitability,gelation time was assessed.The mass fraction of the polymer(poly)was set to 0.6 wt%,and HMTA and HQ were employed as cross-linkers (CL),with mass fractions between 0.05 wt%and 0.2 wt%.The gels(poly:0.6 wt%,CL:0.20 wt%) that were prepared with different polymers are shown in Fig.3,and these gels were aged for 30 days.We found that the strengths of the gels prepared with S00,S30,and S50 polymers reached grades“H”,“G”,“F”,respectively,but the strength of the gel prepared with S70 only reached grade “C”,indicating that the S70 polymer did not form a strong gel under the BSTconditions(130°C,71695 mg/L).
Once I digested this insight my feelings changed from those of a needy19 child to ones of a very proud daughter. Looking at my father more objectively allowed me to view him clearly: he was a man of few words; he was intelligent, kind and extremely modest. Ironically I began to feel closer to him in death than I had while he was alive.
Fig.4 shows the gelation time of the three gelling systems(not including the S70 polymer gel).The gelation time of three gel systems prepared with S00,S30,and S50 polymers were 10-50,21-70,and 24-120 h,respectively.Thus,the three polymer gel types met the field operation requirements.One interesting result was that gelation time increased as the AMPS content increased.One explanation for this phenomenon could be the electrostatic repulsion between the compounds.Sun et al.(2008)found that the cross-linker was negatively charged,and polymers containing AMPS groups were also negatively charged.So the crosslinking reaction was inhabited by the electrostatic repulsion between the AMPS group and cross-linker.Furthermore,the electrostatic repulsion was enhanced with increased AMPS content,thus the gel system containing the S50 polymer (with higher AMPS content)had a longer gelation time.Concerning the phenolic gels,the most suitable polymer was not the most stable one (with the highest AMPS content),polymers containing excessive amounts of AMPS may not form a gel.
3.1.3.Gel strength
Gel strength is a vital index related to plugging ability,and in this section,it was quantitatively characterized by elastic modulus.The mass fraction of the polymer (poly) was set to 0.6 wt%,and HMTA and HQ were employed as cross-linkers (CL) with a mass fraction of 0.10 wt%.The storage moduli of the three gelling systems(not including the S70 polymer) are shown in Fig.6.The S00 polymer gel had the highest elastic modulus,and the variations in elastic modulus with aging time were divided into three sections:the slow ascent stage,the plateau stage,and the abrupt ascent stage.It should be noted that the second abrupt ascent stage was induced by the syneresis of the gel,and the elastic modulus reached 50 Pa after 30 days of aging (marked in red in Fig.5),while the syneresis rate at this time was 35%.The plugging capability of a gel was reduced when the syneresis rate increased;therefore,although the gel containing the S00 polymer had the highest elastic modulus,this gel was not suitable for improving conformance.For the S50 polymer gel,the elastic modulus reached its maximum after 2 days of aging,then gradually decreased.The maximum elastic modulus was only about 5 Pa,which meant that the gel prepared with S50 did not have sufficiently strong plugging capabilities.For the S30 polymer gel,the elastic modulus increased with increased aging time and then remained almost constant (11 Pa).Moreover,the syneresis rate of the S30 polymer gel was nearly zero,and the gel had excellent thermal stability.Through a comparative analysis,the gel prepared with the S30 polymer exhibited excellent thermal stability and had a high elastic modulus.Thus,the S30 polymer was chosen for the development of a heat-resistant and salt-tolerant gel suitable for use in the BST oilfield.

Fig.1.Schematic of the experimental flooding setup.

Fig.2.The viscosity retention rates of the different polymer solutions (The initial viscosities of S00,S30,S50,S70 polymers are 98.35,108.47,60.84,and 73.12 mPa·s).

Fig.3.Gels prepared with different polymers (after aging for 30 days).
3.2.Development of the heat-resistant and salt-tolerant gel
According to the above experimental results,the S30 polymer was selected for developing a suitable gel for use in the BST oilfield.In this section,gelation time,syneresis rate,injection capacity,and plugging capability were evaluated for the development of a heatresistant and salt-tolerant gel.
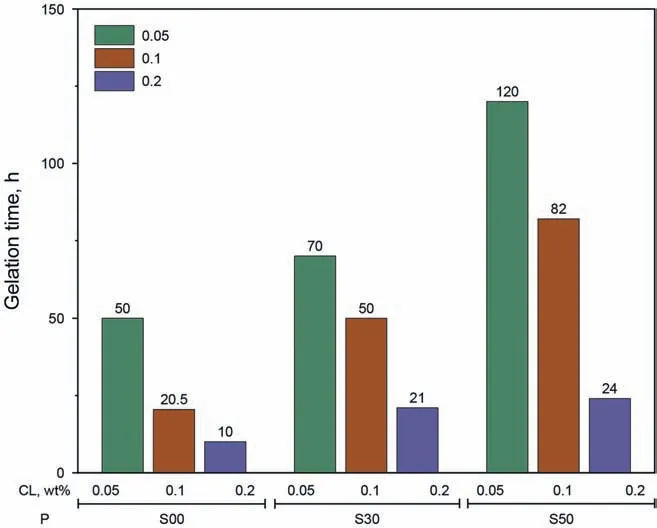
Fig.4.Different polymers prepared as a function of gelation time.
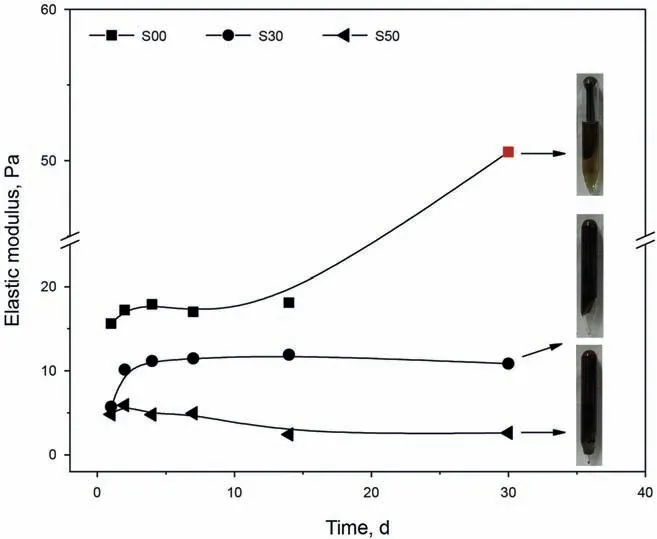
Fig.5.Elastic modulus of different gelling systems.
3.2.1.Gelation time measurements
Gelation time is a vital index for evaluating gelling systems,as gelation time that is too short will negatively affect deep profile control and operational safety.Additionally,the dilution effect may be more extreme when the gelation time is too long.As a result,there are optimal gelation time for gelling systems.In this section,the mass fraction of the polymer was between 0.40 wt%and 1.00 wt%,and the mass fraction of the cross-linker varied from 0.05 wt%to 0.40 wt%.The measured gelation time of the different gelling systems are shown in Fig.6,which indicated that gelation time decreased as the mass fraction of the polymer and cross-linkers increased.When the mass fraction of the polymer or cross-linkers increased,there were more polymer molecules or cross-linker molecules,which provided more crosslinking locations,thus the gelation time was shorter.Therefore,the gelation time of the S30 polymer gels ranged from 9 to 90 h,and the S30 polymer gels were considered suitable for profile control according to the industry standard (Q/SH1020 1493-2014).
3.2.2.Syneresis rate measurements
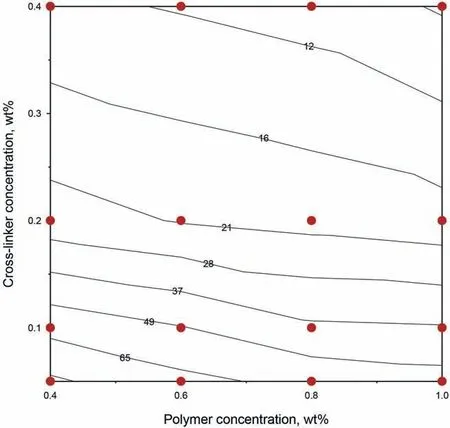
Fig.6.Contour plot of the gelation time of the S30 polymer gels.
Polymers experience hydrolysis during formation,resulting in the destruction of the gel structure and final syneresis.The gel volume usually decreases after syneresis,and plugging capability is reduced.Thus,the syneresis rate is also an important index for evaluating gelling systems.In this study,the mass fraction of the polymer ranged from 0.40 wt%to 1.00 wt%,and the mass fraction of the cross-linker varied from 0.05 wt% to 0.40 wt%.The measured syneresis rates of the different gelling systems are shown in Fig.7,indicating that the syneresis rate decreased as the mass fractions of the polymer or cross-linker increased.This was the result of crosslinking density,and the gel strength increased when the mass fraction of the polymer or cross-linker increased,and eventually syneresis was inhibited.Thus,to achieve a thermally stable gel,the mass fraction of the polymer and cross-linker must exceed 0.60 wt%and 0.10 wt%,respectively.When the two aforementioned conditions were satisfied,the gel exhibited excellent thermal stability(the syneresis rate was lower than 25% after 180 days of aging).
The injection capacity of a gel is an important parameter related to operational safety.The lower the injection capacity,the higher the injection pressure,and an injection pressure that is too high may result in formation fracturing and even operational accidents.In this section,the viscosity and injection pressure gradient of the gels were measured to evaluate injection capacity.In the flooding experiments,thin tubes (inner diameters of 0.8,1.2,and 1.6 mm)were used to simulate fracture,and the injection rate varied from 10 to 100 m/d.Based on the previous gelation time and syneresis rate results,three gelling systems were studied:(A) 0.6 wt% of S30+0.1 wt%of HMTA+0.1 wt%of HQ+0.3 wt%of TH,(B)1.0 wt%of S30+0.1 wt%of HMTA+0.1 wt%of HQ+0.3 wt%of TH,and(C)1.0 wt%of S30+0.4 wt%of HMTA+0.4 wt%of HQ+0.3 wt%of TH.Additionally,the viscosity and injection pressure gradient was measured at 90°C due to restricted experimental conditions.Fig.8 illustrates the viscosity curves of the gel systems at 90°C,which indicated that the gelling systems had lower viscosities.Even when the mass fraction of the polymer and cross-linker reached 1.0 wt%and 0.4 wt%,respectively,the viscosity was only 225.74 mPa·s (at the shear rate of 7.3 s-1).The injection pressure gradient is shown in Fig.9,indicating that the injection pressure gradients were generally low,and the maximum injection pressure gradient was only 10 kPa/m,demonstrating that the selected gelling systems had excellent injection capacities.

Fig.7.The syneresis rates of the different gelling systems.

Fig.8.The viscosity curves for the different gelling systems.
3.2.4.Plugging capability measurements

Fig.9.The injection pressure gradients of the different gelling systems.
The breakthrough pressure gradient is a direct manifestation of the plugging capability of a gel.In this section,the experimental parameters(thin tubes and gelling systems)were identical to those described in Section 3.2.3.The breakthrough pressure gradients of the gels at different water injection rates are shown in Fig.10,indicating that the breakthrough pressure gradient decreased with an increase in the water injection rate and increased inner diameter of the tube,which was consistent with previous research(Ganguly,2009).Moreover,the breakthrough pressure gradient increased with increased polymer and cross-linker concentrations,and the maximum breakthrough pressure gradient reached approximately 3 MPa/m,thus the gels prepared with the S30 polymer exhibited excellent plugging capabilities.
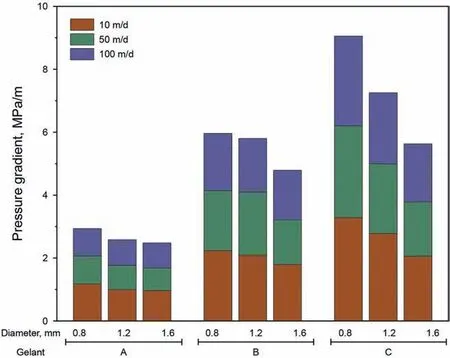
Fig.10.Breakthrough pressure gradients of different gelling systems.
3.3.Stabilization mechanisms of the AMPS group
Hydrolysis of the AM group occurs under high-temperature conditions,generating an AA group that can complex with divalent cations(such as Ca2+).The complexation reaction can improve crosslinking density,which reduces the hydrophilicity of the gels and ultimately leads to syneresis.Based on the aforementioned experimental results,gels prepared with the S30 and S50 polymers(high AMPS content)exhibited better thermal stability than the S00 polymer(low AMPS content).The13C NMR results of three polymer gel types are shown in Fig.11.The results showed that after aging for 5 days,there were no obvious amide peaks in the13C NMR spectrum for the S00 polymer gels,and almost all of the AM moieties experienced hydrolysis,forming AA moieties.For the S30 polymer gels,the peak at δ=176 ppm corresponded to the amide carbonyl carbon,and the peak at δ=187 ppm corresponded to the carboxyl carbonyl carbon.Thus,both AM and AA moieties were present in the S30 polymer gels after 5 days of aging.For the S50 polymer gels,there were no obvious carbonyl peaks in the13C NMR spectra,and hydrolysis of the AM group did not occur.When the aging duration was extended to 30 days,the proportion of AA moieties increased in the S30 polymer gels,indicating more hydrolysis reactions.In addition,the presence of a carboxyl peak in the13C NMR spectrum of the S50 polymer gels meant that the AM group began to hydrolyze after 30 days of aging.Therefore,based on these results,AMPS inhibited the hydrolysis of AM,which in turn improved the thermal stability of the gels.Moreover,the stabilization effect improved as the AMPS copolymer content increased.

Fig.11.The 13C NMR results of three polymer gel types:(a) aging for 5 days;(b) aging for 30 days.The inserts represent the magnified signal between δ=150 ppm and δ=200 ppm.
To visually investigate the stabilization effect of the AMPS group,the microstructure of three polymer gels was analyzed by SEM,as shown in Fig.12.In this section,the mass fractions of the polymer,cross-linker,and antioxidant were set to 0.6 wt%,0.1 wt%,and 0.3 wt%,respectively.As shown in the figure,the S30 and S50 polymer gels had stronger networks than the S00 polymer gel,and small holes were spread throughout the S00 polymer gel network.Besides,the network of the S00 polymer gel was denser compared with gels containing S30 and S50 polymer.This could be because the AA content of S00 polymer was higher than the S30 and S50 polymer after the heat treatment.Due to the increased crosslinking between carboxyl and Ca2+in the S00 polymer gel,the network of the gel was compressed.Thus,the stabilization effect of the AMPS group was observed microscopically.
4.Field applications
4.1.BST oilfield background
The BST oilfield in the northwestern Taklamakan Desert is a fractured carbonate reservoir with edge and bottom water.The reservoir medium includes fracture and matrix media,where the mean porosity and permeability of the matrix are 10% and 12.89×10-3μm2,respectively,and the length and width of the fracture are 3-100 cm and 0.01-1.5 mm,respectively.The midpoint of the pay zone is 4763.19 m,the reservoir temperature is nearly 130°C,and the salinity of the formation brine is approximately 71695 mg/L.The B6H well group in the BST oilfield was selected for profile control treatment,and the B6H well group location is schematically illustrated in Fig.13.The production status of the B6H well group,as of March 2020,is summarized in Table 3,showing that the water content of most of the production wells exceeded 90%,and B3 even reached 97.95%.Thus,conformance improvement was critically needed for enhancing oil recovery in the BST oilfield.
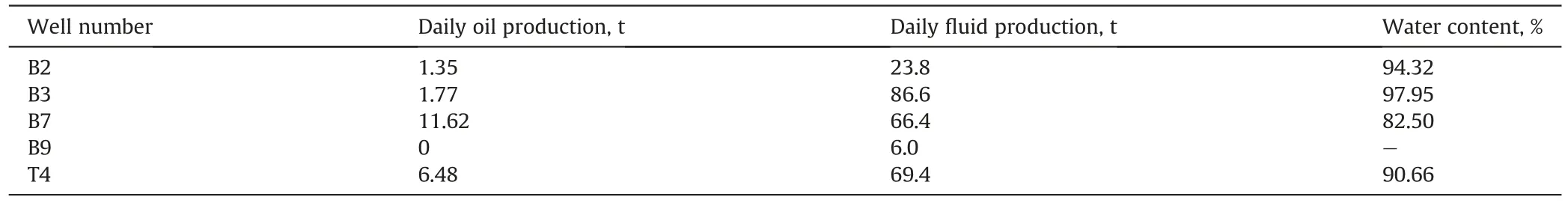
Table 3Production status of the B6H well group.
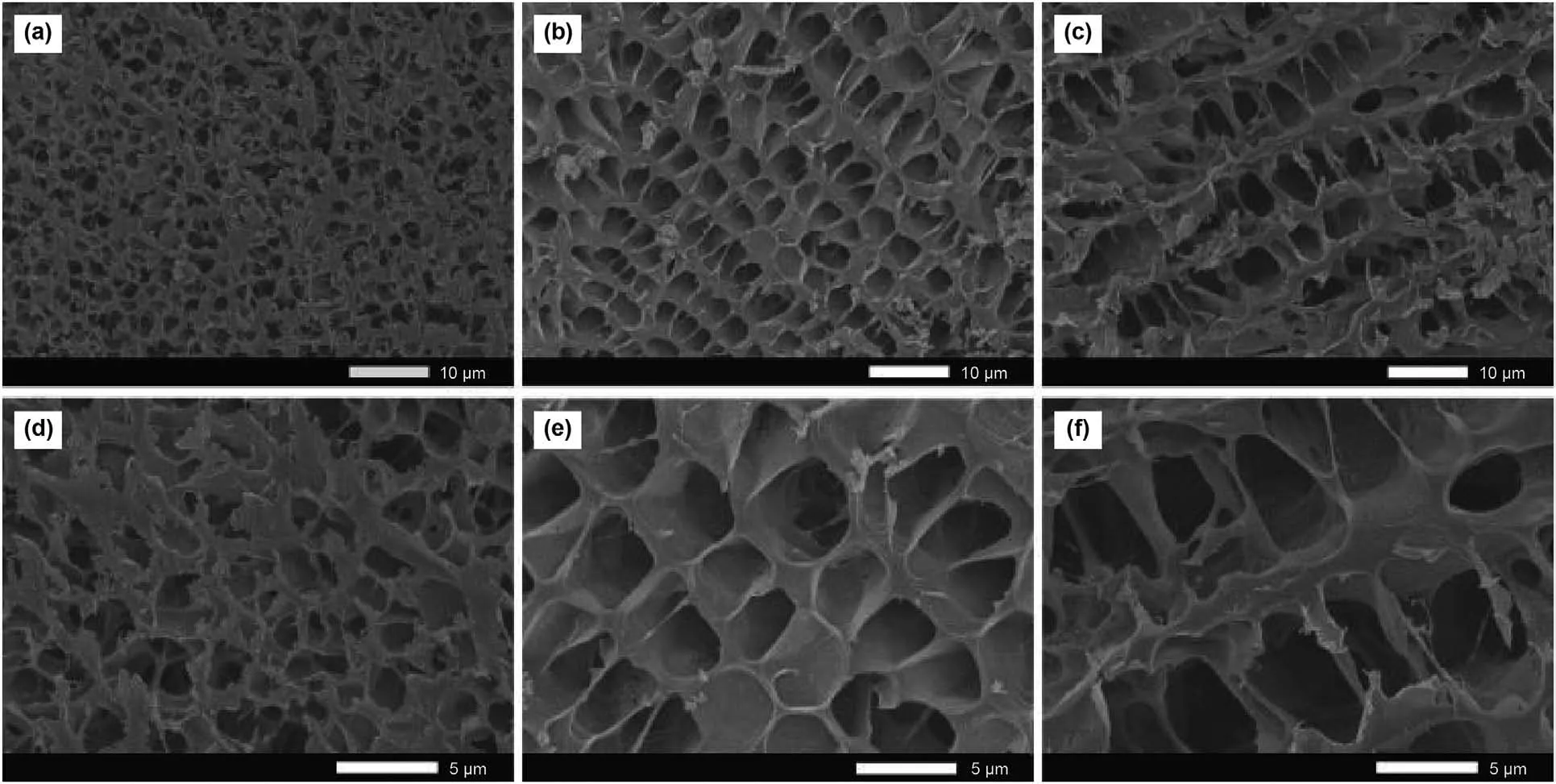
Fig.12.SEM images of different gels after 30 days of aging:(a,d) S00;(b,e) S30;(c,f) S50.

Fig.13.The schematic of the B6H well group.
4.2.Field operations
The polymer gel developed with S30 polymer had better thermal stability compared to S00 polymer gel,and higher gel strength compared to S50 polymer gel.So the S30 polymer gel was selected to conduct the profile control treatments.The field operations were conducted from April 29,2020,to May 3,2020.Fig.14 depicts the tubing pressure,casing pressure,and displacement during operation.The field operation went smoothly,and when 1230 m3of the gelling solution was injected into the formation,the maximum tubing pressure was only about 13 MPa.
The majority of the production wells responded well after profile control treatment except T4 well.The production performance of the B6H well group(not including T4),is shown in Fig.15.The results indicated that most production wells performed well,exhibiting a decrease in water content and an increase in daily oil production.Taking B2 as an example,the water content decreased from 94.32% to 85.25%,and daily oil production increased from 1.35 t/d to 6.35 t/d.Moreover,the cumulative oil production of the B6H well group rose by 1651 tons as of March 2021,confirming that the developed gelling systems can be used for oilfield applications.
5.Conclusions
In this study,AMPS content had varying impacts on polymers and polymer gels.For polymers,the hydrolysis of the AM group was inhibited by AMPS due to steric and electrostatic repulsion effects,and thermal stability improved with increased AMPS content.For polymer gels,the effect of AMPS content was complex.When S00 polymer (without AMPS group) was selected,the developed gel showed poor thermal stability.When the AMPS content was too high,the crosslinking reaction between AM group and cross-linker was hampered due to the electrostatic repulsion,even the S70 polymer (AMPS content:68%) could not form a strong gel.When polymer contained moderate AMPS content,the developed gels showed excellent gelation properties,thermal stability,and strength.Our results showed that the heat-resistant and salttolerant gels fabricated with the S30 polymer (AMPS content:26%)were suitable for use in the BST oilfield(130°C,71695 mg/L).The developed gelling systems had excellent thermal stabilities(the syneresis rate after thermal treatment for 180 days was 0.6%-22.5%),controllable gelation time (9-50 h),good injection capacities (injection pressure gradient:0.228-9.638 kPa/m),and plugging abilities (breakthrough pressure gradient:0.718-3.285 MPa/m).Based upon the13C NMR results,AMPS inhibited the hydrolysis of the AM group,which in turn improved the thermal stability of the gels,and the stabilization effect was enhanced as the AMPS content of the copolymer increased.
The polymer gel developed with S30 polymer had better thermal stability compared to S00 polymer gel,and higher gel strength compared to S50 polymer gel.So the S30 polymer gel was selectedto conduct the profile control treatments,and the results showed that the majority of the production wells responded well,with increased daily oil production and decreased water content.Moreover,the cumulative oil production of the B6H well group rose by 1651 tons,as of March 2021,indicating that the developed gelling systems were good candidates for use in oilfield applications.
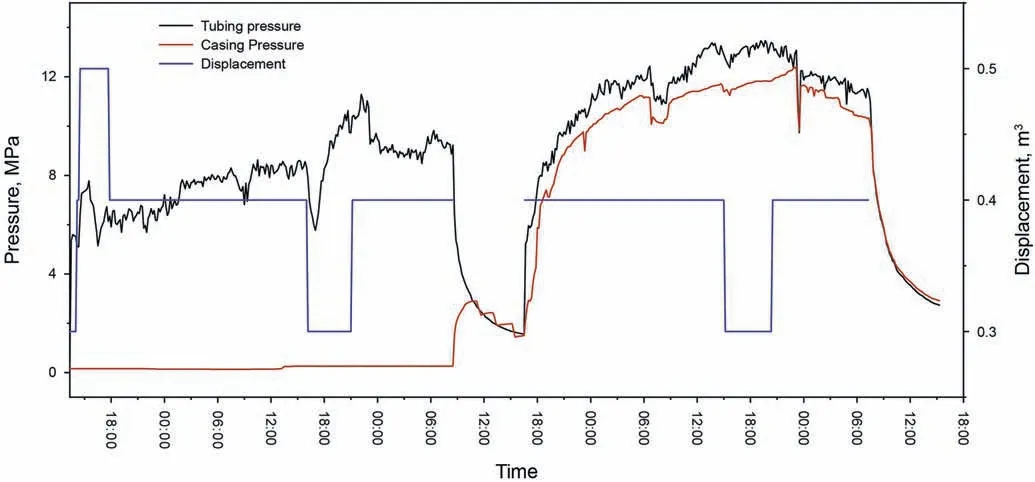
Fig.14.The operation process of the profile control treatments in the B6H well group.
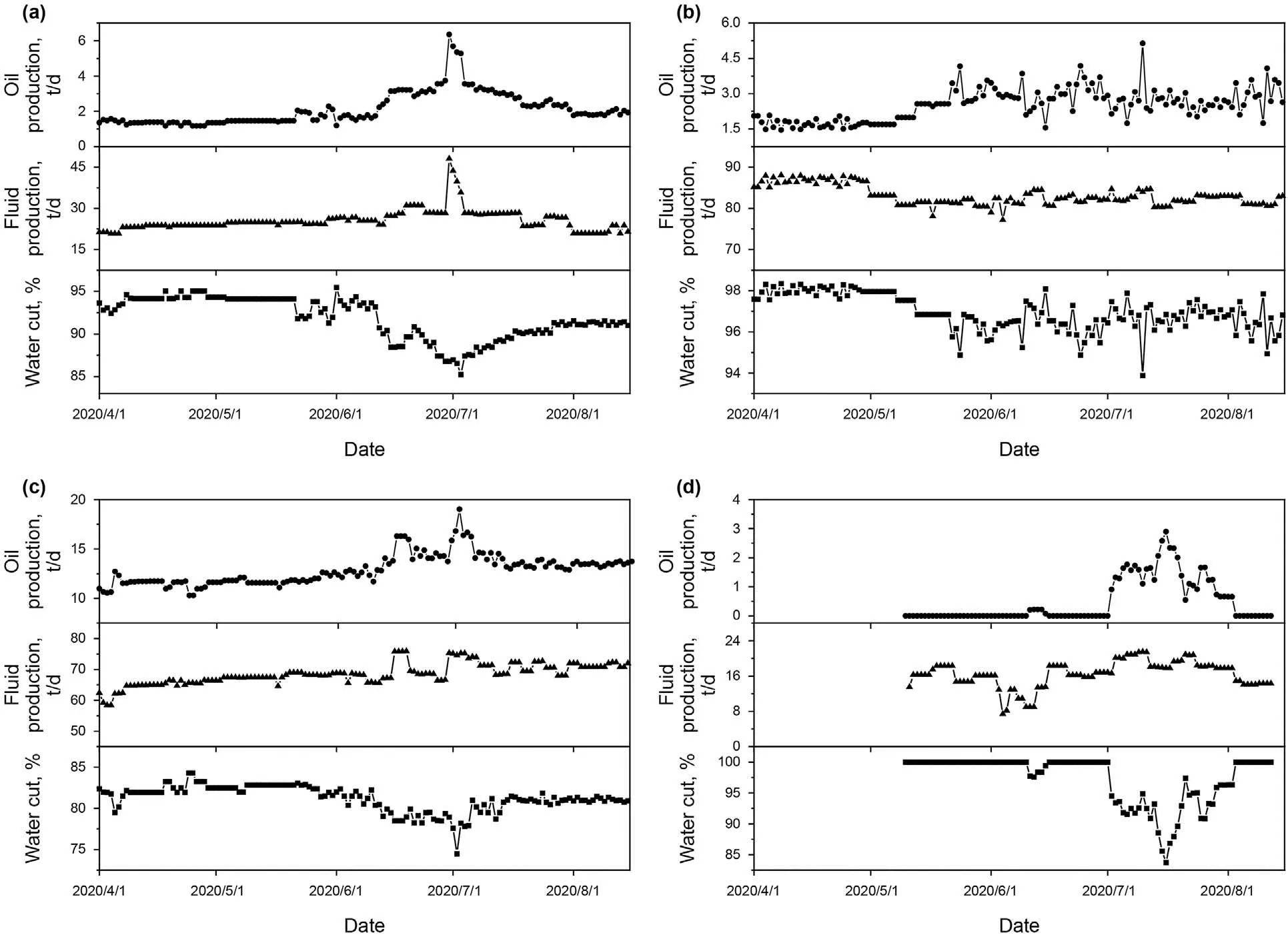
Fig.15.Production performance of the B6H well group:(a) B2;(b) B3;(c) B7;(d) B9.
Acknowledgments
Financial support from the Major Scientific and Technological Project of CNPC under grant number ZD2019-183-007 is gratefully acknowledged.The authors also thank Sinopec Northwest Company of China for the financial support (34400007-17-ZC0607-0095).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.petsci.2022.01.006.
- Petroleum Science的其它文章
- Retraction notice to“Interactions of ferro-nanoparticles(hematite and magnetite) with reservoir sandstone:Implications for surface adsorption and interfacial tension reduction”[Petrol.Sci 17 (2020)1037-1055]
- Carbon nanotube enhanced water-based drilling fluid for high temperature and high salinity deep resource development
- Impacts of inorganic salts ions on the polar components desorption efficiency from tight sandstone:A molecular dynamics simulation and QCM-D study
- Application of nanomaterial for enhanced oil recovery
- New insights into the mechanism of surfactant enhanced oil recovery:Micellar solubilization and in-situ emulsification
- Ce2(MoO4)3 as an efficient catalyst for aerobic oxidative desulfurization of fuels

