Meta-analysis of curative effect of Sacubitril valsartan combined with Qiliqiangxin capsule in the treatment of patients with chronic cardiac failure
Jin-Xuan Wei,Su-Zhen Yang,Chao Song,Xiao-Hang Zhen,Yan-Bo Sui
1Heilongjiang University of traditional Chinese Medicine,Harbin 150040,China.2Qingdao municipal hospital,Qingdao 266011,China.3The second affiliated Hospital of Medical College of Shantou University,Shantou 515041,China.4The first affiliated Hospital of Heilongjiang University of traditional Chinese Medicine,Harbin 150040,China.
Abstract Objective:To systematically review the effect of Sacubitril valsartan combined with Qiliqiangxin capsule on clinical effect,serological index,cardiac function,quality of live,and adverse reactions in patients with heart failure.Methods:Search the databases of CNKI,VIP,WanFang,CBM,DuXiu,ChiCTR,Web of science,The Cochrane Library,PubMed and Embase to collect the randomized controlled trial (RCT) of Sacubitril valsartan combined with Qiliqiangxin capsule in the treatment of patients with heart failure,The search time limit is from the establishment of the database to May 2021.After the literatures were screened,evaluated and extracted by two researchers independently,Meta analysis was carried out with Stata 16.1 software.Results:A total of 18 RCTs,were included,including 1613 patients.The results of the Meta-analysis showed that there was statistical significance in improving the effective rate (OR=2.60,95% CI [2.09,3.24],P < 0.00001),N-terminal pro-brain natriuretic peptide (MD=–468.36,95% CI [–606.80,–329.92],P < 0.00001),left ventricular ejection fraction (MD=5.41,95% CI [4.93,5.89],P < 0.00001),left ventricular end-diastolic diameter (MD=–3.27,95% CI [–3.65,–2.90],P < 0.00001),left ventricular end-systolic diameter (MD=–3.60,95% CI [–4.99,–2.21],P < 0.00001),6-minute walking distance (MD=61.42,95% CI [50.04,72.80],P < 0.00001),Minnesota living with heart failure questionnaire (MD=–11.39,95% CI [–14.50,–8.28],P <0.00001),and traditional Chinese medicine syndrome score scale (MD=–3.62,95% CI [–6.45,–0.80],P = 0.01),but there was no significant difference in cardiac output (MD=0.26,95% CI [–0.02,0.54],P = 0.07) and adverse reactions.Conclusion:The current evidence shows that Sacubitril Valsartan combined with Qiliqiangxin capsule can better improve cardiac function,TCM symptoms and quality of life in patients with heart failure than simple Sacubitril Valsartan.However,there was no significant difference in improving cardiac output between the two groups.However,higher quality RCTs are needed to verify.
Keywords:heart failure;sacubitril valsartan;ARNI;Qiliqiangxin capsule;Meta-analysis
Background
Chronic heart failure (CHF) is caused by various causes of cardiac structural changes,ventricular diastolic and systolic dysfunction,resulting in a decrease in cardiac output,can not meet the needs of metabolism,and then lead to a series of diseases such as fatigue,dyspnea and body fluid retention.With the increasing pressure of modern people's life,irregular diet,work and rest and other reasons,smoking,hyperlipidemia,hyperuricemia,diabetes and other factors can be the pathogenic factors of CHF.In addition,the elderly,men are also positively correlated with CHF events [1–2].As the final stage of various cardiovascular diseases,the incidence of chronic heart failure is increasing year by year.The mortality rate of patients with severe heart failure is as high as 50% within one year,and the 5-year survival rate is similar to that of malignant tumors [3–4].Modern medical research clearly believes that the pathogenesis of CHF is mainly related to cardiac remodeling mediated by the activation of neuroendocrine and cytokine system,therefore,at present,the clinical treatment of CHF is mainly ACEI/ARB,β-blockers,calcium channel blockers,aldosterone receptor antagonists [4–8].Although the combination of these drugs can improve the symptoms of patients,but the long-term indicators such as prognosis,readmission rate and mortality have not been significantly improved,seriously affect the survival and treatment of patients,and bring heavy economic burden to individuals and society [9].Traditional Chinese medicine has played a great role in the treatment of CHF.Studies have shown that traditional Chinese medicine can effectively reduce myocardial injury in CHF in the aspects of stabilizing mitochondrial membrane potential,enhancing the activity of respiratory chain complex,regulating calcium concentration,reducing oxidation,protecting mtDNA and so on [10–16].It has unique advantages in improving prognosis and reducing the incidence of end point events [17–18].
Qiliqiangxin capsule is the first traditional Chinese medicine preparation with evidence-based medical evidence to prove its definite therapeutic effect on CHF.It is one of the most commonly used proprietary Chinese medicines in the treatment of heart failure.It has been listed as recommended drugs by "Guidelines for diagnosis and treatment of Heart failure in China","Expert consensus on Integrated traditional Chinese and Western Medicine for diagnosis and treatment of chronic Heart failure" and "Guidelines for diagnosis and treatment of dilated Cardiomyopathy in China"[19–21].Pharmacological studies have shown that Qiliqiangxin capsule can inhibit excessive activation of neuroendocrine,inhibit cardiomyocyte apoptosis,protect vascular endothelium,block ion channels,improve ventricular remodeling and metabolic remodeling [22].Moreover,it can fully avoid the adverse reactions caused by chemical drugs,such as dizziness,vomiting,diarrhea and so on,which makes up for the defects of modern medicine to a certain extent,improves the efficacy and safety of CHF treatment,and has important clinical significance.In recent years,many studies have shown that in the conventional treatment of chronic heart failure,the intervention effect of Sacubitril valsartan combined with Qiliqiangxin capsule is better than that of Sacubitril valsartan alone.however,a few studies believe that the advantage of integrated traditional Chinese and western medicine is not prominent.Therefore,in this study,Meta analysis was used to comprehensively evaluate the efficacy of shakubar valsartan combined with Qiliqiangxin capsule in the treatment of CHF,in order to provide evidence-based medicine basis for clinic and lay a foundation for follow-up research.
Data and methods
Inclusion and exclusion criteria
Type of study.Randomized controlled trials (Randomized Controlled Trial,RCT).
Participants.Patients with heart failure diagnosed clinically.
Intervention.The control group was treated with routine therapy,while the experimental group was treated with Qiliqiangxin capsule on the basis of the control group.
Outcome indicators.(1) clinical effective rate;(2) N-terminal pro-brain natriuretic peptide (NT-proBNP);(3) left ventricular ejection fraction (LVEF);(4) left ventricular end-diastolic diameter(LVEDD);(5) left ventricular end-systolic diameter (LVESD);(6)cardiac output (CO);(7) 6-minute walking distance (6MWD).
Exclusion criteria.(1) repeated literature;(2) systematic review;(3)the research content is not consistent;(4) the information provided by non-RCT;(5) literature is insufficient and the data is incomplete,which can not be included in the analysis or can not obtain the full text of the literature.
Document retrieval strategy
This research scheme is registered in the Meta analysis registration platform INPLASY,the registration number is:INPLASY202130115[23].The report follows the Cochrane manual and the MOOSE specification for observational Meta analysis.The database of CNKI,WanFang,VIP,CBM,DuXiu,ChiCTR,PubMed,Web of science,The Cochrane Library and Embase was searched to collect the randomized controlled trial (RCT) of Sacubitril Valsartan combined with Qiliqiangxin capsule in the treatment of patients with heart failure.Chinese key words include:Xinlishuaijie,Xinshuai,Manxingxinlishuaijie,Manxingxinshuai,Xingongnengbuquan,Xiongbi,Xiongtong,Xintong,Qiduan,CHF,SV,ARNI,QLQXC,random.English search words include:heart failure,sacubitril valsartan,Qiliqiangxin and so on.Take PubMed as an example,the specific retrieval strategy is shown in Supplementary Material Table 1.
Literature screening and data extraction
Two researchers independently screened the literature,extracted the data and cross-checked,and resolved the differences through discussion or negotiation with the third party.When screening the literature,first read the title,after excluding the obviously irrelevant literature,further read the abstract and the full text to determine whether to include it,and contact the original research author through e-mail to get relevant information when needed.The contents of the data extraction mainly include:(1) the basic information included in the study,including the title,author,publication time,etc.;(2) the baseline characteristics of the study subjects,including the sample number of each group,the patient's age,sex,course of disease,etc.;(3) specific details of intervention measures;(4) key elements of bias risk assessment;(5) outcome indicators and outcome quantitative data.
Methodological evaluation of inclusion in the study
The included study was independently assessed and cross-checked by two researchers using the RCT bias risk assessment tool provided by the Cochrane website.
Statistical analysis.
Stata 16.1 software was used for statistical analysis.The Odds Ratio(OR) was used for the counting data,and the Mean Difference (MD)was used as the effect analysis statistic for the quantitative data,and each effect provided its point estimate and 95% CI.The heterogeneity between the results of the study was quantitatively judged by I2.If there is no statistical heterogeneity among the studies,the fixed effect model is used to merge the data.if there is obvious statistical heterogeneity among the studies,the source of the heterogeneity is further analyzed,and the random effect model is used to combine the data after excluding the obvious clinical heterogeneity.When the clinical heterogeneity was large,subgroup analysis,sensitivity analysis or descriptive analysis were used,and Begg test was used to evaluate publication bias.
Results
document screening process and results
A total of 67 related articles were obtained in the initial examination,and 18 RCT were finally included after layer-by-layer screening,including 1613 patients [4–21].The literature screening process and results are shown in Figure 1.
Basic characteristics of the inclusion study
The basic characteristics of the included study are shown in Table 1,and the results of bias risk assessment are shown in Figure 2.

Figure 1 Specific process of literature screening The database retrieved and the number of documents obtained:CNKI (n=21);VIP (n=16);WANFANG (n=17);CBM (n=9);DuXiu (n=3);ChiCTR (n=0);Web of science (n=0);The Cochrane Library (n=0);PubMed (n=1);Embase (n=0)

Figure 2 Overall and Detailed risk of bias in included studies
Meta analysis results
Clinical effective rate.There were 15 studies [26–36,38–41]reported the clinical effective rate of Shatracurba valsartan combined with Qiliqiangxin capsule in the treatment of heart failure,including 1319 patients.There was no statistical heterogeneity among the results in the heterogeneity test (I2=0%,P=0.986).The fixed effect model combined with grade data analysis (OR=2.60,95% CI=[2.09,3.24],Z=8.520,P<0.00001) showed that the curative effect of the test group was significantly better than that of the control group,and the difference was statistically significant (Table 2).
N-terminal pro-brain natriuretic peptide (NT-proBNP).There were 13 studies [24,26,28,30,32,43,35–41] reported the changes of NT-proBNP before and after treatment,including 1233 patients.There was significant heterogeneity among studies (I2=99.5%,P<0.00001).Combined with clinical practice,due to the large difference in serum NT-proBNP levels at baseline,it may be the main source of heterogeneity in this study.Using the random effect model to combine the data,the serum NT-proBNP of the test group was lower than that of the control group (MD=–468.36,95% CI [–606.80,–329.92],Z=6.63,P<0.00001),suggesting that there was significant difference between the two groups (Table 2).
Left ventricular ejection fraction (LVEF).There were 17 studies reported the changes of LVEF before and after treatment,including 1523 patients [24,25,27–41].There was heterogeneity among the studies (I2=81.8%,P<0.00001).The random effect model was used to combine the data,and the LVEF of the test group was higher than that of the control group (MD=5.41,95% CI [4.93,5.89],Z=22.11,P<0.00001).The results showed that there was statistical significance in the improvement of LVEF between the test group and the control group (Table 2).
Left ventricular end-diastolic diameter (LVEDD).A total of 914 patients were included in 10 studies before and after LVEDD treatment[24,25,27,29–31,33,37,40,41].There was heterogeneity among the studies (I2=45.3%,P=0.058).The fixed effect model was used to combine the data.compared with the control group,the LVEDD of the test group was reduced (MD=–3.27,95% CI [–3.6,2.90],Z=17.20,P<0.00001),suggesting that the test group had statistical significance in improving LVEDD compared with the control group(Table 2).
Left ventricular end-systolic diameter (LVESD).There were 6 studies reported the changes before and after LVESD treatment,including 546 patients [30,31,33,37,39,41].There was significant heterogeneity among the studies (84.3%,P<0.00001).The random effect model was used to combine the data,and the LVESD of the test group was lower than that of the control group (MD=–3.60,95% CI[–4.99,2.21],Z=5.09,P<0.00001).It is suggested that there is statistical significance in the improvement of LVESD between the test group and the control group (Table 2).

Table 1 Baseline characterization of included literatures
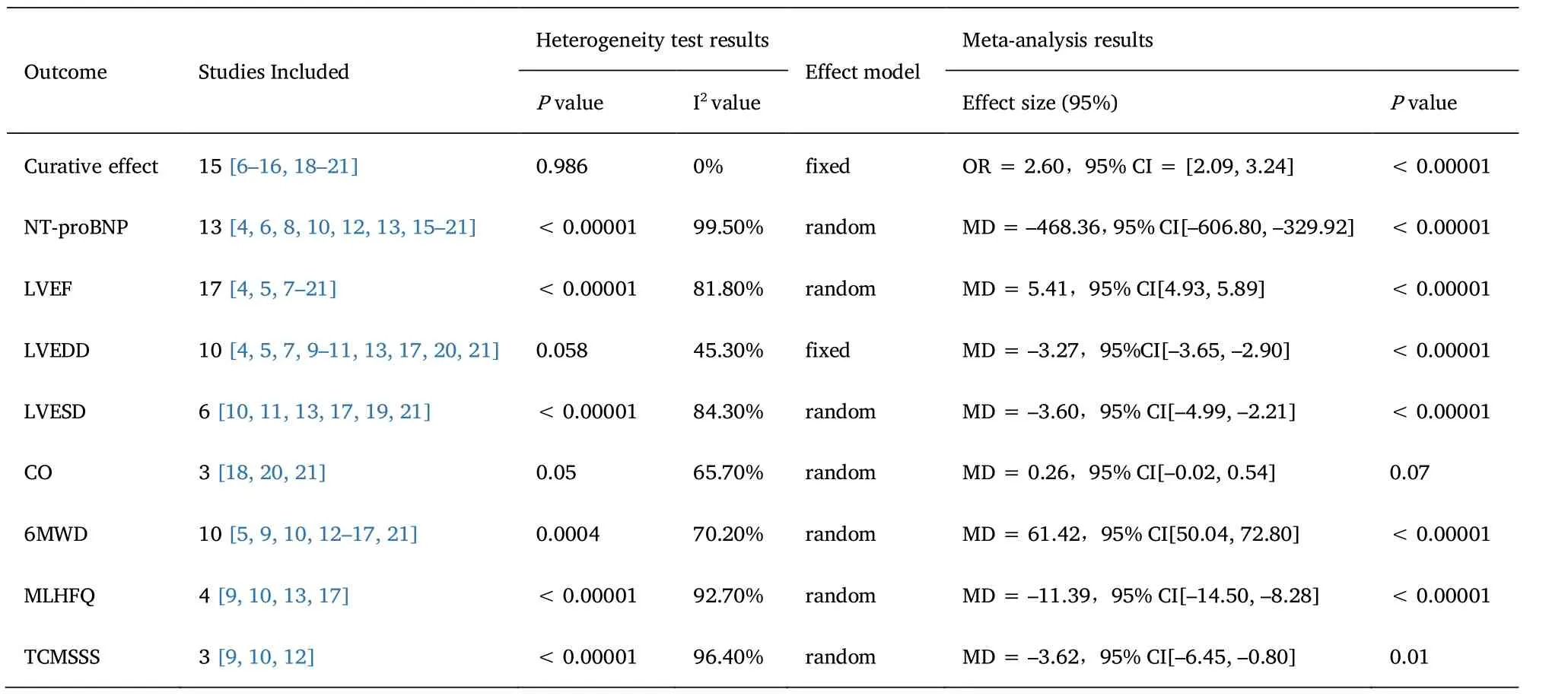
Table 2 Summary of Meta analysis results
Cardiac output (CO).Three studies reported the changes of CO before and after treatment [38,40,41].There was significant heterogeneity among the studies (I2=65.7%,P=0.05).The random effect model was used to combine the data.Compared with the control group,the CO of the test group increased (MD=0.26,95% CI [–0.02,0.54],Z=1.84,P=0.07),suggesting that there was no statistical significance in improving CO between the test group and the control group (Table 2).
6 minutes walking distance (6MWD).There were 10 studies reported the changes of 6MWD before and after treatment,including 897 patients [25,29,30,32–37,41].There was significant heterogeneity among the studies (I2=70.2%,P=0.0004).Using random effect model to combine data (MD=61.42,95% CI [50.04,72.80],Z=10.58,P<0.00001),the results showed that there was statistical significance in the improvement of 6MWD between the test group and the control group (Table 2).
Minnesota Heart failure quality of Life scale (MLHFQ).There were 4 studies reported the changes before and after MLHFQ treatment,including 362 patients [29,30,33,37].There was significant heterogeneity among the studies (I2=92.7%,P<0.00001).The random effect model was used to combine data (MD=–11.39,95%CI [–14.50,8.28],Z=7.18,P<0.00001).The results showed that the experimental group compared with the control group in improving the Minnesota heart failure quality of life scale was statistically significant (Table 2).
Traditional Chinese Medicine Syndrome Score Scale (TCMSSS).
There were 3 studies reported the changes of TCMSS before and after treatment,including 265 patients.There was significant heterogeneity among the studies (I2=96.4%,P<0.00001) [29,30,32].The random effect model was used to combine the data (MD=–3.62,95%CI [–6.45,0.80],Z=2.51,P=0.01).The results showed that there was statistical significance in the improvement of TCM syndromes between the experimental group and the control group (Table 2).
Adverse reactions.Adverse reactions were reported in 8 studies [27,31–33,35,36,38,40],of which 3 studies had no adverse reactions[35,36,38],while in the other 5 studies there were a few mild symptoms such as dizziness [27,31–33,40],headache,nausea,vomiting and palpitations,but there was no statistical significance (P>0.05).
Sensitivity analysis
The sensitivity analysis was carried out by eliminating individual studies one by one,and the results showed that two of the studies had a great influence on the results,considering that it was caused by different courses of treatment,but there was no qualitative change in the combined effect quantity after excluding the above studies.it shows that the results of this study are stable [26,28].
Publication bias test
The publication bias test of the outcome index of "Clinical efficacy"with the largest number of literatures was carried out,and there was no significant change in the results of Meta analysis before and after elimination by single elimination method,suggesting that the results were stable.The funnel plots were drawn by Metabias algorithm,and the results showed that the distribution of the funnel plots was symmetrical (Figure 3).Further,using Egger's test (P=0.150) and Begg's test (P=0.276),P>0.05.It was suggested that the 15 studies selected in this study had less possibility of publication bias [26–36,38–41].

Figure 3 Clinical efficacy funnel plot
Discussion
Reversing myocardial remodeling is one of the most important goals in the treatment of patients with heart failure.As the world's first angiotensin receptor-enkephalinase inhibitor (ARNI),Sacubitril valsartan has a dual inhibitory effect on renin-angiotensin-aldosterone system and natriuretic peptide system.Compared with ACEI,sarkubar valsartan can significantly improve left ventricular end-diastolic meridians,left atrial size and other myocardial remodeling indexes[42–44].To further reduce the mortality rate of CHF has gradually replaced ACEI in clinic and become a class I recommended drug in the 2018 guidelines for heart failure in China [19,45,46].
As the first proprietary Chinese medicine with evidence-based medical evidence to support its definite therapeutic effect on CHF,Qiliqiangxin capsule has opened up a new way to prevent and treat heart failure on the basis of the theory of "collateral disease".The concept of "collateral accumulation" put forward by Academician Wu Yiling is highly similar to the mechanism of myocardial remodeling discovered by modern medicine in recent years,and summed up the prescription rule of "Qi,blood and water are treated separately at the same time"."Qi fen" means Yang Qi deficiency,Qi deficiency and blood stasis are "blood fen",and Yang deficiency can not transform body fluid into "water fen".The "accumulation of collaterals" in Chinese characters means "the breath of meridians stops breathing",that is,compared with Yang deficiency,"phlegm" and "blood stasis" as pathological products will further aggravate the degree of heart failure [47].Qiliqiangxin capsule is used to treat diseases by warming Yang and transforming Qi and warming meridians through Radix Astragali,ginseng,aconite and cassia twig,through Salvia miltiorrhiza,safflower and tangerine peel to regulate Qi,activate blood circulation and remove blood stasis to smooth meridians and collaterals,use semen Lepidopteris,incense plus peel,alisma alisma to reduce swelling,and add Polygonatum odoratum to nourish heart Yin to prevent excessive diuresis.These all Chinese herbal medicines are used to treat the three pathological changes of deficiency of heart-Yang and Qi,stasis and dampness.From the analysis of modern pharmacology,Qiliqiangxin capsule can inhibit the excessive activation of neuroendocrine,protect vascular endothelium,block ion channels and inhibit myocardial remodeling [22].
A total of 18 RCT articles and 1613 patients were included in this study.Meta analysis showed that compared with the control group,there was no significant change in cardiac output,but other cardiac function indexes,activity tolerance,quality of life and symptoms in the treatment group were significantly improved compared with the control group,which was consistent with the results of Gao Yanyan[41].Among them,8 studies mentioned adverse reactions,but all of them were mild reactions,and there was no statistical significance,suggesting that the combination of drugs was safer.
The limitations of this study are as follows:(1) the market time of Sacubitril valsartanis short,there are few studies in combination with Qiliqiangxin capsule in the treatment of heart failure;(2) most of the included studies have not reported whether hidden and blind methods have been assigned;(3) some studies have not reported the level of cardiac function,duration of disease and specific schemes of basic treatment of the subjects,which can not be analyzed by subgroup analysis;(4) there are differences in the measurement methods and tools of the same index,which can lead to certain heterogeneity;(5)there is no long-term follow-up and lack of evidence for long-term effects such as rehospitalization rate,mortality and so on.
To sum up,the current limited evidence shows that the treatment of CHF with Sacubitril Valsartan combined with Qiliqiangxin capsule can effectively relieve the symptoms of heart failure,reverse myocardial remodeling,improve cardiac function and improve quality of life,and there are no serious adverse reactions.The combination of drugs brings a lot of benefits to patients and is worth giving priority to.However,limited to the quantity and quality of the study,the above conclusions need to be carried out more high-quality studies,but also need to observe the rehospitalization rate,mortality and other long-term indicators,in order to further verify the long-term efficacy and safety of shakubar valsartan combined with Qiliqiangxin capsule in the treatment of CHF.
Supplementary Material

Table 1 PubMed search strategy
 Drug Combination Therapy2022年2期
Drug Combination Therapy2022年2期
- Drug Combination Therapy的其它文章
- The potential of complementary and alternative medicine combination chemotherapy in the treatment of non-small cell lung cancer
- Mechanism of Magnoliae Flos and Xanthii Fructus herb pair in treatment of allergic rhinitis based on network pharmacology
- Study on the anti-inflammatory and analgesic effects of white peony root and mucuna pruriens and their combinations in vivo
- Mechanism of Zixinyin oral liquid in the treatment of insomnia based on network pharmacology and molecular docking
- Complex diseases demand novel treatment strategies:understanding drug combination
