Adaptation and validation of pediatric peripheral intravenous catheter insertion and care practices audit tools
Ferika Indarwati , Judy Munday , Samantha Keogh
a School of Nursing and Centre for Healthcare Transformation, Queensland University of Technology (QUT), Brisbane, Queensland, Australia
b School of Nursing, Faculty of Medicine and Health Sciences, Universitas Muhammadiyah Yogyakarta, Yogyakarta, Indonesia
c Faculty of Health and Sports Sciences, University of Agder, Grimstad, Norway
d Mater Research Institute-UQ, University of Queensland, Brisbane, Queensland, Australia
e Alliance for Vascular Access Teaching and Research Group, Griffith University, Queensland, Australia
Keywords:Forward and backward translation Indonesia Nursing care Peripheral intravenous catheters Pediatric Questionnaire
ABSTRACT Objectives: This study aimed to describe the translation process and establish the validity of the three instruments in Indonesian to assess pediatric peripheral intravenous catheter (PIVC) insertion and care practices.Methods: The six-step forward and backward translation method was used to translate the adapted questionnaires.The English version questionnaires included the point prevalence audit checklist, the nurse survey consisting of the nurse PIVC knowledge questionnaire and the nurse PIVC confidence questionnaire, plus a Patient/parent Experience Survey.Data collection was conducted in Indonesia between October 2019 and February 2020.In total,there were six translators (two for each instrument),nine-panel vascular access experts (three for each instrument), and 30 participants (ten for each instrument) of the target population involved in the translation and validation of the three instruments.Three-panel experts rated the content relevance of each instrument using a four-point rating scale.Item level and scale level content validity index and kappa index were calculated.Ten-panel members of the target population evaluated each questionnaire regarding feasibility, clarity, logical sequence, and formatting.Qualitative comments from the panel were also reviewed.Results: The translation process indicated relatively low discrepancies between translators except for semantic equivalence.There were nine, eight, and one semantic discrepancies found in the forward translation of the point prevalence audit checklist, nurse survey, and patient/parent experience survey.The semantic discrepancies were less prevalent in the backward translation, with only one, three, and two items reported during the process.The item validity index for all of the three instruments showed relatively high agreement between experts (I-CVI >0.78, S-CVI/Ave >0.90, S-CVI/UA >0.70, and kappa index >0.74).The face validity was established with the panel reporting that the three instruments were easy to understand and presented logically.However, some re-formatting of the nurse survey and patient/parent experience survey were needed to avoid ambiguity and confusion for the participants.Conclusions: The results indicate that the translated three survey instruments that had been widely used in other developed countries show good content validity in the Indonesian context.They can be used as a reference for further testing in different countries and contribute to understanding the pediatric PIVC audit tools used in future clinical research.
What is known?
· One in three pediatric peripheral intravenous catheters (PIVCs)fail before completion of prescribed treatment.
· Evaluation of PIVC insertion and care practices in pediatric patients is paramount.
· Valid and reliable instruments are needed to assess pediatric PIVC insertion and management practices.
What is new?
· The forward and back translation and validation processes facilitate rigorous adaptation of the instruments into the Indonesian context.
· The Indonesian version of the three instruments: the point prevalence audit checklist,the nursing survey, and the patient/parent experience survey, showed good validity in Indonesian contexts.
· The original and translated instruments can be used as references to audit PIVC insertion and management in pediatric patients.
1.Introduction
Peripheral intravenous catheters(PIVCs)are frequently inserted in hospitalized patients worldwide, including in Indonesia [1].PIVCs are crucial for delivering an array of essential intravenous fluids and medication.However, despite their ubiquity and essential nature, PIVC failure and complications across all settings,including pediatric in developed and developing countries,is high[2].These complications are a source of significant burden for children,families,and health care systems[3-6].Therefore,regular evaluation of PIVC insertion and care practices, including PIVC condition, function, and outcomes, assessment of factors that may influence PIVC outcomes (such as patients’ characteristics and nurses’ knowledge and confidence) patient experience is paramount [7].
To date,very few studies of the PIVC insertion and management practices in pediatric patients have been conducted in Asian countries, including Indonesia.As a result, many pediatric PIVC insertion and management dimensions are still poorly understood in Indonesia [8].Thus, the generation of a comprehensive understanding of the current state of PIVC insertion and care practices for children in Indonesia (including PIVC use, management practice and outcome, nursing knowledge and confidence, and patient experience) is warranted.It is anticipated that this knowledge can contribute to future programs or further research to improve pediatric PIVC outcomes in Indonesia.
To comprehensively understand the current state of PIVCs among pediatric patients in Indonesia, a theoretical framework encompassing all service aspects is important to guide the study.The Donabedian framework of service assessment,which includes structure, process, and outcomes, was considered a suitable framework to answer the aims and objectives of the study and guide the research processes[9].Three instruments were needed to assess the study’s structure, process, and outcomes: a point prevalence checklist,a nurse survey,and a patients/family survey.Valid and reliable instruments are preferred to obtain a comprehensive yet corroborative understanding of the current PIVC [10].Instrument development and validation are important phases in research that are often underestimated [11].Developing instruments for research is a complex process.It involves determining the research objectives and hypothesis, defining research variables and their operationalization, developing items for each of the study constructs and then instrument’ instructions, assessing validity and reliability,as well as pilot testing[12-14].Therefore,in the pursuit of efficiency and consistency, researchers aim to use existing instruments, if one exists with proven validity and reliability [15].Adapting previously developed and validated instruments within research is beneficial for conserving time and energy and unifying the conceptualization of study phenomena, particularly when studies are conducted in different cultures and languages from where original instruments are developed [16].Cross-culturally translated and validated instruments are important to prevent distortion of the original intents of instruments,ensure the validity of the resulting instruments, and enable direct comparison of the research findings from different cultures or countries [14,17].
Therefore, three published survey instruments were used to understand the use, insertion, and maintenance practice and outcomes of PIVC care in pediatric patients.The survey tools include a point prevalence audit checklist [18,19]to assess the peripheral intravenous insertion and maintenance practices; a nurse survey tool to evaluate nursing knowledge[20,21]and confidence[22,23],and a patient/parent experience [24]questionnaire to assess outcomes.Before auditing intravenous catheter insertion and maintenance,the point prevalence audit checklist was used in Australia[25,26]and worldwide[1,19].The nurse knowledge and confidence and the patient/parent experience survey were also used in several other studies to comprehensively evaluate the intravenous catheter management practice [26-28].However, the original instruments were developed and used in English-speaking countries,and there was no current version in the Indonesian language.This paper reports on the translation process and establishes the validity of the three instruments in Indonesian for intended use in a study of pediatric PIVC insertion and care in Yogyakarta, Indonesia.The guideline for reporting reliability and agreement studies (GRRAS)was utilized to report instrument adaptation and validation in this study [29].
2.Methods
2.1.Study design
The forward and backward (FB) translation method was employed to adapt and review the three chosen survey tools into the Indonesian context.The translation and validation of the three instruments were carried out between October 2019 and February 2021.Different types of FB translations have been described and published.They broadly adhere to the same principles and aim to ensure the survey tool’s semantic equivalent with the local language and contexts [30-32].In this study, each instrument underwent the same translation process.The translation process utilized adaptations from Sousa and Rojjanasrirat (2011) method[30].The key steps of the FB process used in this study are illustrated in Fig.1.
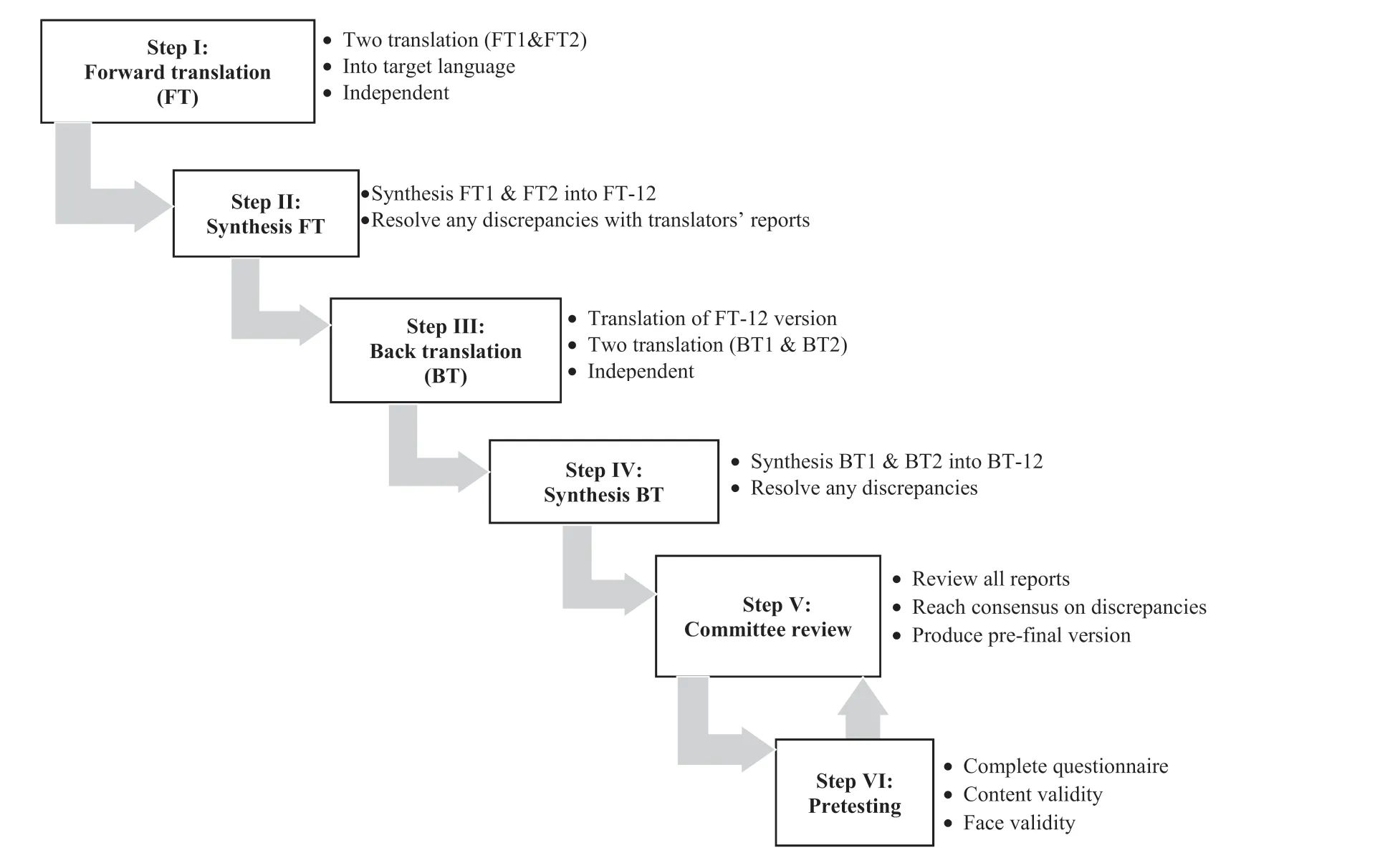
Fig.1. Forward and backward translation process in this study adapted from Sousa & Rojjanasrirat (2011).
In the current study,three adapted instruments were translated:the point prevalence audit checklist, the nurse survey, and patient/parent survey questionnaires.Each instrument has an appropriate method to assess its translated validity and reliability, e.g., item analysis is more suitable for multiple-choice questions such as in the nurse knowledge survey, and kappa inter-rater reliability is more appropriate for the point prevalence checklist [11,13,33].The use of factor analysis such as exploratory factor analysis (EFA) and confirmatory factor analysis (CFA) are commonly undertaken in health research as the first step to build a new scale, to assess the instrument’constructs,to reduce the dimensionality of variables[34-36]possibly, and to confirm hypothesis representing variables in the metrics [37].Factor analysis needs a large sample size (at least 300 participants and five observations for each variable measured) to generate a valid result and diminish error[12,37].The EFA and CFA do not apply to the main FB study’s aim and objectives.The authors did not aim to make a new original questionnaire,but instead,they adapted the already published questionnaires available in the vascular access research field[21,38].
2.2.Instruments
This study includes a point prevalence audit checklist, a nurse survey, and a patients/parents survey questionnaire.The description of each instrument is as follows.
2.2.1.The point prevalence audit checklist
The point prevalence audit checklist consists of 29 items, specifically: patient demographic characteristics; type, number, and purpose of PIVC insertion;insertion location;condition of dressings and other stabilization products;visibility of the insertion site;site location; by whom it was inserted; the evidence of complications,insertion or re-insertion dates; fluid therapy and intravenous medications and documentation on the daily insertion care[18,19].The hospital based Intravenous Access Research Council reviewed the point prevalence checklist in Brisbane, Australia, and then trialed it by two survey teams to test its reliability.After each review,assessment items were modified and reordered to improve clarity and ease of use [18,19].
2.2.2.The nurse survey
The nurse survey consists of two questionnaires:knowledge(23 multiple choice questions) and confidence (19 five-point Likert scales) on PIVC insertion and care.The nurse knowledge questionnaire focuses on patient assessment, insertion [20], maintenance, removal, and documentation [21].The nurse PIVC confidence questionnaire uses a 5-point Likert scale from“strongly agree” to “strongly disagree” to measure nurse confidence in site selections, assessments, procedures, dressing, site cares, removals,and documentation [22,23].The validity and reliability testing of the nurse knowledge and confidence on peripheral intravenous maintenance also indicated general agreement with the questionnaire’s clarity and content from experts [21,23].Further item analysis assessment on the nurse maintenance knowledge questionnaire indicated that the difficulty index and the discriminating power were in the acceptable range(value ranged from 0.4 to 0.8, and value ≥0.35, respectively) [21].
2.2.3.The patient/parent experience survey
The patient’s/parent’s experience survey includes ten questions appraising the number of insertion attempts the child experienced,the difficulty of insertion, the reason why the PIVC insertion was difficult, strategies perceived to assist the PIVC insertion successfully,staff skills,the physiological reaction to insertion such as pain,stress, any concern for the PIVC insertion and care, as well as the PIVC complications and removals [24].The patient and parent experience survey tool was developed and reviewed by five senior clinical and vascular access experts of the Alliance for Vascular Access Teaching and Research(AVATAR)group.The questions went through three rounds of discussion and revision until agreement among experts was reached.The validity and reliability of the questionnaire were in an acceptable range [24].
Some of the translated instruments had two citations adapted from two questionnaires.The two questionnaires were needed to meet the aims and objectives of the study.For example, the nurse knowledge on PIVC insertion and maintenance was adapted from two questionnaires developed by Keleekai et al.[20],which focused on PIVC insertion and Cicolini et al.[21]that focused on the maintenance aspects.The authors obtained permission from the original questionnaire developers on behalf of the research team to use the questionnaires in this study.
2.3.Sample
Two independent bilingual translators were chosen to review forward and backward translated instruments.The inclusion criteria for the translator was that they must be native speakers of Indonesian and proficient in the English language.One translator was a registered nurse with five years’ experience working in pediatric settings in Yogyakarta,Indonesia.The other translator was a certified translator in Indonesia who had lived and studied in the English-speaking country (Australia) for more than five years but was less familiar with medical terminology.The review committee consisted of six nursing scholars: two experts in vascular access research (one from Indonesia and one from Australia), one senior nursing lecturer,the investigator,and the two forward translators.Three pediatric nursing experts[39]in Yogyakarta,Indonesia,who had at least ten years of experience in pediatric clinical settings and were familiar with research,were contacted to review and rate the content validity of items and overall scales of the instruments.
Face validity was conducted by distributing each questionnaire to the target population in Indonesia[26,28].The point prevalence checklist[18,19]was distributed to ten nurse researchers,including four research assistants who will use the checklist in the wider study.Ten registered nurses reviewed the nurse knowledge and confidence questionnaire [20-23]with various working experiences in pediatric settings.In contrast, the patient experience survey [24]was distributed to ten parents whose children had experienced PIVC insertion during hospitalization in Indonesia.The number of experts chosen for content validity was based on Polit and Beck’s[11]guideline,and for face validity assessment followed Cicolini et al.[21].A minimum of three experts for content validity[11]and 10 participants for the face validity of each instrument being evaluated [21].
2.4.Data collection
The review committee, through successive email and WhatsApp™ communication, discussed discrepancies, evaluated whether the translation was conceptually understood in the Indonesian context (conceptual equivalence), correctly reflected the intended English meaning(semantic equivalence),was accepted by targeted respondents (item equivalence); and utilized wording,format,instruction and scaling that could be used in the Indonesian context (operational equality) [40].Back translators were blinded from the original instruments.The final review was conducted to evaluate whether the synthesized version correctly reflected the intended meanings of the original English versions.If there were discrepancies, the committee discussed them with the original developer of the questionnaires and consulted with vascular access researchers and experts until all problems were resolved.The last step(pre-testing)was conducted through content and face validity checking.
The content validity was conducted following an expert review process described by Polit and Beck [11].Three pediatric nursing experts [39]were asked to rate each questionnaire on a 4-point scale, where 1 =not relevant, 2 =somewhat relevant, 3 =quite relevant,and 4 =highly relevant.They were also asked to provide recommendations for each question on a four-point scale, where 1 =delete item, 2 =revise item (major), 3 =revise item (minor),and 4 =keep item as is [11].Items/questions are rated as relevant(either quite relevant or highly relevant) and recommended to be considered for use in the adapted instruments.Items rated as relevant (highly, quite, and somewhat relevant) and revised items(minor or major)were considered as requiring modification.Items rated as not relevant were deleted from the tool [41].Face validity was conducted by asking nurses and parents whether the questions used in the questionnaires are easily understood, logical, and consistent.Also, they were asked to evaluate if the questions are worded and in a format and scale that is feasible for use in the Indonesian context [30,42,43].
2.5.Ethical considerations
This study received ethical clearance from two Institutional Review Boards(reference no.2000000078&reference no.007/ECKEPK FKIK UMY/X/2019).
2.6.Data analysis
Microsoft Excel™was used to collate data and calculate validity index at item-level (I-CVI), scale-level (S-CVI/Average (S-CVI/Ave),and S-CVI/Universal agreement (S-CVI/UA), mean experts proportion agreement, multi-rater kappa, and overall kappa evaluation rating values of each instrument translated in the current study.The I-CVI was calculated by the number of items rated 3 or 4 divided by the number of experts,the S-CVI/Ave was determined by the average of all I-CVI, and the S-CVI/UA was computed by the number of items rated relevant by all panel divided by the number of items.Items with I-CVI equivalent to >0.78 or higher are considered good enough to be included in the final tool [33].The multi-rater kappa coefficient (κ) was then computed using the formula: κ =(I-CVI-Pc)/(1-Pc) [40,41].Pc is the probability of a chance occurrence calculated using the formula: Pc = [N!/A!(N-A)!]×5Nwhere N =number of experts and A =number of raters who agree that the item is relevant[44].Kappa values larger than 0.74 indicate excellent agreement among ratters [45,46].Qualitative comments from the panel were also summarised.The translation issues such as conceptual, semantic, operational, and item equivalence of each question were summarised and described for each instrument.During the translation, process experts were evaluated and classified as strong, slight, and no discrepancy for each instrument’s question.The research team discussed and resolved the differences in three-round separate discussions.The principle of “sense-to-sense translation” was used rather than the literal “word-to-word translation” to find matching conceptual representations of the specific words in Indonesian based on its context, cultural and health systems.In cases where one English term has several meanings, the Oxford English and the Meriam Webster Dictionary were used to identify different meanings and clarify the intentions of the original tools with the developers to confirm the translated term.
3.Results
3.1.The forward and backward translation result
The result of the three instruments’ forward and backward translation processes is described in Table 1.Translation issues were mostly found in the forward translation stage compared to the backward translation stage for all three instruments.The most common problems were found in the semantic equivalence of the questions.
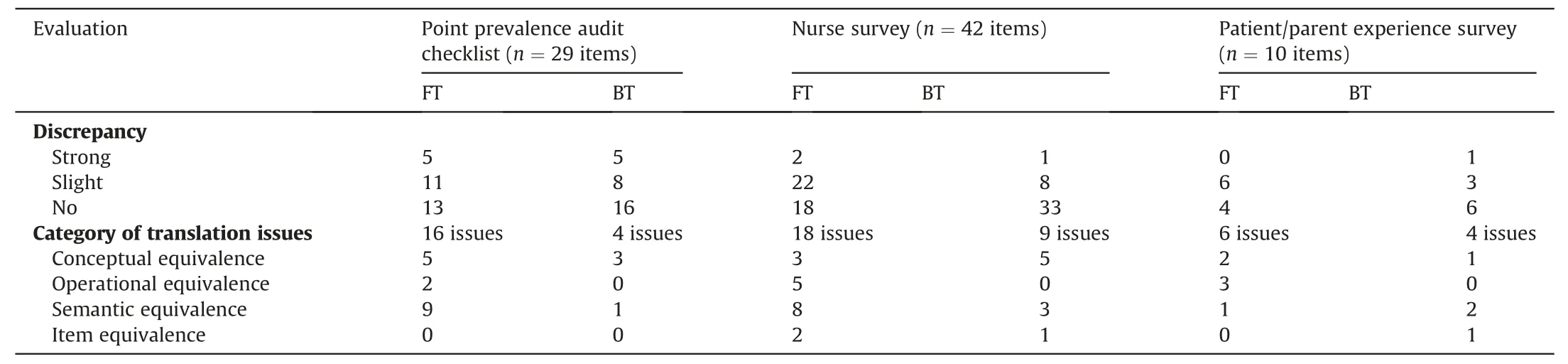
Table 1Forward and backward translation issues for each instrument.
In the point prevalence audit checklist adapted from New et al.and Russel et al.[18,19],the forward translation stage indicated 16 discrepancies between translators.There was difficulty in matching five English terms to the conceptual term in the Indonesian context,for example: “discipline of the inserter,” “bung,” “caps,” “3-way tap”, “ordered.” The term “ordered” could be misunderstood in the Indonesian nursing context.Several items were also identified as being translated differently from the intended English version,such as “splint,” “cord,” “bung,” “caps,” “port,” and “infusate.” For the operational equivalence, two items needed to be clarified: the words“left”and“right”were added for the device position,as was the ability to specify if there was an“other” option.
The FB translation result of the adapted nurse survey: knowledge and confidence questionnaire [20-23]indicated additionalissues compared to the point prevalence tool.Similar to the point prevalence survey tool, the most common issue was the semantic equivalence, where some words were translated differently into Indonesian.These included the terms “peripheral intravenous catheter” or “catheter,” “placement,” words related to the name of the vein, “rotated,” “blanching,” “protocol,” “escalate” and,“advance.” Several operational issues were also resolved, such as the naming of the veins that are appropriate for PIVC insertion in pediatric patients and also re-ordering the PIVC insertion steps,such as two items were also reported by the translators to be at risk of being misunderstood or not being accepted by Indonesian nurses.For example, the answer options could be very difficult to understand.
The patient/parent questionnaire [24]translation process indicated that the main issues were the operational equivalence.For example, it was identified that the format and instruction of the questionnaire might be ambiguous for some people.The phrase peripheral intravenous catheter (English) or “kateter intravena perifer” (Bahasa Indonesia) was also not well understood by laypersons in Indonesia; therefore, it was suggested to use the word“infuse” instead of “kateter intravena perifer.” Another word with potential for misunderstanding, once translated, in Bahasa Indonesia was “concern.” The word “concern” can have two meanings: “responsibility” and “attention/worried” since the original question was intended to see what factors that concern parents most related to their children’s PIVC,the word “attention”was chosen as the most relevance to be used in the questionnaire.
3.2.The content and face validity result
The content validity data from panel experts were analyzed using Microsoft Excel™; the results of the I-CVI for each tool are described in Table 2.The I-CVI of the point prevalence audit checklist [18,19]indicated that most of the items were relevant to measure the intended research aims and objectives.The number of items in the questionnaire rated highly and quite relevant by the experts was 29 items with an average mean CVI was 0.97(Table 2).The kappa values of the 26 questions out of the 29 items also indicated high agreement among raters(κ*>0.74).The other three questions in the point prevalence checklist that had a relatively low agreement among raters underwent revisions before finally being agreed on by all experts to be included in the checklist.The face validity, conducted by distributing the checklist to ten Indonesian pediatric nurses, confirmed that the checklist was easy to understand and could be used as a regular surveillance tool in hospitals.In addition, the formatting style was considered consistent and clear.

Table 2Summary of content validity of three instruments.
The CVI showed a high level of agreement between panel experts, indicating that all of the questions and the nurse survey:knowledge and confidence questionnaire [20-23]were relevant for use in the study.However,reviewers suggested some changes to avoid ambiguity, such as using the same phrase/word for one concept,such as“standard operating procedure and PIVC instead of catheter” for all questions.The feedback from nurses who participated in the face validity testing suggested that several questions and options in the questionnaire were unclear and difficult to understand.Therefore, they suggested some revisions needed to be made to avoid confusion.The format and instruction of the questionnaire were rated as clear and easy to follow.
The pediatric experts’ CVI results indicated that the survey questions were appropriate and relevant to patient and parent experience; however, the face validity assessment suggested that the questionnaire would benefit from reformatting.The reviewers suggested some adjustments to the instruction used in the questionnaire to minimize mistakes.For example, one question in the original questionnaire included an instruction that participants only needed to answer the question if a particular condition was met.The instruction was considered appropriate if the questionnaire was self-administered by participants.However, since the adapted questionnaires were planned to be administered by the researcher, the instruction needed to be changed to avoid confusion.Reviewers also recommended this for questions number 9 and 10 in the parent questionnaire.
4.Discussion
This paper reports the adaptation of the Indonesian version of the point prevalence audit checklist [18,19], nurse survey: knowledgeand confidence tool[20],and patient/parent experience survey[24],from the original English language instrument through a systematic and rigorous forward and backward translation process [30,31].High-quality language translation and semantic validation are foundations for psychometric and statistical testing [13].The FB translation process indicated that the discrepancy between translators was quite low.However, several semantic challenges were identified during the translation process, which the committee addressed.A study conducted in Italy followed the same forward and backward translation methods used in the current study to translate the nurse knowledge questionnaire[21].The tools were checked for semantic and conceptual equivalence, and pilot tested on a local(Italian) population.The difficulties, particularly in the semantic equivalence found in the translation processes, were similar to the current study findings in Indonesia.The panel members recommended several questions to be re-written to avoid misinterpretation and improve clarity [21].Another study conducted in Brazil cross-culturally adapted and assessed the content and semantic validity of the difficult intravenous access(DIVA)score for pediatric patients showed coherent processes and findings to the current study [38].Some items in the DIVA questionnaire, for example, the alternative answer of the Likert scale was, needed to be combined and changed,such as the“not clear at all”and“hardly clear”options were combined as “unclear.” This was to enhance the clarity and reliability of the instrument translated [38].In the current study,several items, such as the answer options in the nurse knowledge questionnaire, were combined to avoid ambiguity and improve psychometric properties.Several translation studies in Indonesia translated an instrument from English to the Indonesian language in different topics that showed similar difficulties in assessing the semantic,operational,item,and conceptual equivalence from the English language into the Indonesian language [47,48].Several words,phrases,and sentences were needed to be changed because they did not match with the local or cultural context or did not fit with the grammatical rules in the Indonesian language[47-49].
Review meetings with the translators and the expert committee regarding the forward and backward translation processes helped identify discrepancies and improve translation quality [30].The committee discovered several discrepancies between the first and second forward translators in the study’s forward translation results.The committee was required to find the appropriate Indonesian terms and expressions for some items specific to pediatric PIVC insertion and care.For example, the term “peripheral intravenous catheter”was not known or well understood in Indonesian.The research team discussed this issue and decided to use “sense to-sense translation”rather than“word-to-word translation”[50]to come up with a conceptual representation of what “peripheral intravenous catheter” means in Indonesian.In the Indonesian language, the word “catheter” is commonly referred to as the urinary catheter.The word “infuse” is appropriate for the peripheral intravenous catheter to refer to PIVC.In cases where one English term had different meanings,such as with the words“catheter”and“guideline,”the Oxford English and the Meriam Webster Dictionary were used to identify different meanings and clarify the intentions of the original tools with the developers to confirm the translated term.This process supports the contextualization of the pediatric PIVC service within Indonesian culture and health systems.It has implications for data collection and the implementation of evidence into practice [51].
Although the content validity index of the three instruments showed a good level of agreement among panel members, additional reformatting of the questionnaires, particularly the nurse knowledge and confidence and parent survey, were needed.The answer options in the nurse knowledge were modified and simplified,whereas,in the parent survey,the language used in the questionnaire’s instructions was changed to minimize potential misunderstanding.Such issues are common in questionnaire adaptation and translation [52].Operational equivalence and suitability, such as instrument instructions, should be given consideration,particularly if the instrument will be administered differently from the original instrument [53,54].The parent survey questionnaire is intended to be administered by investigators in the study,while in the original instrument, the questionnaire is an online survey self-administered by participants.
The inherently comparative nature of health care research to arrive at conclusions and recommendations of practices indicates the importance of using consistent instruments to ascertain consistent comparison of scientific findings among previously conducted studies [15].Likewise, suppose a researcher in the vascular access field is interested in examining the use, insertion and maintenance,outcomes,and patients’experience of peripheral intravenous catheters.In that case, he or his team should necessarily make comparisons of his findings to other studies.By comparing the results generated by several studies conducted on the same topics, the scientific community can then judge the consistency of the findings and make a solid conclusion and or recommendation about the subject matter [15,55].Established research instruments that are valid, reliable, and equivalent in different studies are essential elements of any research conducted in a field to enable dialogue and comparison of the subjects being evaluated [13,15,55].
An example can be drawn from applying the nurse knowledge questionnaire in Indonesia;findings from the nurse survey utilizing the translated nurse knowledge instrument showed that nurses’knowledge on peripheral intravenous catheter maintenance,particularly on complication prevention and management, were still lacking [56].This finding was congruent with other studies conducted in European countries where nurses had limited knowledge of preventing peripheral intravenous catheter infection[21,23,28].In this example, the authors can assure that the results were comparable because they used the same instruments to measure the nurse knowledge.The researcher can then consolidate the conclusions and make solid recommendations to stakeholders such as the health service providers and educational institutions to provide ongoing and tailored training for the nursing staff.
In this report,investigators also described detailed information of each step of the translation process that can facilitate other researchers to translate the pediatric PIVC survey tools into other languages or other measurement tools into the Indonesian language [30].To date, there was no Indonesian version of the point prevalence checklist, the nurse knowledge and confidence, and parent experience related to pediatric PIVC insertion and care.The only tool available in the Indonesian version is the nurse knowledge; however, this was focused only on PIVC insertion and intended for adult patients [57-59].There were no instruments representing nurse knowledge on PIVC insertion and management practice in a single document.Therefore, this study’s findings can also support the interpretation of further psychometric testing and contribute to understanding the pediatric PIVC survey tools used in future studies[13,30,60].
5.Strength and limitations
The three instruments translated in the current study have undergone rigorous translation processes that facilitated a complete linguistic validation of the instruments in the local health system setting, minimized errors, and ensured valid translation results.Furthermore,to our knowledge,no studies were translating a complete tool to assess the comprehensive picture of the current peripheral intravenous catheter insertion and care practice in pediatric patients, particularly in the Indonesian context, which encompassed the structure, process, and outcomes constructs of the service being evaluated.As such, the findings of this study provided a complete tool that clinicians and other stakeholders can use to evaluate the peripheral intravenous catheter insertion and maintenance service in pediatric patients.The pilot testing in the current study has followed the minimum guideline of instruments’assessment by Polit and Beck [11].However, the study is limited with small sample sizes involving three experts and ten-panel members for the content and face validity assessments.Further study using a larger sample size and advanced analysis such as factor analysis could be undertaken to evaluate and establish the psychometric properties of the instruments.
6.Conclusion
This study reports a rigorous and systematic process to translate the English language version of three tools (the point prevalence audit/pediatric PIVC use,management practice and outcome;nurse knowledge and confidence;and patient experience questionnaires)into Indonesian.The process facilitated a complete linguistic validation in the Indonesian context,generated a translated version of the survey tools,and emphasized the importance of understanding the different contexts where an instrument is developed and used.The cross-culturally validated tools provide a fundamental basis to ensure that the pediatric PIVC insertion and care practices in the local contexts are valid and comparable to other countries.Further psychometric statistical testing with larger samples is needed to determine the instruments’ psychometric properties and facilitate a comprehensive understanding of using the tools in the local pediatric PIVC insertion and management practices.
Funding
Nothing to declare.
Data availability statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Credit statement
Ferika Indarwati:Conceptualization, Methodology, Investigation,Validation,Formal analysis,Writing-Original Draft,Writing-Review and Editing.Judy Munday:Conceptualization, Methodology, Writing - Review and Editing.Samantha Keogh:Conceptualization, Methodology, Writing - Review and Editing, Supervision.
Declaration of competing interest
The authors have declared no conflict of interest.
Acknowledgments
The researcher acknowledges the Indonesian Endowment Fund for Education (LPDP) and Universitas Muhammadiyah Yogyakarta that provide scholarship funding for FI PhD study; all translators and experts validators that have given their time to support this study.
Appendix ASupplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijnss.2022.03.005.
 International Journal of Nursing Sciences2022年2期
International Journal of Nursing Sciences2022年2期
- International Journal of Nursing Sciences的其它文章
- Effects of mindfulness meditation on trait mindfulness, perceived stress,emotion regulation,and quality of life in hemodialysis patients:A randomized controlled trial
- Application of rational emotive behavior therapy in patients with colorectal cancer undergoing adjuvant chemotherapy
- The effect of slow deep breathing relaxation exercise on pain levels during and post chest tube removal after coronary artery bypass graft surgery
- The association between frailty of older stroke patients during hospitalization and one-year all-cause mortality:A multicenter survey in China
- Translation and piloting of the Chinese Mandarin version of an intensive care-specific pressure injury risk assessment tool (the COMHON Index)
- Distress management in cancer patients:Guideline implementation based on CAN-IMPLEMENT
